FOXA3: A Novel Tumor Suppressor in Esophageal Squamous Cell Carcinoma
Siang Zhang, Yuxiang Jin, and Qianyu Han contributed equally as co-first authors.
Funding: This project was funded by the National Natural Science Foundation of China (82104229).
ABSTRACT
Background
The forkhead box A (FOXA) family has been extensively studied in cancer research; however, the role of FOXA3 in malignant tumors, particularly esophageal squamous cell carcinoma (ESCC), is not well understood. This study explores the expression and function of FOXA3 in ESCC, assessing its potential as a prognostic marker and therapeutic target.
Methods
This study analyzed FOXA3 expression in ESCC tissues and its correlation with patient prognosis. The effects of FOXA3 overexpression on ESCC cell proliferation, migration, and invasion were examined in ESCC cell lines in vitro. Additionally, an in vivo tumorigenesis assay was performed using subcutaneous injection to assess the impact of FOXA3 overexpression on tumor growth. Statistical analyses were conducted to determine the significance of the results.
Results
FOXA3 expression was significantly reduced in ESCC tissues compared with it in paired adjacent normal tissues, and low FOXA3 expression was significantly associated with poor prognosis in ESCC patients. FOXA3 overexpression markedly inhibited ESCC cell proliferation, migration, and invasion. In addition, overexpression of FOXA3 repressed tumor growth in mice.
Conclusions
These findings indicate that FOXA3 acts as a tumor suppressor in ESCC, and its low expression is linked to poor outcomes. FOXA3 may serve as a potential diagnostic and therapeutic target for ESCC.
Abbreviations
-
- EAC
-
- esophageal adenocarcinoma
-
- ESCC
-
- esophageal squamous cell carcinoma
-
- FOXA3
-
- forkhead box A3
-
- IHC staining
-
- immunohistochemistry staining
-
- qRT-PCR
-
- quantitative real-time polymerase chain reaction
1 Introduction
Esophageal cancer is a common lethal malignant tumor globally, which can primarily be classified into two histological subtypes: esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC), with distinct geographical variance in the incidence [1]. The morbidity and mortality in China are higher than the world average level, and the majority of cases in China are ESCC [2]. The symptoms of esophageal cancer in early stage are subtle; patients are often diagnosed at the advanced stage, thereby losing the opportunity for radical surgery. Furthermore, even after undergoing surgical treatment and other traditional therapeutic approaches, the overall treatment outcome remains unsatisfactory [3, 4]. The current treatment principles for ESCC and EAC are approximately the same, mainly depending on the stage of the tumor, but they are actually two distinct diseases due to their significantly different oncological characteristics [5]. In the context of the rapid development of molecular targeted therapy in malignant tumor and the promising effect it has brought, there is an urgent need to clarify the molecular mechanisms related to the progression of esophageal cancer, particularly ESCC.
The FOX family, known as forkhead box proteins, is transcription factors characterized by a 110-amino-acid length and a highly conserved DNA binding domain, sharing homology with the Drosophila forkhead gene [6]. They are further categorized into 19 subfamilies from FOXA to FOXS based on the sequence of the forkhead box. The Fox family subfamily A (FOXAs) consists of three isotypes: FOXA1/hepatocyte nuclear factor (HNF)3α, FOXA2/HNF3β, and FOXA3/HNF3γ. Since the 110 amino acids, the so-called forkhead box of FOXAs, are structurally similar to the DNA binding motif of linker histones and can directly affect chromatin structure, this property makes them known as “pioneer factors” that bind to promoters and enhancers, enabling chromatin access for other transcription factors to exert a downstream role [7, 8]. The FOXA family is essential for organogenesis and differentiation, such as in the liver, pancreas, lung, and prostate. They can also regulate metabolism by regulating multiple target genes in the liver, pancreas, and adipose tissue to participate in the occurrence and development of disease [9-11]. Additionally, the FOXA family is closely related to the incidence, progression, and metastasis of malignant tumors [12-14]. Currently, FOXA1 and FOXA2 have been extensively studied, while the research on FOXA3 has mainly focused on embryonic development, glucose and lipid metabolism, and related diseases, as well as the emerging fields of liver regeneration and hepatic reprogramming [15, 16]. Up to now, the research of FOXA3 in malignant tumors is still limited compared with other family members.
FOXA1 and FOXA2 both contribute to the development and progression of esophageal cancer and Barrett's esophagus [17-20]. It is reported that FOXA3 is highly expressed in esophageal cancer and Barrett's esophagus, and the invasion and migratory ability of EC cells was significantly reduced following FOXA3 knockdown. Furthermore, FOXA1, FOXA2, and FOXA3 were found to play synergistic roles in the metastasis of esophageal cancer [21].
However, in our preliminary experiment, we discovered that the expression of FOXA3 was downregulated in human ESCC tissues through 11 fresh specimens. Therefore, we hypothesized that FOXA3 might play a role different from that previously reported [21]. In this study, we examined the expression of FOXA3 in ESCC patients, analyzed its correlation with prognosis, and subsequently utilized a series of assays to determine the exact role of FOXA3 in ESCC.
2 Methods
2.1 Patients and Tissue Samples
All tissue samples were collected from ESCC patients who underwent esophagectomy followed by two- or three-field lymph node dissection at our hospital. A total of 93 patients received surgical resection between January 2009 and December 2010. The clinicopathological characteristics of these patients are presented in Table 1. Informed consent was obtained from each patient, and this study was approved by the Ethics Committee of our hospital.
| Clinicopathologic parameter | FOXA3 low (N = 46) | FOXA3 high (N = 47) | p-value |
|---|---|---|---|
| Age | |||
| < 65 | 21 | 24 | 0.753 |
| ≥ 65 | 25 | 23 | |
| Gender | |||
| Male | 38 | 39 | 1.000 |
| Female | 8 | 8 | |
| Size of tumor (cm) | |||
| < 5 | 27 | 25 | 0.745 |
| ≥ 5 | 19 | 22 | |
| T-stage | |||
| T1–T2 | 12 | 11 | 0.953 |
| T3–T4 | 34 | 36 | |
| N-stage | |||
| N0 | 13 | 29 | 0.002 |
| N1–N3 | 33 | 18 | |
| Differentiation | |||
| G1 | 7 | 7 | 1.000 |
| G2–G3 | 39 | 40 | |
| p-TNM stage | |||
| I–II | 16 | 28 | 0.029 |
| III–IV | 30 | 19 | |
2.2 Cell Lines
ESCC cell lines (TE-1 and Eca-109) were obtained from Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences. All cells were cultured in DMEM high-glycemic medium supplemented with 10% fetal bovine serum at 37°C with 5% CO2.
2.3 Quantitative Real-Time PCR (qRT-PCR)
Total RNA was extracted from tissues and cells using TRIzol Reagent (Sigma). Reverse transcription and qRT-PCR were performed with the Bestar one-step qRT-PCR kit (SYBR Green). β-actin was used as an internal control. The primers at exon–exon junctions were designed using Primer Blast. During the design process, we changed the Exon junction span option to Primer must span an exon–exon junction, which means that at least one primer crosses the junction between exons. In this way, even if genomic DNA contaminates cDNA, it will not be amplified, ensuring accurate quantification. The primer sequences used in this paper were shown in Table 2.
| Gene | Forward primer sequence (5′-3′) | Reverse primer sequence (5′-3′) |
|---|---|---|
| FOXA3 | CTGGCCGAGTGGAGCTACTA | AGGGGGATAGGGAGAGCTTA |
| β-actin | AATCGTGCGTGACATTAAGGAG | ACTGTGTTGGCGTACAGGTCTT |
2.4 Immunohistochemistry (IHC) Staining
ESCC tissue slides were deparaffinized, rehydrated, and subjected to heat-induced antigen retrieval. Subsequently, they were blocked with 5% BSA. The slides were then incubated overnight at 4°C with a primary antibody against FOXA3 (Sigma, rabbit, 1:1000) and subsequently with a secondary antibody for 30 min. The expression levels, along with the staining intensity, were assessed based on the German immunohistochemical score (GIS). The percentage of positive cells was classified as 0 (negative), 1 (< 10% positive cells), 2 (11%–50% positive cells), 3 (51%–80% positive cells), or 4 (> 80% positive cells). Staining intensity was classified as 0 (no staining), 1 (weak), 2 (moderate), or 3 (strong). The final immunoreactive GIS was defined as the product of both grading results (percentage of positive cells × staining intensity). According to scores averaged, the expression of FOXA3 was divided into two groups: high expression group [2, 3] and low expression group (0, 1).
2.5 Lentivirus Infection
Recombinant lentiviral vectors containing green fluorescence protein (GFP) were obtained from HanBio Technology Co. Ltd. (Shanghai, China). The resultant plasmid was sequenced to confirm the authenticity of the insert. A total of 5 × 105 Eca-109 and TE-1 cells were seeded on six-well plates and further incubated for 12 h to reach 30% confluence, and then infected with lentiviral vectors expressing either vector control (LV-Con) or LV-FOXA3 by replacing the infection medium containing recombinant vectors at a multiplicity of infection (MOI) of 20 plaque-forming units (pfu) per cell. Three days later, the GFP density contained by lentivirus was detected to assess the infection efficiency, and cells were harvested for qRT-PCR and Western blot.
2.6 Western-Blot Assay
The protein was extracted from cell lysis using RIPA lysis buffer, and equal amounts of proteins were separated by SDS-PAGE and transferred to nitrocellulose filter membranes. Then, the membranes were blocked with 5% skimmed milk for 1 h and incubated with the primary antibody against FOXA3 (ab108454, 1:500, Santa Cruz Biotechnology) or GAPDH (1:5000, Santa Cruz Biotechnology) at 4°C overnight. After being washed three times, the membranes were incubated with the secondary antibody for 1 h. The blots were visualized with the enhanced chemiluminescence substrate.
2.7 Colony Forming Assay
Each group of cells was seeded onto petri dishes and maintained for 2 weeks, respectively. Then, cells were fixed in 4% paraformaldehyde and stained with crystal violet. The visible colonies were counted manually after taking photographs.
2.8 Cell Counting Kit 8 (CCK-8) Assay
Cells were seeded onto the 96-well plates at a density of 1000 cells per well and incubated for 24, 48, and 72 h at 37°C. Cells were then incubated with 10-μL CCK-8 solution (R&D Systems) for 2 h. Cell counts were measured in triplicate by measuring absorbance at 450 nm using the microplate readers (BioTek Instruments). And then the cell proliferation curve was plotted according to the OD value.
2.9 5-Ethynyl-2′-Deoxyuridine (EdU) Assay
Cell proliferation was measured using the EdU assay kit (RiboBio, China). First, cells were seeded onto the 96-well plates and incubated with EdU (50 μM) solution for 2 h at 37°C. Cells were then fixed in 4% paraformaldehyde for 30 min and permeabilized with 0.5% Triton X-100 for 10 min at room temperature. After washed with PBS, cells were incubated with 1× Apollo staining reaction (100 μL) in the dark for 30 min. Subsequently, cells were stained with Hoechest33342 (100 μL) for 30 min to visualize cell nucleus. The images were obtained using the fluorescence microscope (OLYMPUS, Japan). Cell proliferation was analyzed based on the percentage of EdU-positive cells for each sample.
2.10 Wound Healing Assay
Cells were seeded on 6-well dish at a density of 3 × 106/mL and grown until reaching 90% confluence. A 200-μL pipette tip was used to create a straight scratch in each well, and the images were captured at 0 and 48 h under a microscope to observe wound closure.
2.11 Transwell Assay
Tumor cell migration assay was performed using a Transwell chamber with 8-μm pores in 24-well plates. Cells (2 × 105/mL) were suspended in serum-free medium and seeded into upper chambers, while the lower chambers were filled with medium containing 10% FBS. After 24 h of incubation, cells remaining in the upper chamber were removed. Cells that had migrated onto the membrane were fixed and stained with crystal violet. Quantification was based on counting the cells in five randomly selected fields under a microscope at 100× magnification.
For the invasion assay, similar steps were followed using Transwell inserts pre-coated with Matrigel (8 μm, BD Biosciences) to simulate the extracellular matrix. All other procedures were the same as the migration assay.
2.12 Tumorigenesis by Subcutaneous Injection
All animal procedures were approved by the Medical Experimental Animal Care Commission in Navel Military Medical University. Six-week-old nude mice were randomly divided into two groups: Eca-109 (LV-Con) and Eca-109 (LV-FOXA3). Each group received a subcutaneous injection of cells (2 × 106/mL) into the back. Six weeks after the injection, the mice were sacrificed, and the tumors were excised and weighed.
2.13 Statistical Analysis
Data were expressed as the mean ± standard error of the mean (SEM). Categorical variables were analyzed using the Chi-squared test, while continuous variables were assessed with the Mann–Whitney U test. Overall survival (OS) analysis was conducted using univariate and multivariate Kaplan–Meier survival analysis and Cox regression analysis. OS was described as the time frame between date of surgery to the date of death or the last follow up date: July 2015. Statistical significance level was p-values < 0.05. Statistical analyses were achieved using the SPSS version 22.0.
3 Results
3.1 FOXA3 Is Down-Regulated in Human ESCC Tissues
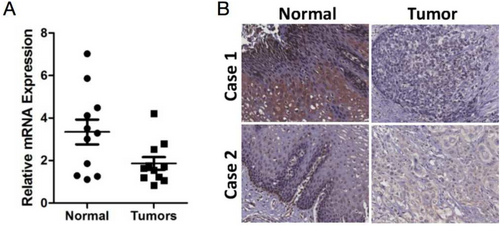
We initially collected 11 human ESCC tissue samples and paired adjacent normal tissues to detect the expression of FOXA3 by RT-PCR. As shown in Figure 1A, FOXA3 expression was significantly lower in human ESCC tissues compared to the paired adjacent normal tissues (p = 0.022). The results from IHC staining also revealed the staining intensity of FOXA3 in 68/93 of normal tissues was noticeably stronger than that in tumors, as shown in Figure 1B. All these results suggested that FOXA3 might play an important role in the progression of ESCC.
3.2 Association of FOXA3 Expression With Clinicopathological Characteristics
To further explore the role of FOXA3 in the progression of ESCC, we analyzed the correlation between FOXA3 expression levels and the clinicopathological characteristics of ESCC patients (age, gender, tumor size, histology grade, TNM stage, T grade, and lymph node metastasis status). All of ESCC patients were divided into two groups according to FOXA3 expression level. As shown in Table 1, FOXA3 expression was inversely correlated with lymph nodes metastasis (p = 0.002) but did not show a significant association with age, gender, tumor size, or differentiation. In addition, FOXA3 was correlated with TNM stage (p < 0.05). However, due to the small number of cases in stages I and IV (5 cases each) and the fact that the primary difference between stages II and III was lymph node metastasis (N0 vs. N1–3), the correlation between FOXA3 expression and pTNM stage requires further investigation with more early- and advanced-stage cases.
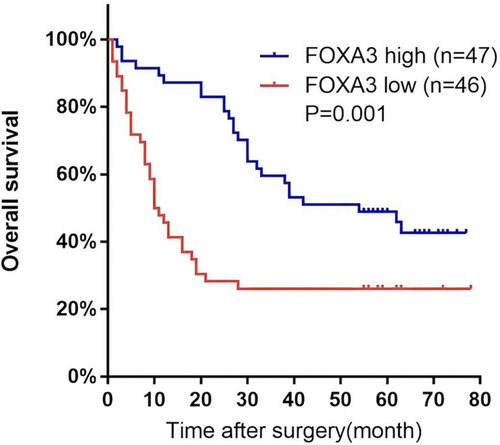
Kaplan–Meier survival analysis indicated that higher FOXA3 expression was associated with better overall survival in ESCC patients (p = 0.001, Figure 2). Univariate Cox regression analysis revealed that both lymph node metastasis and low FOXA3 expression were significantly associated with poorer survival (p = 0.001 and p = 0.002, respectively, Table 3). Multivariate Cox regression analysis confirmed that lymph node metastasis and FOXA3 expression were independent prognostic factors for overall survival (p = 0.044 and p = 0.032 respectively, Table 4).
| Clinicopathologic parameter | HR | 95% CI | p-value |
|---|---|---|---|
| Gender | 1.055 | 0.548–2.030 | 0.872 |
| Age | 1.309 | 0.786–2.178 | 0.302 |
| Size of tumor | 0.693 | 0.411–1.168 | 0.169 |
| T-stage | 0.965 | 0.538–1.732 | 0.906 |
| Lymph node metastasis | 2.327 | 1.370–3.953 | 0.002 |
| Differentiation | 1.234 | 0.586–2.601 | 0.580 |
| FOXA3 | 0.422 | 0.251–0.709 | 0.001 |
| Clinicopathologic parameter | HR | 95% CI | p-value |
|---|---|---|---|
| Lymph node metastasis | 1.811 | 1.016–3.228 | 0.044 |
| FOXA3 | 0.539 | 0.306–0.948 | 0.032 |
3.3 FOXA3 Inhibits the Proliferation of ESCC Cells
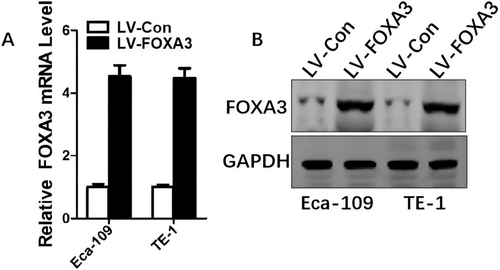
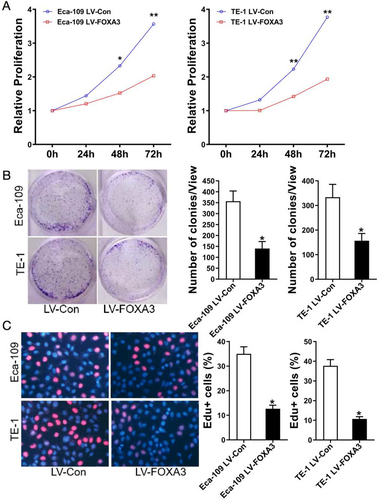
To investigate the functional role of FOXA3 in ESCC, we generated stable FOXA3-overexpressing cell lines and confirmed the upregulation of FOXA3 expression at both the mRNA and protein levels (Figure 3A,B). The influence of FOXA3 on ESCC cell viability was investigated using the CCK-8 assay. As shown in Figure 4A, FOXA3 overexpression significantly decreased the viability of ESCC cells. To further validate these findings, we performed colony formation and EdU assays, and the results demonstrated that FOXA3 overexpression markedly reduced ESCC cell proliferation (Figure 4B,C). These results collectively indicated that FOXA3 inhibited the proliferation of ESCC cells.
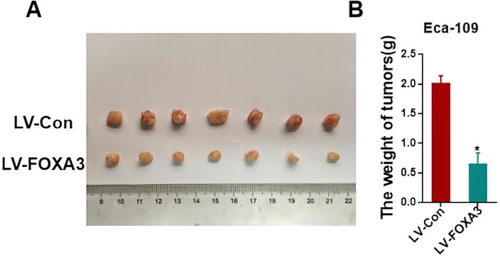
3.4 FOXA3 Inhibits the Tumorigenicity In Vivo
To investigate the effects of FOXA3 on tumorigenicity in vivo, Eca-109 cells transfected with FOXA3-overexpressing vectors were injected into the backs of nude mice. As shown in Figure 5, the average tumor weight in the FOXA3 overexpression group (0.6586 ± 0.1764 g) was significantly lighter than that in the control group (2.021 ± 0.1162 g, p < 0.05). This result suggested that FOXA3 could suppress tumor growth in vivo.
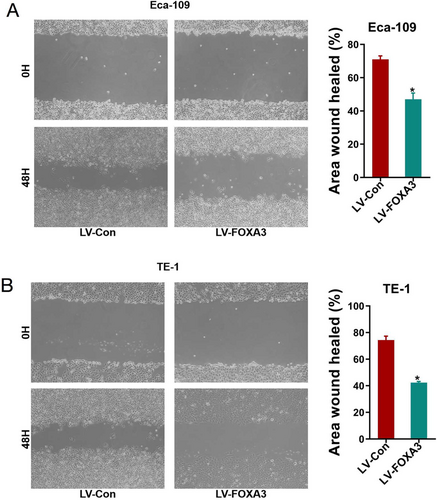
3.5 FOXA3 Inhibits Migration and Invasion of ESCC Cells
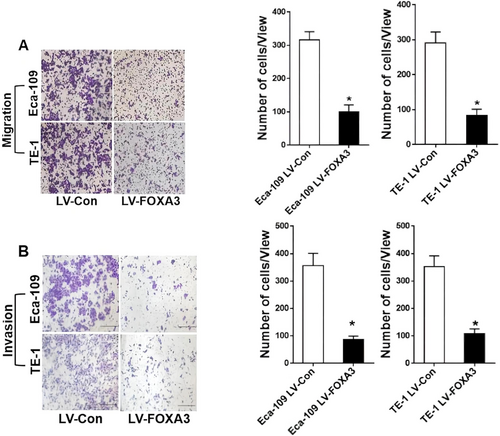
Given the correlation between lower FOXA3 expression and lymph node metastasis, we hypothesized that FOXA3 might also play a role in tumor metastasis. Tumor metastasis itself is the greatest cause of death in cancer patients and is an extremely complex, multi-step process that involves the migration and invasion of cancer cells, which is the key step in cancer metastasis. Thus, we evaluated the effect of FOXA3 overexpression on ESCC cell migration and invasion. As shown in Figure 6, FOXA3 upregulation significantly reduced cell migration. The results of the Transwell migration assay (Figure 7A) further confirmed that fewer cells migrated in the LV-FOXA3 group compared to the control group. Similarly, the invasion assays (Figure 7B) demonstrated a significant reduction in the invasive potential of both Eca-109 and TE-1 cells after FOXA3 overexpression. These results were in accordance with our clinical data, indicating the suppressive role of FOXA3 in the metastasis of ESSC.
4 Discussion
ESCC ranks as the seventh most common cancer globally and the sixth leading cause of cancer-related deaths [1]. Despite advancements in diagnosis and surgical treatment, ESCC prognosis remains unsatisfactory [22]. Therefore, identifying new molecular markers and effective therapeutic targets for ESCC is urgent. The detection of tumor-specific biomarkers, which is the goal of this study, could prove useful in the diagnosis and treatment of ESCC.
It has been reported that FOXA members play a key role in esophageal cancer. FOXA3 is an important member of the hepatocyte nuclear factor family and is prominently expressed in the liver [23]. Some studies have shown contradictory roles for FOXA3 in different cancers. For example, FOXA3 promoted hepatoblastoma progression by regulating HNF1A, AFP, and ZFHX3 expression [24]. Meanwhile, it was previously described that FOXA3 inhibited cancer stemness and partially potentiates chemosensitivity in colorectal cancer cells [25]. Notably, a previous study showed that upregulated FOXA3 promoted metastasis in esophageal cancer [21], contrasting with our preliminary results indicating downregulated FOXA3 mRNA expression in human ESCC tissues (n = 11).
In this study, the expression level of FOXA3 in ESCC tissues (n = 93) was examined using immunohistochemistry. Subsequently, we investigated the correlation of FOXA3 with clinicopathologic characteristics of ESCC patients. Kaplan–Meier survival analysis indicated that ESCC patients with low FOXA3 expression in general had worse prognosis than those with high FOXA3 expression. Univariate and multivariate Cox regression analysis indicated that low FOXA3 expression was an independent risk factor for the prognosis of ESCC patients. The clinical data also showed that low FOXA3 level was correlated with lymph node metastasis.
To further explore whether FOXA3 acts as a tumor suppressor in ESCC, we conducted functional assays including CCK-8, colony formation, and EdU assays, all demonstrating that FOXA3 overexpression significantly inhibited ESCC cell proliferation. Subsequently, using a xenograft mouse model with Eca-109 cells, we observed that FOXA3 markedly inhibited tumor growth in vivo.
Despite our patient cohort consisting exclusively of post-operation cases without distant metastasis, lower FOXA3 expression remained strongly associated with lymph node metastasis, suggesting FOXA3's potential dual role in inhibiting both proliferation and metastasis in ESCC. Tumor metastasis itself is the biggest cause of death in cancer patients and is an extremely complex process involving multiple steps [26, 27]. Furthermore, the ability of cell invasion and migration is considered the most crucial to tumor metastasis [28]. Notably, cell migration and invasion assays further supported FOXA3's suppressive effect on ESCC metastatic potential.
Taking these results together, FOXA3 may exert tumor-suppressive roles in ESCC by inhibiting cancer cell proliferation and invasion. However, intriguingly, previous studies indicate FOXA3's involvement in promoting esophageal cancer metastasis [21]. To analyze that, it is worth noting that the esophageal cancer case data in the GSE6059, GSE13898, and Oncomine datasets chosen in the above study did not distinguish the specific pathological type of esophageal cancer, and 96 pairs of surgically removed tumor tissues also covered multiple pathological types involving not only ESCC, but also undifferentiated carcinoma, and Siewert type I gastroesophageal junction adenocarcinoma. Furthermore, the specific number of each pathological type was not mentioned. Since ESCC and EAC are obviously two different diseases according to their oncogenic characteristics and all tissue specimens we chose for our study are all ESCC, it is understandable that the two studies have inconsistent results.
The limitation of this study is that there was only subcutaneous tumorigenesis assay that was done on nude mice, and the effect of FOXA3 on tumor metastasis in vivo has not been studied yet. The lack of research on the mechanism of how FOXA3 is involved in the process of tumor proliferation, and metastasis is another disadvantage, which may also partly explain the inconsistency with the results of previous studies.
The specific role of FOXA3 may be controversial, but there is no doubt that FOXA3 is quite crucial for the development and progression of ESCC, and FOXA3 is expected to become a molecular biomarker, a prognostic indicator, and an effective therapeutic target for ESCC. Our research suggests that targeting FOXA3 to increase FOXA3 expression may be a potential therapeutic strategy for ESCC. Therefore, in the next step, animal models of tumor metastasis need to be constructed to clarify the role of FOXA3 on metastasis in vivo, and further research should be carried out to seek the signaling pathways and molecular networks involved in the function of FOXA3 in ESCC.
5 Conclusion
In summary, we have reached the preliminary conclusion that FOXA3 downregulation was significantly correlated with a poor prognosis of ESCC and FOXA3 might play a suppressive role in the growth and metastasis of ESCC. These results indicate that FOXA3 is a potential diagnostic and therapeutic target for ESCC. In addition, targeting FOXA3 to increase its expression in patients may be a potential therapeutic strategy for ESCC.
Author Contributions
Xuewei Zhao served as the guarantor of integrity of the entire study. Siang Zhang contributed to the study concepts, study design, data acquisition, and manuscript preparation. Lei Xue assisted with the study design and manuscript review. Yuxiang Jin and Qianyu Han conducted the experimental studies and participated in manuscript editing. Qianyu Han also handled data analysis and statistical analysis. All authors reviewed the manuscript.
Acknowledgments
The authors have nothing to report.
Ethics Statement
The experimental protocol was established, according to the ethical guidelines of the Helsinki Declaration and was approved by the Ethics Committee of Changzheng Hospital (approval number: 2020sl004). Informed consent was obtained from each patient.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




