Safety and efficacy of pentazocine–midazolam combination for pain and anxiety relief in radiofrequency ablation therapy for hepatocellular carcinoma
Declaration of conflict of interest: None.
Abstract
Background and Aim
Radiofrequency ablation (RFA) therapy is frequently used as first-line treatment for small hepatocellular carcinoma (HCC). RFA is often associated with pain; however, no definitive solution has been established for its relief. We retrospectively analyzed the safety and efficacy of the combination of pentazocine and midazolam to relieve pain experienced by HCC patients undergoing RFA.
Methods
We studied 77 patients with 98 HCCs treated with RFA between January 2015 and August 2019. Patients were divided into two groups: the sedative-free group, which included those who received pentazocine alone, and the pentazocine–midazolam group, which included those who received a combination of pentazocine and midazolam. The degrees of analgesia and sedation were evaluated using the numerical rating scale (NRS) and the Richmond Agitation-Sedation Scale (RASS), respectively. Other parameters such as treatment time, awakening time, midazolam dosage, vital signs, local recurrence rate, and time to recurrence were also examined.
Results
The median NRS score and RASS score were significantly lower in the pentazocine–midazolam group. Ninety-five percent of patients in the pentazocine–midazolam group had no memory of the RFA session. The treatment time and awakening time were prolonged for the pentazocine–midazolam group. No significant differences in oxygen saturation, recurrence rates, and time to local recurrence were observed between groups.
Conclusion
A combination of pentazocine and midazolam is safe and effective for pain and anxiety relief experienced by patients undergoing RFA for local treatment of HCC.
Background
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide. It ranks fourth in terms of cancer-related deaths. According to the 2018 global statistics, approximately 841 000 new cases and 782 000 deaths occur annually. The incidence and mortality rates of HCC are two to three times higher for men, with the highest number of cases occurring in East Asia and Southeast Asia.1 HCC, often secondary to chronic hepatitis B and C, is the fifth leading cause of death in Japan.2 Hepatectomy and ablation are recommended as first-line curative treatments for small HCCs.3, 4 Ablation is increasingly preferred over invasive surgery because of the aging demographic profile of HCC and metastatic liver tumors. Because of the high recurrence rate of HCC, multiple ablations are often required.5-12 The duration and frequency of ablation therapy are calculated based on the size and number of tumors. It is important to reduce any pain associated with the procedure to improve patient compliance for retreatment. Several studies have reported pain reduction methods using analgesics and sedatives for patients undergoing radiofrequency ablation (RFA) for local treatment of liver tumors; however, no definitive solution has been established.13-17 During this study, we retrospectively examined the safety and efficacy of a combination of pentazocine and midazolam to relieve any pain and anxiety associated with RFA therapy for HCC.
Materials and methods
Patients
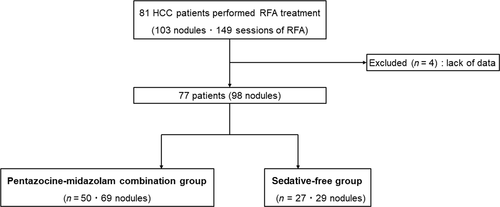
From January 2015 to August 2019, RFA was performed for 81 patients diagnosed with HCC at Jikei University Hospital and Fuji City General Hospital. We performed 149 RFA sessions for 103 HCC nodules; of these, we retrospectively analyzed 98 nodules from 77 patients who could be followed up after RFA. This study was not randomized or blinded. From May 2015 to August 2019, RFA was performed for 27 consecutive cases at Jikei University Hospital with pentazocine alone. Additionally, at Fuji City General Hospital, RFA was performed for 50 consecutive cases from January 2015 to September 2017, using a combination of pentazocine and midazolam. Patients were not selected based on tumor size, age, or tumor localization. HCC was diagnosed using dynamic computed tomography (CT), dynamic magnetic resonance imaging (MRI), contrast-enhanced ultrasonography (US), and abdominal angiography. The diagnosis was confirmed by observing early enhancement during the arterial phase, and a washout during the portal phase or a perfusion defect using dynamic CT, dynamic MRI, contrast-enhanced US, CT-assisted hepatic arteriography, and conventional CT scans acquired during CT arterial portography. Fifty of the patients with 69 nodules were categorized into the pentazocine–midazolam group and the remaining 27 patients with 29 nodules were categorized into the sedative-free group. This study was approved by the Ethics Committee of Jikei University School of Medicine for Biomedical Research (approval no. 31-023 [9522]). Informed consent regarding study participation was officially obtained using a web page.
Radiofrequency ablation
RFA treatment sessions were performed by three hepatologists. The following instruments were used: RF3000 generator (Boston Scientific Japan Co., Tokyo, Japan), 18G LeVeen™ needle electrode (Boston Scientific Japan Co.), Cool-tip™ RFA system (Covidien, Boulder, CO, USA), and VIVARF system (STARmed Co., Korea). When using the RF3000 generator, ablation was initiated using power of 30 W with a 2-cm needle and 40 W with a 3-cm needle. The output was increased by 10 W/min until roll-off occurred because of the increase in tissue impedance. RFA was repeated after a 1-min interval. Using the Cool-tip needle, ablation was started at 30 W for the 2-cm needle and 40 W for the 3-cm needle. The output was increased by 5 W every 30 s to obtain a maximum output of 100–120 W. By gradually increasing the output from a low level, it was possible to perform thermal coagulation over a sufficient period of time. If the final temperature of the tissue exceeded 60°C, then ablation was completed with a single break. The VIVARF system is a relatively new device with a six-stage variable needle. RFA was performed in the continuous mode with initial powers of 20, 30, and 40 W for the 1.5-, 2-, and 3-cm needles, respectively. The output was increased manually, as was the Cool-tip RFA system.
Intravenous anesthesia
Sedative-free group
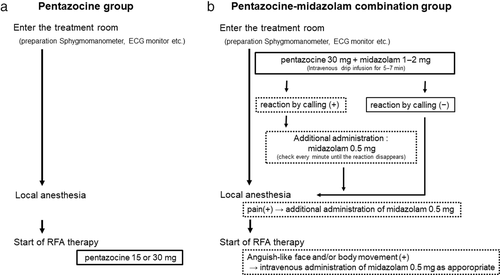
On admission to the treatment room, each patient was subjected to abdominal ultrasonography to localize the lesion, followed by local disinfection of the skin while preparing for RFA. After local anesthesia, pentazocine (15–30 mg) was administered before RFA commencement (Fig. 1a). During therapy, the patient's vital signs, including blood pressure, pulse rate, and oxygen saturation, were checked every 5 min using an electrocardiogram monitor. For patients with decreased oxygen saturation, oxygen administration and airway insertion were performed as appropriate. Patients were able to hold their breath on command when the needle was punctured.
Pentazocine–midazolam group
Immediately after admission to the treatment room, each patient was administered an intravenous infusion of pentazocine (30 mg) and midazolam (1–2 mg). Simultaneously, the preparations for RFA and local disinfection of the skin were performed. At the end of the infusion, the consciousness of the patient was determined by a response call. If there was no response to the call, then we proceeded to administer local anesthesia. Conversely, if the patient responded to the call or opened the eyes, then 0.5 mg midazolam was administered intravenously and repeated every 1 min until the patient was no longer responsive. If a pain reaction, such as frowning, was observed during local anesthesia, then 0.5 mg midazolam was administered intravenously again and repeated until the pain response disappeared. Similarly, during RFA, if the patient had a facial expression or body movement suggestive of pain, then an additional 0.5 mg of midazolam was administered intravenously as appropriate (Fig. 1b). With adequate administration of midazolam, the patients became oblivious to the procedure from the time of local anesthesia. Changes in blood pressure, pulse rate, and oxygen saturation were monitored throughout the procedure. Oxygen administration and oral pharyngeal airway insertion were performed as appropriate for patients with decreased oxygen saturation. Because of sedation, it was not feasible for patients to hold their breath on command.
Evaluation
We assessed the pain caused by RFA therapy using different scoring systems. Analgesia was assessed on the day after RFA therapy through patient feedback using the numerical rating scale (NRS). The NRS, which has been validated as an international evaluation tool, divides the degree of pain felt into 11 points using a scale of 0–10, with 0 indicating no pain and 10 indicating the worst pain imaginable.18-20 The degree of sedation was evaluated using the Richmond Agitation-Sedation Scale (RASS), which was devised by Sessler et al. in 2002.21 It consists of a total of 10 criteria (−5 to +4) and is considered to have a numerical structure that avoids the complexity of summing multiple subscale scores. Scores of 0 to +4, which indicate calmness and restlessness, can be evaluated by observing the patient's behavior. Scores of −1 to −3 are determined based on whether and for how long the patient makes eye contact in response to the call. If the patient does not respond to the call, then physical stimuli can be used to assess scores of −4 and −5. Compared with other sedation scoring systems, the RASS also has the advantage of being able to assess the patient within a shorter amount of time.22 We used the RASS for this study because it was the simplest and most reliable sedation evaluation scale available. Subsequently, we evaluated the extent of tumor ablation using dynamic CT or contrast-enhanced MRI within 2–3 days of RFA therapy. Local treatment for liver cancer was terminated if a sufficient safety margin of 5 mm or more was confirmed, and additional RFA sessions were performed soon thereafter for patients with insufficient safety margins. Intrahepatic recurrence of HCC was classified as local tumor progression and intrahepatic distant recurrence. During this study, local recurrence was defined as recurrence along the peripheral margin of the ablation region.23, 24
Follow-up and endpoints
The primary endpoint of this study was patient distress during RFA therapy. The secondary endpoints were the safety and efficacy of pentazocine–midazolam during RFA therapy and the subsequent likelihood of local recurrence of HCC.
Statistical analysis
A statistical analysis was performed using SPSS version 20 statistical software (IBM Corp., Armonk, NY, USA). Each variable was represented by its median. We performed the data analysis using the Mann–Whitney U test, Pearson's chi-squared test, and log rank test (Mantel–Cox). Statistical significance was set at P < 0.05.
Results
Baseline patient characteristics
Figure 2 shows the diagnoses of the patients included in this study. We excluded four patients who were difficult to follow-up. The baseline characteristics of all HCC patients enrolled in this study are shown in Table 1, and the median of each data point is provided. The baseline point was defined as the day of commencement of RFA therapy. The median age of all patients was 74 years (range, 37–87 years), and 77.9% of patients (60 patients) were male. The distribution of patients in the Child–Pugh A category was as follows: 41 patients (53.2%) had a score of 5 points and 21 patients (27.3%) had a score of 6 points. The Barcelona clinic liver cancer staging for both groups is provided. In the sedative-free group, 18 cases (67%) were stage 0 and 7 cases (26%) were stage A. However, 20 cases (40%) of the pentazocine–midazolam group were stage 0 and 25 cases (50%) were stage A. The median tumor diameters were 14 mm (range, 10–45 mm) and 15 mm (range, 6–51 mm), respectively; there was no significant difference. Additionally, there was no significant difference between the two groups regarding areas difficult to treat with RFA. Serum albumin levels were significantly lower in the pentazocine–midazolam group (3.7 g/dL; range, 2.3–3.7 g/dL) than in the sedative-free group (3.9 g/dL; range, 3.1–4.5 g/dL). No other obvious differences in patient background characteristics were observed.
| Sedative-free group | Combination group | P value | |
|---|---|---|---|
| Age | 75 (51–83) | 72 (37–87) | 0.806‡ |
| Sex: male/female, n | 20/7 | 40/10 | 0.550† |
| Body weight (kg) | 61 (35–100) | 60 (32–104) | 0.627‡ |
| AST (IU/L) | 38 (15–79) | 35 (11–130) | 0.763‡ |
| ALT (IU/L) | 33 (8–90) | 24 (2–155) | 0.230‡ |
| T.Bil (mg/dL) | 0.9 (0.4–3.4) | 0.6 (0.2–2.6) | 0.006‡ |
| ALP (IU/L) | 355 (183–607) | 321 (187–792) | 0.366‡ |
| γGTP (IU/L) | 45 (19–211) | 39 (13–806) | 0.349‡ |
| Alb (g/dL) | 3.9 (3.1–4.5) | 3.7 (2.3–3.7) | 0.022‡ |
| BUN (mg/dL) | 13 (9–92) | 15 (6–79) | 0.187‡ |
| Cr (mg/dL) | 0.8 (0.6–1.61) | 0.8 (0.5–6.8) | 0.185‡ |
| WBC (/μL) | 4400 (2100–7600) | 4800 (2700–11 200) | 0.174‡ |
| Hb (g/dL) | 13.2 (8.6–16.5) | 12.9 (9–17.4) | 0.669‡ |
| Plt (104/μL) | 11.7 (5.2–40) | 13.9 (4.8–101) | 0.577‡ |
| PT (%) | 87 (63–100) | 80 (6–100) | 0.184‡ |
| AFP (ng/mL) | 4 (1–52) | 7 (0.5–120) | 0.003‡ |
| PIVKAII (mAU/mL) | 21 (10–549) | 27 (8–649) | 0.848‡ |
| HBV/HCV/AL/PBC/NBNC, n | 4/9/5/3/6 | 0/32/10/0/8 | 0.002† |
| Child–Pugh: A/B/C/non LC, n | 25/2/0/0 | 38/10/0/2 | 0.178† |
| BCLC staging: 0/A/B/C/D, n | 18/7/2/0/0 | 20/25/5/0/0 | 0.078† |
| Number of total tumors, n | 29 | 69 | 0.01‡ |
| Tumor location: S1/2/3/4/5/6/7/8, n | 0/0/0/2/5/10/3/9 | 0/2/8/10/13/8/12/16 | 0.058† |
| Tumor size (mm) | 14 (10–45) | 15 (6–51) | 0.871‡ |
| Difficult area of RFA treatment, n (%) | 5 (17.2) | 3 (4.3) | 0.086† |
- BCLC staging, Barcelona Clinic Liver Cancer staging; RFA, radiofrequency ablation.
- † Pearson's chi-squared test.
- ‡ Mann–Whitney U test.
NRS evaluation after RFA therapy
The median NRS scores were 5.0 (range, 1–10) and 0 (range, 0–2) for the sedative-free and pentazocine–midazolam groups, respectively, with significantly higher scores observed in the former (P < 0.001) (Table 2).
| Sedative-free | Combination group | P value | |
|---|---|---|---|
| Numerical rating scale | 5.0 (1–10) | 0 (0 to 2) | <0.001† |
| Richmond agitation-sedation scale | 2.0 (1–2) | −5.0 (−5 to 2) | <0.001† |
| Satisfaction with sedation (%) | |||
| Rate of complete forgetting of treatment | 0 | 95 | <0.05‡ |
| The dose of midazolam (mg) | |||
| Pretreatment dose | 0 | 2.0 (0.5 to 6.0) | <0.001† |
| Total dose | 0 | 3.0 (0.5 to 20) | <0.001† |
| Generator of RFA treatment, n | |||
| RF3000/Cool-tip RF System/VIVA RF generator | 27/2/0 | 0/62/7 | <0.001‡ |
| Number of pass | 1 (1–3) | 1 (1 to 6) | 0.267† |
| Treatment time (min) | 30 (10–85) | 55 (20 to 168) | <0.001† |
| Ablation time (s) | 364 (165–1532) | 470 (70 to 1500) | 0.064† |
| Time to awakening (min) | 0 | 155 (0 to 610) | <0.001† |
| Oxygen administration, n (%) | 7 (25.9) | 42 (84) | <0.001‡ |
| Oral pharyngeal airway insertion, n (%) | 0 (0) | 9 (18) | 0.019‡ |
| Vital signs | |||
| The rate of change (post/pre) | |||
| Systolic blood pressure | |||
| Rate of increase | 1.14 (0.92–1.55) | 1.16 (0.97 to 1.68) | 0.267† |
| Rate of decrease | 0.95 (0.46–1.25) | 0.88 (0.39 to 1.13) | 0.004† |
| Pulse rate | 1.07 (0.94–1.45) | 1.15 (0.89 to 1.63) | 0.018† |
| Oxygen saturation | 0.97 (0.89–1.00) | 0.97 (0.30 to 1.0) | 0.857† |
| Local recurrence rate, n (%) | 5 (17.2) | 13 (18.8) | 0.852‡ |
| time to recurrence (days) | 577 (197–926) | 282 (80 to 1191) | 0.143† |
- RFA, radiofrequency ablation.
- † Mann–Whitney U test.
- ‡ Pearson's chi-squared test.
RASS evaluation during RFA therapy
The median RASS scores were 2.0 (range, 1–2) and −5.0 (range, −5 to 2) for the sedative-free and pentazocine–midazolam groups, respectively, with significantly lower scores observed in the latter (P < 0.001) (Table 2). With the preemptive use of midazolam, it was possible to maintain a sedative state close to a temporary deep trance, thus leaving patients unresponsive to calls and physical stimuli.
Pain reduction effect during RFA therapy
Regarding pain relief during RFA therapy, 95% of the patients in the pentazocine–midazolam group had no memory of the RFA session itself. Compared with the sedative-free group, the pentazocine–midazolam group had significantly less treatment-related distress (P < 0.05) (Table 2).
Sedation dose, treatment time, and time to awakening
The median pretreatment dose of midazolam in the pentazocine–midazolam group was 2 mg (range, 0.5–6 mg) before RFA therapy, and the median total midazolam dose at the end of RFA therapy was 3 mg (range, 0.5–20 mg). The treatment time was calculated from the start of pentazocine or pentazocine–midazolam infusion until the end of RFA. The median treatment times were 30 min (range, 10–85 min) and 55 min (range, 20–168 min) in the sedative-free group and the pentazocine–midazolam group, respectively, and the treatment time was significantly longer in the pentazocine–midazolam group (P < 0.001). The times from the end of treatment to awakening were 0 and 155 min (range, 0–610 min), respectively. Time to awakening was significantly longer in the pentazocine–midazolam group (P < 0.001). However, the ablation time did not differ between groups (Table 2).
Changes in vital signs during RFA therapy
Changes in systolic blood pressure and pulse rate before and during the RFA session were analyzed across the sedative-free group and pentazocine–midazolam group. The rate of increase in systolic blood pressure was not significantly different between groups (1.14 vs 1.16). However, the rate of decrease was significantly greater in the pentazocine–midazolam group (0.95 vs 0.88; P = 0.004). The rate of change in the pulse rate was also greater in the pentazocine–midazolam group (1.07 vs 1.15; P = 0.018). There was no clear difference in the rate of change in oxygen saturation between groups (0.97 vs 0.97). Both oxygen administration and the oral pharyngeal airway insertion rate were significantly higher in the pentazocine–midazolam group (26% vs 84%; P < 0.001) than in the sedative-free group (0% vs 18%; P = 0.019) (Table 2).
Recurrence rate and time to recurrence after RFA therapy
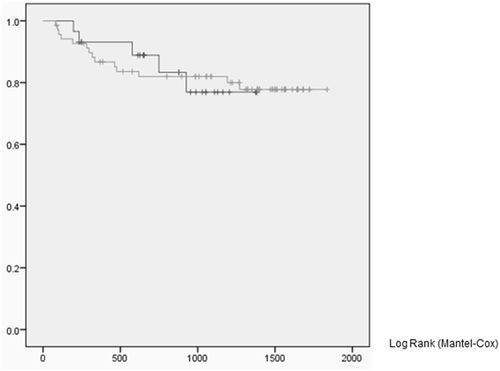
The local recurrence rates were 17.2% in the sedative-free group and 18.8% in the pentazocine–midazolam group, with no significant difference between groups. The times to local recurrence were 577 days (range, 197–926 days) and 282 days (range, 80–1191 days) in the sedative-free group and pentazocine–midazolam group, respectively; no significant difference was observed (Table 2, Fig. 3).
Discussion
RFA is an effective treatment for eradicating early-stage HCC using nonsurgical methods; however, patients often experience distress, such as anxiety and intense pain.25-27 Pentazocine and morphine have been used as analgesics,15, 28, 29 and benzodiazepines, such as midazolam, and systemic anesthetics, such as propofol, have been used as sedatives.16, 30 Studies have shown that propofol has a greater sedative effect than midazolam because of its smooth recovery.16 However, the routine use of propofol requires the cooperation of anesthesiologists and is not practical. The efficacy of dexmedetomidine (DEX), a highly selective α2-adrenoreceptor agonist, has also been reported.31-34 Because DEX leads to less respiratory depression than benzodiazepines, its use enables physicians to obtain an appropriate sedative effect while maintaining communication with the patient.35 DEX can be administered intravenously during endoscopic examinations and procedures such as endoscopic submucosal dissection.36-39 However, it is necessary to adjust the volume and manage the syringe pump during the period of administration to maintain hemodynamic control. Therefore, DEX is not only expensive but also inconvenient.35, 39, 40
Midazolam is widely used for sedation during endoscopic examination and other procedures in hospitals. During a study involving postoperative patients, a single intravenous administration of midazolam (0.06 mg/kg) was able to produce an effect equivalent to level 4 (quick response to loud voices and tap) of the Ramsay Sedation Scale in 45.5% of cases.41 Furthermore, as mentioned in the second edition of the Japanese Endoscopy Guidelines, it has been shown that the amnestic effect of midazolam is significantly higher than that of other benzodiazepines.42, 43 There have been reports of deep sedation with respiratory depression during endoscopy after the administration of midazolam (4–5 mg) combined with pethidine hydrochloride (70–100 mg).(44) Therefore, the dosage of midazolam infusion, the selection of concomitant analgesics, and the monitoring of patients during treatment are equally important. The dose of midazolam used during this study was relatively low. However, a significant reduction in patient discomfort, including pain and anxiety, was observed when compared with the sedative-free group. Kanogawa et al. examined the sedation effects of propofol and midazolam in RFA therapy and reported high rates of deep sedation (88.5% and 90.5%, respectively). However, the rates of complete analgesia in both groups were as low as 32.5% and 35.1%, respectively. Therefore, researchers consider the use of propofol and midazolam to be inadequate for reducing pain and anxiety associated with RFA therapy.16 In contrast, during our study, we were able to obtain a good sedative effect, with 95% of patients in the pentazocine–midazolam group having no memory of RFA therapy. During a previous study, sedation was often used from the time of RFA initiation subsequent to needle puncture.15, 16 During our study, sedatives were administered to the pentazocine–midazolam group soon after the patient was admitted to the treatment room. As a result, the patients did not perceive the pain of injection of the local anesthetic. All these factors translated to high patient satisfaction and pain relief.
The ablation time was relatively short, and there was no statistical difference between groups in this study; however, a certain amount of time is necessary to initiate treatment. Time is required to determine the puncture site using abdominal ultrasound, disinfect the skin, and prepare the RFA equipment. Patients are placed in an environment where RFA therapy is to be performed while they are awake, resulting in anxiety and fear. During this study, the treatment time was approximately 20 min longer for the pentazocine–midazolam group than for the sedative-free group. We surmised that this preparatory period was more than sufficient for the patients to develop anxiety. We showed that although midazolam has a short plasma half-life and is less likely to provide anesthesia after treatment, it also has a retrograde amnestic effect and is extremely effective for reducing pain during RFA therapy.
It should be remembered that even if the dose of midazolam is relatively small, the systolic blood pressure may tend to decrease during its administration when used in combination with an analgesic. The decrease in oxygen saturation associated with deeper sedation can be prevented by concurrent administration of 1–2 L of oxygen. However, because there have been cases of rapid oxygen desaturation without a decrease in blood pressure, preparedness for airway management using an oropharyngeal airway is necessary. During this study, we had to use the oropharyngeal airway in 18% of patients in the pentazocine–midazolam group; however, it did not necessarily improve the oxygen saturation of patients who received high doses of midazolam. Oxygen saturation rapidly improved when airway management was combined with oxygen administration. Furthermore, RFA therapy was completed for all cases.
It has also been suggested that patient cooperation, such as breath-holding and respiratory regulation, aids in achieving a safe and satisfactory puncture for RFA therapy. Regardless, we observed no significant difference in the local recurrence rate of HCCs between groups. Because the pentazocine–midazolam group appeared to be deeply sedated based on the RASS scale scores (−5.0; range, −5 to 2), the patient's own respiratory control could not be applied therapeutically. However, a few seconds of respiratory pause occur during the transition from expiration to inspiration. Positioning of the puncture needle can be safely performed during the end-expiration phase.
Although this was a retrospective study, RFA-treated patients at Fuji City General Hospital and Jikei University Hospital were followed up during the study period. We observed that there were far too many patients in the pentazocine–midazolam group at Fuji City General Hospital, whereas the majority of patients at Jikei University Hospital were in the sedative-free group. This bias can be considered the main limitation of this study. Also, regarding the generator, there are many Cool-tip RF systems at Fuji City General Hospital and RF3000 systems at Jikei University Hospital, which was also a limitation. Pentazocine has an analgesic effect within 2–3 min and lasts for 3–4 h when administered by intravenous drip.45 Therefore, it is considered that there is no effect on analgesia in both groups. However, in the pentazocine–midazolam group, midazolam, which has a short half-life and a retrograde amnestic effect, is administered immediately after admission to obtain a sedative effect and an amnestic effect. Evaluations using this method are biased.
Conclusion
Administration of pentazocine with midazolam significantly reduces pain and anxiety while maintaining safety and efficacy for patients undergoing RFA therapy. The end result is increased patient compliance with RFA repeat treatment.
Acknowledgments
We thank the medical staff of The Jikei University School of Medicine and Fuji City General Hospital who were involved in data collection.




