Invasive aspergillosis causing gastric necrosis and perforation: A case report
Abstract
Aspergillosis is an opportunistic infection commonly seen in immunocompromised patients. Patients with hematological malignancies, postorgan transplantation, or those with comorbid conditions are susceptible to the development of invasive aspergillosis. Lungs are the main portal of entry and are thus most commonly involved. Aspergillosis can involve the gut, causing vascular thrombosis leading to ischemia and necrosis of the gut wall, resulting in perforation. Primary gastric involvement has been rarely seen, with few case reports in the literature. We report a rare case of primary invasive gastric aspergillosis in a 60-year-old diabetic and cirrhotic woman, who presented with clinical features of perforation peritonitis. Exploratory laparotomy was performed, and a 6 cm × 6 cm perforation with necrotizing inflammation was found in the distal stomach, pylorus, and duodenum. Distal gastrectomy with Billroth II reconstruction was performed. Pathology demonstrated septate fungal hyphae invading the gastric wall transmurally. The morphology was compatible with those of Aspergillus spp. Liposomal amphotericin B was started immediately after surgery based on the presence of unusually large areas of necrosis and perforation with blackish exudate covering the ulcer base. Unfortunately, the patient succumbed to rapidly progressive fungal septicemia despite early surgical intervention and critical care management. We recommend that any large confluent areas of gastric ulceration and necrosis with blackish exudates in an appropriate setting should evoke suspicion of invasive fungal infection. These patients should be started on prophylactic broadspectrum antifungal therapy immediately, which may be switched over to specific therapy once the diagnosis is confirmed.
Introduction
Aspergillus spp. are ubiquitous saprophytic molds that can be transmitted with inhalation or ingestion of spores. Invasive aspergillosis (IA) is an opportunistic infection commonly seen in immunocompromised patients. The common species responsible for the majority of infections are Aspergillus fumigatus, Aspergillus flavus, and Aspergillus niger. They can involve diverse organ systems, causing respiratory, rhinocerebral, cutaneous, eye, and gastrointestinal infections. Lungs are the predominant organ involved in 90–98% of patients.
Gastric aspergillosis is very rare, occurring in isolation or usually as a part of disseminated aspergillosis. Invasive disease predominantly occurs in classical immunosuppressive conditions like HIV, organ transplant recipients, bone marrow transplant recipients, prolonged steroid users, and patients with hematological malignancies.1, 2 Besides these classical risk factors, liver cirrhosis, diabetes, tuberculosis, and chronic lung disease are newly recognized risk factors for IA.1 We report a rare case of invasive gastric aspergillosis with perforation peritonitis in the background of liver cirrhosis and diabetes.
Case details
A 60-year-old obese (BMI: 38.7 kg/m2) female patient from a rural area of North India presented with complaints of acute pain in the abdomen and abdominal distension for the last 7 days and an inability to pass stool for the last 3 days. She was a known case of type II diabetes mellitus for the last 6 years and was being treated with insulin for the same. There was no associated fever, jaundice, or any history suggestive of gastrointestinal bleeding. On examination, she was sick looking, with tachycardia (pulse of 100 beats/min), tachypnea (respiratory rate of 34 min−1), and hypotension (blood pressure 100/60 mmHg). Her abdomen was distended, with diffuse guarding and rigidity suggestive of peritonitis. Laboratory investigations demonstrated the following (values in parentheses indicate normal range): hemoglobin 9.2 g/dL (12–14 g/dL), white blood cell count 18 000/mm3 (4000–11 000/mm3), platelet count 32 000/mm3 (150 000–450 000/mm3), total bilirubin 0.9 mg/dL (0.3– 1.2 mg/dL), aspartate aminotransferase 722 IU/L (2–40 IU/L), alanine aminotransferase 169 IU/L (2–41 IU/L), alkaline phosphatase 249 IU/L (42–128 IU/L), albumin 2.13 g/dL (3.4–4.8 g/dL), sodium 142 meq/L (135–145 meq/L), potassium 3.6 meq/L (3.5–5 meq/L), urea 121 mg/dL (10–50 mg/dL), creatinine 1.0 mg/dL (0.5–1.2 mg/dL), and blood glucose (random) 170 mg/dL.
Chest X-ray was normal. X-ray abdomen in erect position showed no air under the diaphragm. Contrast-enhanced computed tomography (CECT) abdomen was performed, which demonstrated pneumoperitoneum and thickened duodenal wall, with decreased enhancement and intramural air foci (pneumatosis intestinalis) suggestive of gastric ischemia with necrosis (Fig. 1a). The visualized lungs appeared normal.
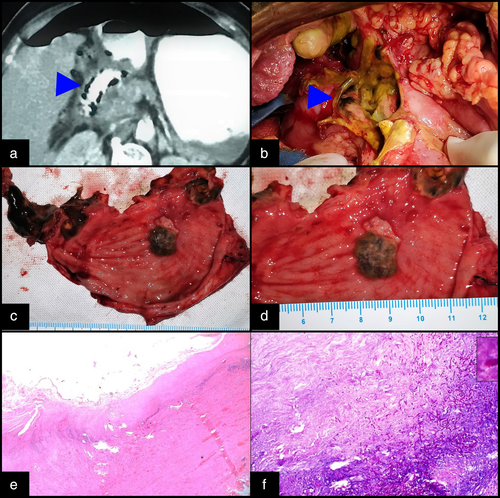
The patient was resuscitated with intravenous fluids and started on piperacillin-tazobactam. Inotropic support (noradrenaline) was required for hypotension. In view of severe sepsis and circulatory compromise that did not respond to initial fluid boluses, bilateral flank drains were placed to drain out the infected fluid and achieve partial source control. These yielded an output of around 500 mL of biliopurulent fluid in each drain. She responded favorably to resuscitation and drainage and was taken for emergency laparotomy around 10 h after the initial presentation. On exploration, there was gross peritoneal contamination with 700–800 mL of biliopurulent fluid. The liver was cirrhotic with a multinodular appearance. There was a solitary large perforation (6 cm × 6 cm) in the anterior wall of the distal stomach involving the pylorus and the first part of the duodenum (Fig. 1b). The tissue at the edges of the perforation site was necrotic with unhealthy blackish exudates covering it. Distal gastrectomy with Billroth II reconstruction was performed with hand-sewn closure of the duodenal stump. Cut section of the resected stomach showed multiple deep ulcers with punched out edges and blackish slough covering them (Fig. 1c,d). Histopathology demonstrated transmural necrotizing inflammation with the presence of multiple aggregates of fungal septate hyphae and acute branching pattern, confirming the morphology of Aspergillus spp (Fig. 1e,f).
Postoperatively, she was managed in the surgical intensive care unit. Liposomal amphotericin B was started in view of suspicion of fungal sepsis. However, despite optimal critical care and supportive management, she succumbed to septicemia with multiorgan dysfunction syndrome on postoperative day 2. Involvement of other organs could not be confirmed as consent for autopsy was not given.
Discussion
Aspergillus is a saprophytic mold that is found ubiquitously in the natural environment. In humans, it is responsible for opportunistic invasive fungal infections that can commonly affect immunocompromised hosts. A. fumigatus is the most common species implicated, with A. niger, A. flavus, and A. terreus less commonly seen. Patients with hematological malignancies, organ transplant or stem cell transplant recipients, patients receiving chemotherapy, those on long-term corticosteroid treatment, and those with HIV infections are predisposed to developing IA.1 Patients with diabetes, chronic obstructive pulmonary disease, chronic kidney disease, or liver cirrhosis are also more likely to develop IA.1
Aspergillus enters the body via the respiratory route. The lungs are involved in the overwhelming majority (90–98%) of cases of IA. Indeed, IA is almost exclusively considered a pulmonary disease with secondary hematogenous dissemination.2 Gastrointestinal involvement occurs secondarily as a feature of disseminated disease. Primary gastric IA is exceedingly rare, with only a few case reports in the literature. Kazan et al.,3 in a retrospective review of gut aspergillosis, found only three cases of gastric aspergillosis over a 13-year period at nine major hospitals in Europe. Chakrabarti et al.1 conducted a literature review of IA in developing countries and did not report any case of primary gastric IA. Our patient likely had primary gastric IA as the lungs were not involved in any preoperative imaging.
The stomach is not normally conducive to the growth of fungi because of its acidic environment. However, decreased gastric acid secretion, disruption of the gastric mucosal barrier, gastric ulcer, and mucosal ischemia can predispose to the colonization of Aspergillosis spp.4 Once colonized, Aspergillus hyphae can penetrate through the mucosa in susceptible hosts. Their angiotropic properties enable them to invade the vascular wall and proliferate within the lumen of blood vessels.5 This causes thrombosis and hemorrhagic infarcts, leading to local necrosis and perforation. They can also disseminate to distant organs hematogenously in the form of septic emboli.2
The morphological features of gastric perforation caused by fungi differ significantly from those caused due to peptic ulceration. Fungal perforations are secondary to ischemia and necrosis of the gastric wall, whereas peptic perforations occur due to chronic transmural inflammation. Hence, the former tend to be large, with necrotic edges and poor demarcation between normal and diseased stomach. There is a distinct lack of fibrosis and thickening of the gastric wall. Peptic ulcers display characteristic white slough, whereas fungal ulcers display black slough and may be covered with fungal hyphae.6
The diagnosis of gastrointestinal aspergillosis is difficult both clinically and radiologically. In our case, we suspected fungal involvement perioperatively based on the findings of large perforation with necrotic tissues in a patient who had multiple predisposing factors. Yang et al.7 described the characteristic radiological features of gastric IA. Contrast-enhanced CT imaging may show decreased wall enhancement and nodular, irregular collections of intramural gas bubbles in the gastric wall, representing emphysematous gastritis. Similar findings were seen in our patient. Emphysematous changes are notable because, unlike Clostridium or Pseudomonas spp., Aspergillus is not a gas-forming organism. It is possible that gastric wall necrosis caused by IA could be followed by a gas-forming bacteria superinfection.
Aspergillus is not the only invasive fungus to involve the stomach. Candida and Mucor have been reported to cause similar presentations. Mucormycosis is by far the most common fungus causing gastric involvement and perforation.8, 9 Clinically, mucormycosis and aspergillosis are difficult to differentiate because both occur commonly in immunocompromised conditions, are angiotropic, and cause necrotizing inflammation. However, they may be differentiated on histopathology. Aspergillosis hyphae are septate, with dichotomous branching at an acute angle. Conidial heads may be observed. In mucormycosis, broad, thin-walled, hyaline aseptate hyphae with right angle branching patterns are seen.4, 8
Once IA is suspected or confirmed, specific antifungal treatment has to be started urgently. Serological markers like B-glucan and galactomannan are nonspecific for gut aspergillosis. Hence, empirical therapy has to be considered. As per the guidelines of the Infectious Disease Society of America, empirical broadspectrum antifungal therapy with amphotericin-B or caspofungin is strongly recommended. Voriconazole is the drug of choice for IA.10 IA remains a killer infection. In spite of improvements in diagnosis and an aggressive treatment approach, the mortality rate of IA remains high (30–70%).11
To summarize, this case report highlights the finding of primary gastric IA in a patient with diabetes and liver cirrhosis who presented with a large gastric perforation with necrosis. To the best of our knowledge, such a presentation has not been previously reported from any tropical country. One should be aware of, and have a high index of suspicion for, invasive fungal infection in high-risk patients with gastric perforation and consider prophylactic antifungal therapy for the same.




