Reprogramming of Fatty Acid Metabolism in Acute Leukemia
ABSTRACT
Fatty acids are essential biomolecules that support several cellular processes, such as membrane structures, energy storage and production, as well as signal transduction. Accordingly, changes in fatty acid metabolism can have a significant impact on cell behavior, such as growth, survival, proliferation, differentiation, and motility. Therefore, it is not surprising that many aspects of fatty acid metabolism are frequently dysregulated in human cancer, including in highly aggressive blood cancers such as acute leukemia. The aims of this review are to summarize the aspects of fatty acid metabolism that are specifically coopted in acute leukemia as well as current preclinical strategies for targeting fatty acid metabolism in these cancers.
1 Introduction
Cellular fatty acids contribute to a wide array of cellular and physiological processes, and fatty acid metabolism imbalances are found in a number of diseases and disorders, including human cancer (Fahy et al. 2011; Huang and Freter 2015; Li et al. 2023; Liu et al. 2022). Cellular fatty acids are a diverse group of biomolecules comprised of a non-polar hydrocarbon (i.e., acyl) chain attached to a carboxyl group. While the majority of cellular fatty acids are utilized to synthesize complex lipids, many free fatty acids also have unique bioactive properties. Fatty acid diversity is driven by acyl chain length and the number of double bonds. Based on the number of carbons in the aliphatic tail, fatty acids are subdivided into: short chain- (SC-, 2–6 carbons), medium chain- (MC-, 7–12 carbons), long chain- (LC-, 13–21 carbons), and very long chain- (VLC-, ≥ 22 carbons) fatty acids (Wang et al. 2021; Kihara 2012). Fatty acids are also categorized based on the number of double bonds within the aliphatic tail: (1) Saturated fatty acids (SFA, no double bonds), (2) monounsaturated fatty acids (MUFA, one double bond), and (3) polyunsaturated fatty acids (PUFA, two or more double bonds).
Mammalian cells are able to import numerous fatty acids from the extracellular environment; however, they can also synthesize certain SFA and MUFAs de novo. For example, Fatty Acid Synthase (encoded by FASN) utilizes acetyl-CoA as an initiator, malonyl-CoA as a two-carbon donor, and NADPH as a reducing agent to drive the synthesis of palmitate, a 16-carbon SFA (Chirala and Wakil 2004; Röhrig and Schulze 2016; Tabe, Konopleva, and Andreeff 2020; Tabe and Konopleva 2023). Furthermore, the human Stearoyl-CoA desaturases (encoded by either SCD1 or SCD5) are able to convert SFAs into MUFAs (Kubota and Espenshade 2022; Igal and Sinner 2021). Conversely, the sole source of mammalian intracellular PUFAs comes from the importation of diet-derived omega-3 (ω-3) or omega-6 (ω-6) PUFAs. Once imported into cells, PUFAs can be further enzymatically modified (e.g., alterations in chain length or the number of double bonds) to generate an array of PUFAs that influence multiple cellular processes, such as inflammation as well as membrane composition and flexibility (Harayama and Shimizu 2020). Although mammalian cells cannot synthesize PUFAs de novo, under certain conditions, free PUFAs can be liberated from phospholipids by a series of enzymes called phospholipases, to meet short-term demands (Park et al. 2012; Aloulou et al. 2018).
While certain aspects of fatty acid distribution and utilization are frequently dysregulated in many human cancers, the contribution of dysfunctional fatty acid metabolism to the pathogenesis of acute leukemia is becoming increasingly recognized. This review explores the mechanisms by which fatty acid metabolism contributes to leukemogenesis as well as discusses emerging preclinical strategies for therapeutically targeting fatty acid metabolism in acute leukemia.
2 Fatty Acid Distribution in Acute Leukemia
Acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) are two forms of highly proliferative blood cancers that are occur in both children and adults. AML arises when hematopoietic stem and progenitor cells incur mutations that cause them to grow limitlessly, adopt myeloid-progenitor characteristics, and lose their ability to terminally differentiate into mature blood cells (Döhner et al. 2022). Although new therapies have recently become available, AML remains the deadliest form of leukemia in both children and adults. ALL arises from the aberrant expansion of immature lymphocytic progenitors that are either of B-cell or T-cell origin, B-ALL and T-ALL, respectively (Terwilliger and Abdul-Hay 2017). While certain forms of ALL are treatable, chemotherapy resistance and disease relapse remain a challenge for many ALL patients. As a result, identifying molecular features with high therapeutic targetability remains a major priority in both AML and ALL.
As seen in several solid cancers, fatty acid biology has been found to be altered in both AML and ALL patients. Early analyses of lipid content in the plasma of patients with non-Hodgkin's Lymphoma or acute leukemia indicated that acute leukemia patients displayed altered lipids levels (i.e., dyslipidemia) trending toward elevated levels of triglycerides and very-low density lipoproteins with a concomitant decline in high-density lipids (Spiegel et al. 1982; Munir et al. 2014). Other studies that considered factors such as disease type, age, sex, and body mass index found that ALL patients displayed elevated levels of triglycerides and very-low-density lipoproteins whereas decreased high-density lipids were observed in AML patients (Usman et al. 2015). Furthermore, both of these patterns were selectively seen in females, however, male AML patients displayed an overall decrease in total cholesterol and low-density lipoproteins (Usman et al. 2015).
Alterations in sphingolipid biology have also been found in AML patients and certain sphingolipid regulatory enzymes promote leukemogenesis and chemotherapy resistance (Wątek et al. 2017; Ung et al. 2022; Stefanko et al. 2017). A number of interesting observations regarding fatty acid metabolism in AML arose from a study by Thomas Pabst and colleagues that evaluated an array of free fatty acids and lipids in 20 AML patients and 20 healthy individuals (Pabst et al. 2017). Using three different mass spectrometry approaches, they observed that AML patients displayed a global reduction in plasma fatty acids, including specific declines in sphingolipids, phosphocholines, triglycerides, and cholesterol esters compared to healthy donors. They also reported that certain free fatty acids such as palmitic, palmitoleic, oleic, linoleic, and arachidonic acids, as well as a few specific complex lipids, were increased in AML patients relative to controls. Lastly, from targeted lipidomic analysis of PUFAs and downstream eicosanoids, Pabst and colleagues reported that the arachidonic acid (AA) biosynthesis pathway was significantly elevated in the serum of AML patients and AA levels correlated with worse prognostic factors. Collectively, these studies highlight how the lipidome of acute leukemia patients is significantly altered compared to healthy individuals and raise several key questions such as: (1) how are leukemia cells utilizing fatty acids and lipids?; (2) what are the central sources of fatty acid and lipids in acute leukemia cells?; and (3) can aberrant fatty acid metabolism be targeted in acute leukemia?
3 Fatty Acid Utilization in Acute Leukemia
Altered lipid metabolism is a common feature across multiple forms of human cancer, and many mechanisms of fatty acid and/or lipid utilization have been discovered. In this section, we will focus on summarizing three well-defined examples of fatty acid utilization in acute leukemia; however, additional mechanisms exist.
3.1 Fatty Acid Oxidation
Dysfunctional metabolism is regarded as a hallmark of cancer, and it is driven by the increased energy and biomass needs of rapidly proliferating tumor cells (Hanahan 2022; DeBerardinis, Lum, and Thompson 2006; Menendez and Lupu 2007). Although many tumor cells are highly dependent on glucose uptake, a phenomena often referred to as the Warburg Effect, glucose-derived carbons are not solely utilized to supply the tricarboxylic acid (TCA) cycle and drive oxidative phosphorylation (OXPHOS). A wide range of cancers exhibit elevated rates of glutaminolysis (the utilization of glutamine to supply TCA intermediates) as well as fatty acid oxidation (aka. FAO or β-oxidation of fatty acids)—a process that yields significantly higher adenosine triphosphate (ATP) molecules than the breakdown of glucose (Ma et al. 2018; Carracedo, Cantley, and Pandolfi 2013; Boroughs and DeBerardinis 2015; Houten and Wanders 2010). Fatty acid oxidation occurs in both the mitochondria (mFAO) and the peroxisome (pFAO) (Houten and Wanders 2010; Houten et al. 2016; Tannoury et al. 2024; Ding et al. 2021; Violante et al. 2019). Both processes begin with the importation of fatty acids that have been activated via the addition of coenzyme A (referred to as acyl-CoAs). Upon importation, acyl-CoAs are subjected to a series of enzymes (which are unique to each organelle) that oxidize the β-carbon of fatty acyl-CoAs, resulting in the liberation of acetyl-CoA, which can be injected into the TCA to drive OXPHOS-mediated energy production (Figure 1). Though both mFAO and pFAO generate one acetyl-CoA molecule for each round of β-oxidation, acyl-CoAs in the peroxisome are not oxidized to completion. Acyl-CoAs that are shortened in the peroxisome are subsequently transported to the mitochondria for further oxidation (Tannoury et al. 2024). Additionally, while mFAO primarily oxidizes LCFAs, a multitude of fatty acids, including VLC-, LC-, MC- and branched chain-fatty acids can be oxidized in the peroxisome (Ding et al. 2021; Violante et al. 2019).
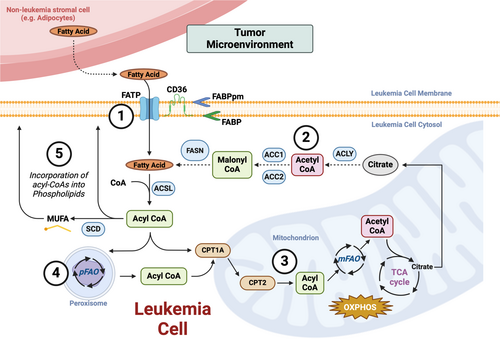
Like many solid cancers, AML cells rely on FAO to supply their energy needs and resist first-line chemotherapies (Tabe, Konopleva, and Andreeff 2020). Initial studies on the role of FAO in leukemia revealed that multi-drug resistant acute leukemia cells exposed to limiting glucose conditions are able to rapidly utilize oleic acid to drive mitochondrial respiration (Harper et al. 2002). Additional studies confirmed that very long chain fatty acids, which would be initially oxidized and shortened in peroxisomes, are used to source palmitate for oxidation and that pharmacological blockade of FAO impedes leukemia cell proliferation and sensitizes these cells to first-generation BCL2 inhibitors and Nutlin3a (Samudio et al. 2010; Ricciardi et al. 2015; Tcheng et al. 2021). Several studies have also implicated FAO as a driver of resistance to FDA-approved chemotherapies in AML and ALL. For example, AML cells that have developed resistance to the upfront chemotherapy, cytarabine (Ara-C), or the BCL2 inhibitor, Venetoclax, have switched their primary mitochondrial fuel source from glycolysis to FAO (Farge et al. 2017; Stevens et al. 2020). Furthermore, AML cells bearing IDH mutations engage multiple FAO-activating signal transduction pathways to sidestep targeted therapies (Stuani et al. 2021). In T-ALL, increased FAO is also associated with resistance to glucocorticoid resistance (Olivas-Aguirre et al. 2023).
Strategies for targeting FAO in cancer have focused on either pharmacologically interfering with the enzymes involved in the oxidation of fatty acids or the carnitine shuttle, which is the molecular system that ferries fatty acids from the cytoplasm into the mitochondria (Figure 1) (Carracedo, Cantley, and Pandolfi 2013; Longo, Frigeni, and Pasquali 2016; Bremer 1983). Direct inhibitors of fatty acid oxidation (e.g., via inhibition of the very-long chain acyl-CoA dehydrogenase, VLCAD) or the carnitine shuttle component, carnitine palmitoyltransferase 1 A or CPT1A (using Etomoxir or ST1326), impede leukemia cell proliferation and sensitize them to cytotoxic agents (Table 1) (Samudio et al. 2010; Ricciardi et al. 2015). Etomoxir is also able to overcome resistance to the combination of Venetoclax and 5-azacytidine in AML and cooperates with l-asparaginase in the treatment of ALL (Stevens et al. 2020; Hermanova et al. 2016). Similarly, ST1326 (aka. Teglicar) cooperates with Venetoclax to kill AML cells (Mao et al. 2021). Despite preclinical effectiveness, these compounds have either shown significant unwanted toxicities (e.g., Etomoxir) or have yet to be evaluated in clinical human trials of acute leukemia (Holubarsch et al. 2007).
| Targeting agents | Compounds | Fatty Acid metabolic process | Disease | Preclinical efficacy |
|---|---|---|---|---|
| CPT1A inhibitors | Etomoxir, ST1326 | Fatty acid mitochondrial shuttling | AML | Cell lines and PDX models |
| VLCAD inhibitors | Avocadyne (AYNE) | Fatty acid oxidation | AML | Cell lines and PDX models |
| Ceramidase inhibitors | LCL-204 | Ceramide metabolism | AML | Cell lines and PDX models |
| SPHK targeting agents | SKI-178, SKI-349, MP-A08 (inhibitors) and FTY720 (agonist) | S-1-P Signaling | ALL & AML | Cell lines, GEMMs of ALL, and CDX and PDX models of AML |
| SCD1 inhibitor | SSI-4, SW203668 | MUFA biosynthesis | ALL & AML | Cell lines, AML patient samples, and PDX models of ALL |
| FADS inhibitors | CP-24879 (FADS1/2i), sc-26126 (FADS2i) | PUFA biosynthesis | AML | Cell lines, AML patient samples, and GEMMs of AML |
| CD36 inhibitors | SMS121, FA-152 anti-CD36 antibody | Fatty acid import | AML | AML cell lines, CDX models of AML |
- Abbreviations: CDX, cell line-derived xenograft; GEMM, genetically engineered mouse model; PDX, patient-derived xenograft.
3.2 Synthesis of Complex Lipids
Fatty acids are also utilized in the synthesis of complex lipids such as fats (i.e., glycerides), glycerophospholipids, and sphingolipids. Similar to certain free fatty acids, plasma levels of several lipids are significantly altered in acute leukemia patients. For example, plasma levels of triglycerides (TAGs—the most abundant lipids in mammals) are variable across acute leukemia patients and are significantly lower in AML patients (Spiegel et al. 1982; Pabst et al. 2017). TAGs arise from excess fatty acids (saturated and unsaturated) being incorporated, through esterification reactions, into a glycerol backbone, which can be subsequently stored for future energy use (Cohen and Spiegelman 2016; Li et al. 2022). It has been speculated that the decreased levels of TAGs in AML patients are the result of enhanced FAO; however, future studies are needed to definitively establish the existence of such a phenomenon.
The plasma levels of several glycerophospholipids (commonly referred to as phospholipids) have also been found to differ between acute leukemia patients and healthy patients. As the name implies, glycerophospholipids are comprised of a glycerol backbone bound by two fatty acids (saturated and unsaturated) and a phosphate group. The phosphate group can also be bound by additional molecules and is the distinguishing characteristic that subdivides glycerophospholipids. For example, phosphatidylserines have a serine molecule bound to the phosphate group, whereas the phosphate groups of phosphatidylcholine and phosphatidylinositol are bound by choline and inositol sugar molecules, respectively. Glycerophospholipids are key constituents of the lipid bilayers that make up the various cellular and organelle membranes and thus, disruptions in glycerophospholipid metabolism can drastically alter cell biology (Wang and Tontonoz 2019). While studies comparing the phospholipid composition of leukemia cells and healthy hematopoietic cells are limited, plasma levels of phosphatidylcholine are significantly lower in AML patients compared to healthy individuals (Pabst et al. 2017). Given their high rates of cellular duplication, acute leukemia cells likely have higher demands for glycerophospholipids to maintain membrane production. Therefore, it stands to reason that acute leukemia cells also display elevated levels of phospholipid synthesizing enzymes. However, further functional studies are needed to confirm these possibilities and, if so, determine whether such dependencies can be therapeutically exploited.
Cholesterols are another form of complex lipids that initially seemed to be a promising therapeutic target in acute leukemia. Mammalian cells are able to both biosynthesize and import cholesterol from the microenvironment; however, the former is an energy expensive process. In the mid-1970s, a class of fungi-derived compounds called statins were discovered to block de novo cholesterol synthesis. Shortly thereafter, statins were approved for the treatment of patients with elevated cholesterol (Endo 2010). Preclinical studies in the late 1990s-early aughts showed that statins have anti-leukemia activity in both AML and ALL cells and render these cells sensitive to other chemotherapies in vitro (Sheen et al. 2011; Infante, Heasman, and Ridley 2011; Dimitroulakos et al. 1999; Li et al. 2003). However, clinical trials testing the second-generation statin, Pravastatin, in combination with standard first-line AML chemotherapies, did not show sufficient efficacy to warrant Phase-3 trials (Advani et al. 2014; Shadman et al. 2015). These clinical failures are likely the result of leukemia cells importing cholesterol to bypass the need to synthesize cholesterol de novo (Banker et al. 2004). Notably, a recent retrospective study found that statins are associated with improved survival in patients suffering from myelodysplastic syndrome, as well as a reduced risk of evolving to AML (Afzal et al. 2023).
3.3 Cell Signaling
In addition to their roles in membrane biology, several lipids have been found to play key roles in the signal transduction of acute leukemia cells. A prime example are sphingolipids, which support cellular membrane integrity and drive signal transduction pathways that influence leukemia cell growth and survival (Futerman 2006; Hannun and Obeid 2002; Spiegel and Milstien 2002; Snider, Alexa Orr Gandy, and Obeid 2010). Sphingolipids represent a unique and diverse class of lipids that contribute to a wide array of pro- and anti-leukemogenic cellular processes. For example, the accumulation of ceramides, which are conjugates of sphingosine and fatty acids, are associated with apoptosis, whereas sphingosine-1-phosphates (S-1-P) exert pro-survival effects on cells (Ung et al. 2022; Lewis et al. 2018). Not surprisingly, several enzymes involved in sphingolipid metabolism are aberrantly expressed in acute leukemia and functionally important. For instance, certain AML samples display elevated expression of the ceramide-catabolizing enzyme acid ceramidase (AC) and are functionally dependent on AC (Tan et al. 2016, 2019). Several components of the S-1-P signaling cascade are also dysregulated and support acute leukemia. For example, Sphingosine kinase 1 (encoded by SPHK1)—the enzyme that catalyzes the phosphorylation of sphingosine—is upregulated in certain AML and ALL patients and functionally supports leukemogenesis (Lewis et al. 2018; Dick et al. 2015; Wallington-Beddoe et al. 2019). Moreover, Sphingosine kinase 2 is required to drive leukemogenesis in a mouse model of B-ALL (Wallington-Beddoe et al. 2014). The S-1-P transporter, S1PR3 (Sphingosine-1-Phosphate Receptor 3) has also emerged as a potential point of therapeutic intervention of AML. First, transgenic expression of S1PR3 has been shown to promote the development of AML in mice (Vorbach et al. 2020). Second, a recent study published by Xie and colleagues has shown that S1PR3 expression varies across AML and even within distinct populations with an individual tumor (Xie et al. 2021). Many normal karyotype AMLs arise from the acquisition of a combination of cooperating gene mutations and these tumors often display significant clonal heterogeneity. Specifically, the bulk of AML cells contain all the given mutations while a smaller fraction of cells may contain one or a subset of these mutations (often referred to as leukemia sub-clones) (Sykes et al. 2015; Majeti 2014). Separate studies have shown that certain acute leukemia tumors contain a sub-population of malignant cells that display gene expression signatures akin to hematopoietic stem cells (HSCs) and are able to propagate leukemia when transplanted into immune-compromised mice—this population of cells are often referred to as leukemia stem cells (LSCs), leukemia-initiating cells (LICs) or leukemia progenitors (LPs) (Thomas and Majeti 2017; Lane and Gilliland 2010; De Grandis, Mancini, and Aurrand-Lions 2018) Interestingly, Xie and colleagues showed that the S-1-P transporter, S1PR3, is differentially expressed on LSCs compared to more myeloid-like AML cells (Xie et al. 2021). They also showed that S1PR3 expression is elevated in AMLs display more myeloid-like tumors, which are commonly associated with resistance to the combination of Venetoclax and 5-azacytidine (Xie et al. 2021).
Importantly, these findings and others have sparked strategies for targeting sphingolipid metabolism in a number of blood malignancies (Table 1) (Lewis et al. 2018). For example, the S1PR3 agonist (FTY720) reduces LSC function by inducing tumor necrosis factor-alpha (TNF-α)-based inflammatory signaling (Xie et al. 2021). A chemical inhibitor of AC, LCL-204, has been shown to induce type I apoptosis in AML cell lines by regulating MCL-1 expression. Furthermore, as a single agent, LCL-204 delays the onset of disease in a mouse model of AML and significantly decreases circulating blasts in a patient-derived xenograft (PDX) model of AML (Tan et al. 2016). Pharmacological targeting of sphingosine kinases has also shown promise in preclinical models of ALL. In line with SPHK2 playing a supportive role in ALL, small molecules targeting sphingosine kinase 2 show cooperativity with genotoxic agents in eliminating ALL cell lines (Evangelisti et al. 2014). Additionally, chemical inhibitors targeting SPHK1 and/or SPHK2 display synergistic cooperativity with imatinib in ALL cell lines bearing the t(9;22)-p190 chromosomal rearrangement (Wallington-Beddoe et al. 2019). Sphingosine kinase inhibitors have also shown efficacy in AML models. For example, the SPHK inhibitors, SKI-I and SKI-II, promote AML cell line cytotoxicity in vitro and reduce leukemogenesis in a cell-line xenograft model (Powell et al. 2017; Paugh et al. 2008; Yang et al. 2015). Other SPHK inhibitors such as SKI-178, SKI-349, MP-A08 have also shown anti-leukemia activity (Dick et al. 2015; Powell et al. 2017; Hengst et al. 2020). While these strategies have shown promise in preclinical testing, they have yet to be fully evaluated in clinical trials.
Another well-defined example of fatty acids that contribute to signal transduction are the phosphorylated phosphatidylinositols (called phosphoinositides), which are key messengers in the phosphatidylinositol-3-kinase (PI3K) signaling network. PI3K is almost universally dysregulated in human cancer, including acute leukemia; however, we will not delve into this topic as it has been reviewed extensively elsewhere (Fruman et al. 2017; Jabbour et al. 2014). Another example of a class of fatty acids that can act as mediators of cell signaling, are the arachidonic acid-derivatives called are eicosanoids (Figure 2), which influence a wide range of physiological processes including inflammation, allergy and immune responses, cell growth, and blood pressure (Calder 2020; Wang and Dubois 2010). The role of eicosanoids and other PUFA-derived lipid mediators in AML will be discussed later on.
4 Fatty Acid Sources in Acute Leukemia
Given the unique fatty acid and lipid dependencies observed in human cancer, much effort has been made to identify the molecular mechanisms that source these biomolecules. Here, we will discuss the currently known mechanisms by which acute leukemia cells maintain sources of intracellular fatty acid and lipid levels.
4.1 De Novo Fatty Acid Synthesis
To meet their high biomass demands and supply metabolite and nutrient pools, many tumor cells rely on both importation and de novo synthesis of metabolites. Similar to many solid cancer cells, acute leukemia cells utilize a combination of importation and de novo synthesis to maintain intracellular fatty acid levels and support fatty acid oxidation (Röhrig and Schulze 2016; Tabe, Konopleva, and Andreeff 2020; Tabe and Konopleva 2023; Samudio et al. 2010). Fatty acid synthesis is catalyzed by a multifunctional enzymatic system called fatty acid synthase (FAS, encoded by FASN), which converts and elongates the acetyl-CoA-derived metabolite, malonyl-CoA into long-chain fatty acids such as palmitate (Figure 1). Compared to various healthy human blood cells, including HSCs, AML cells display significantly higher levels of FASN (Humbert et al. 2021). Furthermore, leukemia cells from patients with relapsed ALL display higher FASN expression relative to therapy responders (Ghaeidamini Harouni et al. 2020).
Cytoplasmic acetyl-CoA utilized in fatty acid synthesis is derived from glucose. Essentially, ATP Citrate Synthase/Lyase (encoded by ACLY) liberates glucose-derived acetyl-CoA from the tricarboxylic acid (TCA) cycle intermediate, citrate, which is subsequently carboxylated by Acetyl-CoA Carboxlases (aka. ACC1 and ACC2, encoded by ACACA and ACACB, respectively) to generate malonyl-CoA (Figure 1) (Dominguez, Brüne, and Namgaladze 2021). Low expression of ACLY is associated with favorable outcomes in AML, and although yet to be peer-reviewed, ACLY expression seems to be elevated in T-ALL (Wang et al. 2019; da Silva-Diz et al. 2024). On the contrary, ACACA expression is significantly lower in AML patient samples relative to normal bone marrow, and enforced expression of a degradation-resistant form of ACC1 suppresses leukemogenesis in a mouse model of MLL-AF9-driven AML (Ito et al. 2021). These results suggest that although leukemia cells ramp up fatty acid synthesis, there may be a threshold that, once surpassed, antagonizes leukemogenesis.
However, tumor cells from many human cancers, are able to accumulate and store excess fatty acids in lipid droplets, which are single membrane organelles that contain neutral lipids, such as TAGs and cholesterol (Jin et al. 2023). Under conditions of high energy demand, cells can readily liberate fatty acids from lipid droplets to support fatty acid oxidation and OXPHOS (Jin et al. 2023). Although our understanding of lipid droplet formation in acute leukemia remains limited, recent studies suggest that lipid droplets may contribute to the pathogenesis of AML (Nisticò and Chiarella 2023). Specifically, the elevated expression of TPD52, which promotes fatty acid storage and lipid droplet formation, is associated with worse patient outcomes (Ha et al. 2019). Furthermore, perturbation of autophagy promotes lipid droplet formation and diminished OXPHOS in AML cells, suggesting that lipolysis of lipid droplet fatty acids is needed to support mFAO in AML (Bosc et al. 2020). However, the therapeutic potential for targeting lipid droplet biology in acute leukemia has yet to be investigated.
4.2 De Novo Fatty Acid Desaturation
Fatty acids generated by FAS are not only stored in triglycerides and lipid droplets for future energy use but they can also be elongated and/or desaturated to form a variety of distinct fatty acids (Röhrig and Schulze 2016). The stearoyl-CoA desaturase SCD, which desaturates SFA to generate MUFAs, is rapidly emerging as a potential therapeutic target in acute leukemia (Table 1). A retrospective analysis of gene expression profiles revealed that gene signatures related to elevated SCD expression are associated with poorer outcomes (Kim et al. 2023). Consistent with this observation, Jean Emmanuel-Sarry's group recently showed that inhibition of SCD could have therapeutic potential in AMLs bearing internal tandem duplications of the FLT3 gene (FLT3-ITD) (Sabatier et al. 2023), which are commonly associated with worse outcomes in AML (Kiyoi, Kawashima, and Ishikawa 2020; Daver et al. 2019). Briefly, the authors showed that FLT3-mediated activation of the transcription factor C/EBPα (both FLT3 and C/EBPα are recurrently mutated in AML) leads to SCD-mediated production of MUFAs that are subsequently incorporated into membrane phospholipids. The authors also showed inhibition of the FLT3-C/EBPα axis results in decreased MUFA membrane incorporation followed by ferroptotic cell death. The authors then combined FLT3 and glutathione peroxidase 4 (GPX4) inhibitors to trigger ferroptosis in FLT3-mutant AML cells (Sabatier et al. 2023). Additionally, Paolo Gallipoli's group has observed that elevated SCD1 expression is associated with worse outcomes in AML and that SCD1 expression correlates with FASN expression (Dembitz et al. 2024). Using a clinical-grade SCD inhibitor called SSI-4, the authors also showed that patient-derived AML cells display high levels of unsaturated fatty acids and are highly sensitive to SCD inhibition. Furthermore, they showed that SSI-4 cooperates with anthracyclines to significantly delay the onset of disease in multiple mouse models of AML, importantly without significant unwanted toxicity to healthy mouse tissue. Another study examining the role of SCD1 in ALL discovered that SCD1 expression is particularly enriched in ALL cells localized in the central nervous system (CNS) (Savino et al. 2020). They also showed that pharmacological inhibition of SCD1 (SW203668) significantly reduced ALL infiltration into the CNS in PDX models of ALL. While clinical testing is needed, these studies highlight the therapeutic potential of targeting SCD alone or as an adjuvant therapy in acute leukemia.
4.3 PUFA Biosynthesis
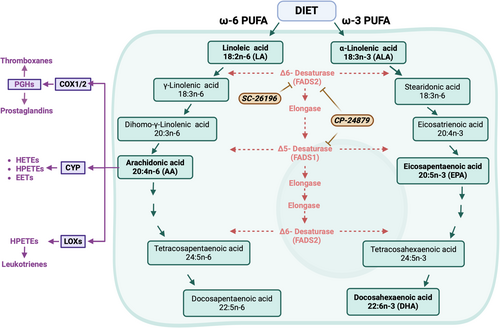
PUFAs are crucial for maintaining health and offer protection against diseases such as osteoarthritis, cancer, and autoimmune disorders (Kapoor et al. 2021; Lee et al. 2016). PUFAs containing more than two double bonds are considered to be important bioactive nutrients that regulate many physiological conditions but are also frequently dysregulated in human cancer (Lee and Park 2014). Although PUFAs are strictly sourced from diet in mammals, once imported into the cell, PUFAs can be further enzymatically modified (e.g., elongated and/or desaturated) to generate a diverse array of bioactive molecules. α-linolenic acid (ALA) is the dietary source of ω-3 fatty acids that are subsequently used to generate other bioactive PUFAs such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (Figure 2). The dietary source used to synthesize bioactive ω-6 fatty acids, such as AA, is linoleic acid (LA) (Figure 2). However, AA can also be liberated from membrane phospholipids by phospholipase A2 (Wang and Dubois 2010).
ω-3 fatty acids such as EPA and DHA are generally considered anti-inflammatory mediators, whereas AA and other AA-derived PUFAs are perceived to be largely pro-inflammatory mediators (Azrad, Turgeon, and Demark-Wahnefried 2013). As such, ω-3-derived fatty acids are associated with health benefits and decreased cancer risk, and certain ω-3 fatty acid derivatives are even being evaluated as potential anticancer compounds (Freitas and Campos 2019; Murray 2024). Consistent with this paradigm, in vitro administration of EPA and/or DHA has shown anti-leukemia effects in a variety of acute leukemia cell lines (summarized by Moloudizargari and colleagues [Moloudizargari et al. 2018]). Furthermore, dietary EPA supplementation significantly reduced disease burden in experimental mouse models of MLL-AF9-driven AML and BCR-ABL-driven chronic myelogenous leukemia (Finch et al. 2015). As an adjuvant to Ara-C, increased dietary DHA concentration significantly delays the onset of disease in the lymphoblastic leukemia mouse model, L1210 (Cha et al. 2002). While these data are promising, ω-3 fatty acid derivatives need to be clinically evaluated, possibly as adjuvant therapies.
Conversely, ω-6 PUFAs, such as AA, are largely considered tumor-promoting compounds. AA is a precursor for several enzymes, such as cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P-450 (CYP) (Wang and Dubois 2010). Cyclooxygenase 1 (COX1 encoded by PTGS1) and cyclooxygenase 2 (COX1 encoded by PTGS2) convert AA into Prostaglandin H2 (PGH2), which can be further metabolized into other prostaglandins (e.g., PGE2, PGF2α, PGD2, and PGI2) or thromboxanes (e.g., TXA2), which influence inflammatory responses and vasoconstriction, respectively. The LOX enzymes (e.g., ALOX5 or ALOX12) utilize ω-6 PUFAs, such as AA, to generate hydroperoxyeicosatetraenoic acids (HPETEs), which are used in leukotriene synthesis, whereas the CYP pathway produces hydroxyeicosatetraenoic (HETEs) and epoxyeicosatetraenoic acids (EETs) in addition to HPETEs (Figure 2).
Levels of AA as well as its precursors gamma-linolenic acid (18:3 n-6) and 8,11,14-eicosatrienoic acid (20:3 n-6) are elevated in the plasma of AML patients, particularly in those with higher blast counts and risk (Pabst et al. 2017). Furthermore, the expression and enzyme activity of certain forms of phospholipase A2 are also elevated in AML blasts (Runarsson et al. 2007; Fiancette et al. 2009). In contrast to AA, the plasma of AML patients displays lower levels of PGE2 and 15-keto-PGF2α compared to that of healthy individuals (Pabst et al. 2017). COX1 expression is elevated in both AML and ALL patients, and the COX inhibitor, indomethacin, reduces leukemia-initiating cell frequency in a mouse model of AML driven by MLL-AF9 (Truffinet et al. 2007; Wang et al. 2010). However, the COX inhibitor, aspirin, does not impact the incidence of hematological malignancies in humans (Walter et al. 2011). Additional studies suggest that PGE2 may contribute to the pathogenesis of acute leukemia by acting on the tumor microenvironment. Specifically, PGE2 generated from mesenchymal stem cells (MSC) may contribute to chemotherapy resistance in AML (Carter et al. 2016). Additionally, inflammatory prostaglandins could contribute to leukemogenesis by suppressing the immune system. For example, PGE2 can suppress certain aspects of dendritic cell biology while simultaneously stimulating immunosuppressive cell populations such as myeloid-derived suppressor cells and regulatory T-cells (Tregs) (reviewed by Loew et al [Loew et al. 2019]). While the role of PGE2-mediated regulation of the immune system in AML remains unknown, elevated Treg counts are associated with diminished chemotherapy responses, and COX2 inhibition reduces AML cell-mediated Treg conversion (Szczepanski et al. 2009).
Once ALA and LA have been imported into the cell, they undergo a series of enzymatic steps that elongate and further desaturate these PUFA to generate EPA and AA, respectively (Figure 2). Fatty acid desaturase 1 (FADS1) and 2 (FADS2) desaturate both ω-3 and ω-6 PUFAs and both enzymes have been previously implicated in human cancer (Heravi et al. 2022; Kothapalli et al. 2023; Mustonen and Nieminen 2023). Several PUFA products of FADS2 are significantly increased in childhood ALL compared to healthy controls (Agatha, Häfer, and Zintl 2001). Angelo D'Alessandro's group first showed that leukemia cells from patients with relapsed AML display increased fatty acid desaturation as well as increased FADS1 and FADS2 expression compared to de novo AML patients (Culp-Hill et al. 2023). We reported that FADS1 expression is elevated across multiple AML subtypes and is associated with poorer outcomes (Kanefsky et al. 2024). We also showed that inhibition of FADS1 antagonizes the growth and survival of mouse and human AML cells without harming healthy mouse bone marrow cells in vitro and that FADS1 knockdown delays disease onset in a mouse model of AML driven by MLL-AF9 (Kanefsky et al. 2024). Interestingly, Culp-Hill et al. found that genetic or pharmacological inhibition of FADS1, FADS2 or both sensitized AML cells to the combination of 5-azacytidine and venetoclax (Culp-Hill et al. 2023). We, too, observed that chemical inhibition of FADS1 and FADS2, using the dual desaturase inhibitor CP-24879, significantly impaired the growth of patient-derived AML cells ex vivo (Kanefsky et al. 2024). Mechanistically, Culp-Hill and colleagues showed that pharmacological inhibition of FADS1 and FADS2 (using CP-24879) or FADS2 alone (using sc-26196) decreases the production of ω-6 PUFAs and carbon flux through the TCA cycle (Culp-Hill et al. 2023). Notably, they also observed that FADS inhibition significantly perturbed glycolytic flux, which is consistent with a previous study showing that FADS enzymes regulate NAD+ recycling (Kim et al. 2019).
Although we did not assess the impact of FADS1 interrogation on glycolysis and the TCA cycle, we did observe that shRNA-mediated inhibition of FADS1 significantly reduced global fatty acid desaturation and specifically decreased the incorporation of 20:4 fatty acids (AA is a 20-carbon fatty acid with 4 double bonds) into select membrane phospholipids (e.g., phosphatidylserine and phosphatidylethanolamine) and storage lipids (e.g., triglycerides and cholesterol esters) (Kanefsky et al. 2024). Interestingly, we also found that genetic or chemical inhibition of FADS1 resulted in activation of the STING pathway, which is an antiviral signaling network (Corrales et al. 2016; Vashi and Bakhoum 2021), and that deletion of STING mitigated certain anti-leukemia effects of FADS inhibition (Kanefsky et al. 2024). Aiming to therapeutically exploit these observations, we combined a STING agonist, diAZBI (Ramanjulu et al. 2018), with CP-24879 and found that the two compounds synergistically eliminated cultured AML cells but only marginally cooperated on patient-derived AML cells—this difference was largely due to the remarkable effectiveness of the STING agonist alone on patient-derived AML cells (Kanefsky et al. 2024). What remains to be determined is the precise mechanism by which FADS enzymes regulate cellular metabolism and antiviral signaling. Furthermore, while these preclinical studies position FADS enzymes as potential targets in AML (Table 1), PUFA biosynthesis plays a crucial role in many organ systems. Therefore, clinical testing will be needed, likely with the assistance of biomarkers and careful dosing, to determine whether targeting PUFA biosynthesis in AML is efficacious and safe.
4.4 Fatty Acid Uptake—The Tumor Microenvironment
The nutrient-rich tumor microenvironment plays a crucial role in the pathogenesis of many cancers, including acute leukemia, and is now a recognized hallmark of tumorigenesis (Hanahan 2022; Menter and Tzankov 2022; Dander et al. 2021). In particular, tumor cells rely on surrounding tissue to meet their fatty acid and lipid demands (Corn, Windham, and Rafat 2020). Both AML and B-ALL arise and reside in the bone marrow microenvironment (Menter and Tzankov 2022; Dander et al. 2021; Garcia-Gimenez and Richardson 2023). This microenvironment can be subdivided into various niches, each of which is made up of distinct cellular populations, such as adipocytes and MSCs (Allert, Müller-Tidow, and Blank 2024). Several studies have shown that acute leukemia cells reprogram and rely on local adipocytes, which specialize in storing fatty acids and lipids, to support leukemogenesis and promote chemotherapy resistance (Tabe, Konopleva, and Andreeff 2020). In coculture models of ALL, 3T3-L1-derived adipocytes significantly reduce the effectiveness of several chemotherapies in vitro (Behan et al. 2009). Furthermore, a high-fat diet, which increases bone marrow adipocytes, reduces the effectiveness of vincristine in a mouse model of ALL (Behan et al. 2009). Follow-up studies showed that ALL cells promote the release of free fatty acids (predominantly MUFAs) from adipocytes, which they subsequently utilize to produce triglycerides and phospholipids as well as fuel mFAO (Tucci et al. 2021).
The first detailed description of the bone marrow microenvironment contributing to fatty acid metabolism in AML showed that MSCs promote a shift in leukemia cell metabolism toward fatty acid oxidation (Samudio et al. 2010). Several years later, three studies emerged that highlighted the contribution of adipocytes to leukemia cell metabolism. Craig Jordan's group showed that LSCs residing in the bone marrow expressed high levels of the fatty acid transporter CD36 and stimulated local adipocytes to release fatty acids that the leukemia cells subsequently utilized to fuel fatty acid oxidation (Ye et al. 2016). Similarly, Stuart Rushworth and colleagues showed that AML cells promoted lipolysis of bone marrow adipocytes but also found that genetic or pharmacological inhibition of FABP4 (fatty acid binding protein 4), which facilitates fatty acid transport and uptake, suppresses AML biology in vitro and in vivo (Shafat et al. 2017). In line with these observations, Marina Konopleva and colleagues also found that human bone marrow-derived adipocytes sourced fatty acids to support AML fatty acid oxidation and cell survival (Tabe et al. 2017). Interestingly, increased expression of CD36 was recently found to be associated with extramedullary leukemia, chemotherapy resistance, as well as reduced event-free and overall survival. Functionally, the authors of this study also showed that CD36 inhibition reduced extramedullary leukemia and cooperated with chemotherapy in vivo (Farge et al. 2023). To therapeutically capitalize on these observations, a CD36 inhibitor called SMS121 was recently developed to block AML cell lipid uptake and reduce leukemia viability in vitro (Åbacka et al. 2024).
While human cells are able to generate certain short-chain fatty acids (SCFAs) such as acetate and butyrate, the gut microbiota remains a major supplier of SCFAs. The SCFAs butyrate and propionate have notable effects on AML through their impact on gut microbiota and intestinal barrier function. First, Wang et al. reported that butyrate levels are lower in the feces of AML patients, leading to reduced intestinal barrier function and subsequent release of lipopolysaccharide (LPS) into circulation thereby supporting leukemogenesis (Wang et al. 2022). Remarkably, butyrate supplementation restored intestinal barrier function, blocked lipopolysaccharide (LPS) absorption resulting in reduced disease burden in mouse models of AML. Similarly, propionate, another major SCFA, has also been found at reduced levels in AML patients and supplementation suppresses disease progression in vivo (Wei et al. 2024). High propionate levels are also associated with mitochondrial fission and enhanced mitophagy and supplementation of propionate promotes cell death through mechanisms such as mitophagy and ACSL4-mediated ferroptosis, which elicits anti-leukemia immunity (Heravi et al. 2022).
The gastrointestinal microbiome also plays a significant role in managing infectious complications during induction chemotherapy for AML (Rashidi et al. 2020). Chemotherapy often leads to gut dysbiosis, reducing overall microbial diversity and SCFA levels, which correlates with an increased risk of infections. This highlights the importance of maintaining gut microbiota health to mitigate the risks associated with chemotherapy and improve patient outcomes (Rashidi et al. 2020; Galloway-Peña et al. 2016).
From these collective studies, leukemia cells alter multiple aspects of fatty acid metabolism to support their biosynthesis needs. However, whether reprogramming or differential utilization of these fatty acids is a leukemia driving process or a passive adaptation of disease progression and chemotherapy resistance remains to be determined. Regardless, what is clear, it that disrupting many aspects fatty acid metabolism has severe consequences of leukemia cells highlighting that fatty acid metabolic reprogramming remains a point of therapeutic interest in leukemia.
5 Current Limitations and Future Prospects in Acute Leukemia Fatty Acid Metabolism
While many preclinical strategies for targeting various aspects of fatty acid metabolism in hematological malignancies and solid cancers have been investigated (Table 1), clinical translatability has been limited. For example, while etomoxir showed preclinical potential in sensitizing leukemia cells to chemotherapy, its high cost and potential toxicity in muscle and liver tissues have limited its clinical development (Bitar 2001). ST1326, an aminocarnitine derivative is highly selective for the CPT1a isoform, has shown preclinical efficacy but has yet to be evaluated clinically in acute leukemia. Interestingly, an oral formulation of ST1326 has shown promise in Phase 2 studies for Type 2 diabetes with a favorable safety profile (Samudio and Konopleva 2015). A major consideration in translating compounds that target fatty acid metabolism, is that although many tumor cells reprogram several aspects of fatty acid metabolism, these processes often play critical roles in many healthy tissues. Therefore, it is necessary to evaluate how new fatty acid targeting strategies impact healthy tissue in small and large animal models. Additionally, targeting the upstream regulators that promote fatty acid metabolic reprogramming in cancer cells may be an alternate strategy to directly targeting fatty acid metabolizing enzymes. For example, rather than targeting FAO enzymes, an alternative therapeutic entry point could be to first identify and target the upstream molecular pathways that shift AML energy metabolism from glycolysis and glutaminolysis towards FAO in response to chemotherapy in AML.
Another critical gap in knowledge in the study of fatty acid metabolism is pinpointing the precise fatty acid species (and complex lipids) that contribute to leukemogenesis and chemotherapy resistance. For example, many studies of fatty acid energy metabolism utilize palmitate as the substrate for FAO. While palmitate is one of the most abundant physiological fatty acids, other fatty acids such as other LCFAs (e.g., 18-carbon stearic acid) that may also be mono- or poly-unsaturated, may contribute to FAO. Identifying such fatty acids relies on isotopologue tracing assays, however, such studies are accompanied by their own limitations. For example, many fatty acids must be conjugated to carrier proteins (e.g., Bovine Serum Albumin) to be imported. The varying stability of fatty acids in culture complicates the accurate measurement and tracking of fatty acid isotopologues. Another challenge is that the use of fetal calf serum in cell cultures, which is rich in fatty acids and complex lipids, can influence exogenous fatty acid uptake resulting in artifactual data that may not align with in vivo realities. For example, the high abundance of lipids in serum used to supplement cell culture media can interfere with the uptake and assessment of fatty acids by cells. Also, cell culture systems often fail to replicate the complexity of the in vivo microenvironment, such as the substantial presence of adipocytes in the bone marrow. Developing more stable tracing molecules is essential to improve the accuracy and reliability of tracing studies by minimizing the impact of molecular instability. Additionally, optimizing serum-free or more physiologically relevant culture systems is important to better mimic in vivo conditions, including the role of adipocytes in the bone marrow, thus enhancing the relevance of in vitro findings. Advanced analytical techniques are also needed to better account and correct for the effects of serum lipids and other interfering factors on cellular uptake and metabolism studies. Integrating sophisticated in vivo models and techniques will help validate in vitro findings and provide a more accurate representation of physiological conditions. Lastly, refining assay methods and developing new methodologies that address the challenges of biomolecule instability will improve the precision and scope of tracing and metabolic studies.
6 Conclusion
The goal of this review has been to provide an updated overview of the studies that provide us with our current understanding of how fatty acid and lipid metabolism contribute to the pathogenesis of acute leukemia. Additionally, we have delineated how dysregulated lipid biosynthesis pathways, such as enhanced lipogenesis and altered lipid uptake, support various facets of leukemia cell biology and therapy resistance. We also delved into the dualistic role of PUFAs in cancer, elucidating their tumor-promoting and anticancer properties. We have also touched upon current preclinical strategies for targeting fatty acid metabolism and sphingolipid biology in acute leukemia and emphasized the immediate need but also the limitations for clinical evaluation of these exciting potential therapies.
Acknowledgments
S.M.S. is supported by the National Cancer Institute (R01-CA227830, R01-CA273127) and the American Cancer Society (RSG-18-195-01-DDC). J.S. is a recipient of a graduate fellowship from the McDonnell International Scholars Academy as well as an American Society of Hematology (ASH) Graduate Student Award. J.K. was supported by the ASH Minority Hematology Graduate Award.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
No data was generated for this article however, the figures have been generated with a paid academic subscription to Biorender.




