Primary cilium-mediated signaling cascade suppresses age-related biliary fibrosis
Abstract
The primary cilium is increasingly recognized as a crucial player in the physiology of biliary epithelial cells (BECs). However, the precise role of primary cilia in the development of age-related biliary fibrosis remains unclear. Herein, using cilium-deficient mice, we demonstrate that disruption of ciliary homeostasis in BECs in aged mice leads to significant bile duct proliferation, augmented biliary fibrosis, and heightened indicators of liver injury. Our RNA-sequencing data revealed a dysregulation in genes associated with various biological processes such as bile secretion, fatty acid metabolism, and inflammation. Loss of primary cilia also significantly enhanced signaling pathways driving the development of biliary fibrosis. Our findings collectively suggest that loss of primary cilia in the BECs of aged mice initiates a cascade of signaling events that contribute to biliary fibrosis, highlighting the primary cilium as a potential therapeutic target in the treatment of fibrosing cholangiopathies.
1 INTRODUCTION
Biliary fibrosis is a pathological wound healing response to chronic injury of biliary epithelial cells (BECs), characterized by progressive scarring of the biliary system, which can lead to cirrhosis, liver failure, and in some cases, necessitate liver transplantation (Amarachintha et al., 2022; Lendahl et al., 2021; Zhang et al., 2022). This progressive condition significantly contributes to the global burden of liver disease, responsible for approximately two million deaths per year worldwide (Asrani et al., 2019). Importantly, both the incidence and severity of biliary fibrosis increase significantly with age, establishing a crucial link between aging and exacerbated fibrotic processes in the biliary system (Lindor et al., 2009; Mayo, 2022). Despite this association, the molecular mechanisms driving age-related biliary fibrosis remain largely unexplored.
Primary cilia are small, hair-like structures protruding from the surface of almost all mammalian cells, including cholangiocytes, the epithelial cells lining the bile ducts (Breslow & Holland, 2019; Chen et al., 2022; Frassetto et al., 2018; Ge et al., 2022; Ran et al., 2021; Y. Yang et al., 2021). Functioning as sensory organelles, they play pivotal roles in cell signaling and tissue homeostasis (Qi & Zhou, 2021; Shapiro et al., 2018; Song et al., 2022). The importance of primary cilia in maintaining bile duct health is evident in ciliopathies, disorders arising from dysfunctional cilia (Hellen et al., 2023; Lucas et al., 2020; Ma et al., 2022). Several ciliopathies are associated with liver diseases, including biliary fibrosis (Mansini et al., 2018). Intraflagellar transport 88 (IFT88), a component of the IFT system, is vital for cilium formation and maintenance (Coveney, Samvelyan, et al., 2022; W. Li et al., 2022; Y. Yang et al., 2021). Mutations or disruptions in IFT88 have been implicated in cilia dysfunction, leading to the development of ciliopathies, including polycystic liver and kidney disease (Coveney, Zhu, et al., 2022; R. Fujii et al., 2021; Shao et al., 2020; Y. Wu et al., 2023; S. Yang et al., 2023). Cholangiocyte cilia are believed to be critical in sensing and responding to changes in bile flow and composition (Amarachintha et al., 2022). Dysfunction of cilia, due to defects in IFT88 or other ciliary components, can impair these essential cholangiocyte functions, thereby disrupting bile duct homeostasis (Lendahl et al., 2021; Mayo, 2022; Ogawa et al., 2021). Nevertheless, the exact mechanisms linking cilia dysfunction to the progression of age-related biliary fibrosis remain unclear, emphasizing the need for more in-depth investigation.
In this study, we found that disruption of ciliary homeostasis in BECs stimulates biliary fibrosis and liver injury in aged mice. High-throughput RNA sequencing revealed that the immune response process was activated in IFT88-deficient mice. Moreover, stress-related pathways such as cyclic adenosine monophosphate (cAMP) and extracellular signal-regulated kinase (ERK) signaling were stimulated, while processes related to bile secretion and fatty acid catabolism were suppressed. Therefore, cilia deficiency, by impairing bile duct function and promoting cellular stress, contributes to the development of biliary fibrosis. Our study highlights the importance of ciliary homeostasis in bile duct health and provides fresh insights into the pathogenesis of age-related biliary fibrosis.
2 MATERIALS AND METHODS
2.1 Animal experiments
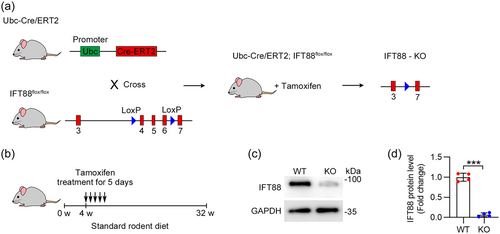
Ift88fl/fl mice, harboring loxP sites flanking exons 4–6 of the IFT88 gene, were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). These mice were bred with mice carrying the tamoxifen-inducible, ubiquitously expressed Cre/ERT2 recombinase-encoding Tg (Ubc-Cre/ERT2) transgene. Subsequently, heterozygous Ubc-Cre/ERT2/+; ift88fl/+ mice were crossed with homozygous ift88fl/fl mice to generate Ubc-Cre/ERT2; ift88fl/fl mice. To inactivate IFT88 function, the mice were administered with tamoxifen at a concentration of 20 mg/mL. All mice were backcrossed onto a C57BL/6J background and maintained on it. Genotyping of both Cre-positive and flox mice was performed using polymerase chain reaction (PCR). The usage of mice in this research study adhered to the relevant institutional and/or national guidelines for animal care and use, and was approved by the Animal Care and Use Committee of Nankai University. The mice were housed in a controlled environment free from pathogens, with a 12-h light-dark cycle and ad libitum access to food and water.
2.2 Reagents
Tamoxifen (TAM, cat #T5648). The hematoxylin and eosin (H&E) staining kit (G1120) and Sirius red staining kit (G1471) were purchased from Solarbio. The primary antibodies used in this study are as follows: rabbit anti-α-smooth muscle actin (α-SMA) (Abcam, ab5694; IF 1:500), rabbit anti-vimentin (Abcam, ab92547; IF 1:500), mouse anti-IFT88 (ProteinTech, 13967-1-AP; IB 1:1000), mouse anti-CK19 (Abcam, ab254186; IF 1:1000), rabbit anti-ARL13B (ProteinTech, 17711-1-AP; IF 1:1000), mouse anti-ac-tubulin (Sigma, T7451, IF 1:1000), rabbit anti-phospho-Raf1 (Ser338) (Abcam, ab51042 IB 1:1000), rabbit anti-RAF1 (Servicebio,GB11543, IB 1:500), rabbit anti-phospho-MEK1/2 (Ser217/221) (Cell Signaling, 9154, IB 1:1000), rabbit anti-MEK1/2 (ProteinTech, 11049-1-AP, IB 1:10,000),rabbit anti-phospho-ERK1 (Thr202/Tyr204) + ERK2 (Thr185/Tyr187) (Servicebio, GB11004; IB 1:500), rabbit anti-ERK1/2 (Servicebio, GB11560, IB 1:3000), and rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Abways, AB0036; IB 1:2000). HRP-conjugated goat anti-rabbit (AB0101; IB 1:5000) and goat anti-mouse (AB0102; IB 1:5000) secondary antibodies were obtained from Abways. Alexa Fluor 488- and 568-conjugated secondary antibodies were purchased from Life Technologies.
2.3 Immunoblotting
Protein isolation was performed with RIPA lysis buffer. Concentration was determined using BCA protein assay. Briefly, the protein sample was separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes and then blocked with 5% fat-free milk at room temperature for 2 h and incubated with primary antibody overnight at 4°C. After washing five times, the membrane was incubated with secondary antibodies at room temperature for 1 h. Protein signals were detected using immunoblotting detection reagents (Millipore, WBKLS0500). The experiment was repeated for at least three times, and the intensity of immunoblots was measured by densitometry using the ImageJ software (National Institutes of Health, Washington, DC, USA).
2.4 Histopathology and immunofluorescence microscopy
Liver samples were fixed with 4% paraformaldehyde for 24 h for histopathological analysis. After fixation, the samples were dehydrated and embedded in Tissue-Tek OCT (Sakura) on dry ice slabs. The embedded samples were then cut into frozen sections with a thickness of 8 µm. H&E or Sirius red staining were performed on the paraffin-embedded sections, following the standard protocols established in our laboratory as described previously (Ran et al., 2022). For immunofluorescence staining, the slides were fixed once again with 4% paraformaldehyde for 20 min, permeabilized with 0.5% Triton X-100 in phosphate-buffered saline for 20 min, and blocked with 4% bovine serum albumin for 1 h. The slides were then incubated overnight at 4°C with the appropriate dilution of antibodies against CK19, ARL13B, or Ace-tubulin. HRP-conjugated secondary antibodies (mouse or rabbit) were applied at room temperature for 1 h. Staining was visualized using DAPI (Sigma-Aldrich) for 5–10 min. The length of cilia and the percentage of ciliated cells were quantified using Image J software (National Institutes of Health).
2.5 Determination of biochemical indexes
Serum was collected to measure alkaline phosphatase (ALP), total bilirubin (TBIL), aspartate transaminase (AST), and alanine transaminase (ALT) levels. The activities of ALT and AST in the serum were determined using the ALT activity kit (Solarbio, BC1555) and the AST activity kit (Solarbio, BC1565) following the manufacturer's instructions. Serum ALP and TBIL levels were measured using commercial kits (Pointe Scientific Inc.; ALP: A7516150; bilirubin: B7576120).
2.6 RNA sequencing
Liver tissue was collected from 32-week-old IFT88-KO mice and WT littermates (n = 4 mice per group) for total RNA extraction. After extraction, the RNA was subjected to high-throughput RNA-seq (Novogene). The raw data were collected and analysed according to the methods described in previous study (Umbarkar et al., 2022). Briefly, gene ontology (GO) analysis was performed through the Gene Ontology Consortium website (http://geneontology.org/), and pathway analysis was performed through the Kyoto Encyclopedia of Genes and Genomes (KEGG) website (https://www.kegg.jp/). The fold changes in differentially expressed genes (DEGs) were analysed according to the reads per kilobase of transcript per million mapped reads (RPKM).
2.7 RNA isolation and real-time quantitative polymerase chain reaction (qRT-PCR)
At harvest, liver tissue was snap frozen in liquid nitrogen and stored at −80°C until further processing. Total RNA was isolated from frozen liver tissue using TRIzol® reagent (Life Technologies) according to the manufacturer's protocol, and 1 µg RNA was reverse-transcribed by the SuperScript III kit. qRT-PCR was then performed with the Power SYBR Green PCR Master Mix Kit (Applied Biosystems). GAPDH was used as the loading control, and the results were calculated with the 2−ΔΔCt method. Sequences of primers are summarized in Supplementary Table S1.
2.8 Statistical analysis
All data were derived from a minimum of three independent experiments unless otherwise specified. Statistical analysis was performed using GraphPad Prism 8.0 (GraphPad Software). To determine significant differences between two groups, an unpaired two-tailed Student's t-test was applied. Results are presented as mean ± standard deviation. Statistical significance is denoted as follows: ns, not significant; *p < 0.05; **p < 0.01; ***p < 0.001.
3 RESULTS
3.1 Construction and validation of IFT88-deficient mice
To investigate whether the disruption of ciliary homeostasis is involved in biliary injury and fibrosis in aged mice, we generated mice with floxed IFT88 alleles and a tamoxifen-inducible Cre/ERT2 recombinase under the control of the ubiquitin C promoter to induce whole-body IFT88 deletion. To induce IFT88 deletion, Ift88fl/fl; Ubc-Cre/ERT2 mice were subjected to a tamoxifen regimen starting from the fourth week postbirth, continuing for 5 consecutive days, and the mice were maintained on a standard rodent diet until they were 32–34 weeks old before they were killed (Figure 1a,b). The deletion of IFT88 in the livers of Ift88fl/fl; Ubc-Cre/ERT2 mice was confirmed by immunoblotting (Figure 1c,d). These results indicate that IFT88 was efficiently deleted after tamoxifen injection.
3.2 Disruption of ciliary homeostasis by IFT88 depletion in BECs
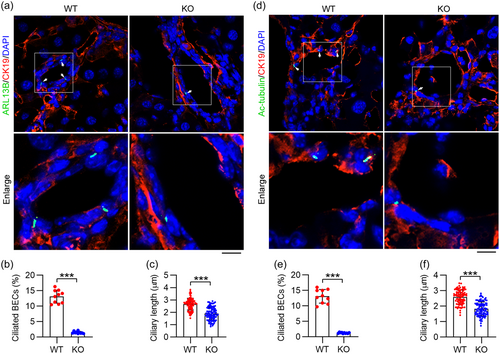
Primary cilia localized on the apical side of BECs play pivotal roles in BEC polarization and in sensing flow within the bile ducts (Fabris et al., 2019). Previous studies have noted a reduced number of primary cilia in BECs from cases of biliary atresia (Lendahl et al., 2021). To evaluate the presence of primary cilia on BECs after IFT88 depletion, liver tissues were stained with antibodies against cytokeratin 19 (CK19, a marker of BECs), and ADP ribosylation factor-like GTPase 13B (ARL13B, a marker of cilia) (Hor & Goh, 2019). We found that depletion of IFT88 reduced the number of cilia in BECs and shortened the ciliary length (Figure 2a–c). To further corroborate this phenotype, we immunostained BECs with another ciliary marker, acetylated tubulin (ac-tubulin). Our data showed that IFT88 depletion decreased both the number and the length of cilia in the BECs (Figure 2d–f). These findings confirm that the deletion of IFT88 disrupts ciliary homeostasis in BECs.
3.3 Cilium-deficient aged mice are more susceptible to biliary fibrosis
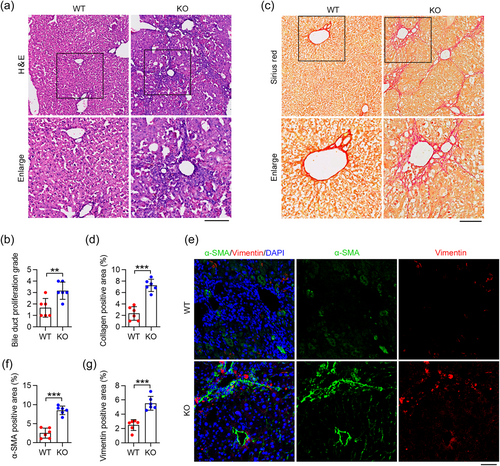
We aimed to investigate whether the IFT88 depletion and the subsequent disruption of ciliary homeostasis contribute to the development of biliary fibrosis in aged mice. Utilizing H&E staining, we observed a well-structured hepatic lobule in the wild type (WT) group. In contrast, the IFT88-knockout (KO) group exhibited notable bile duct proliferation, accompanied by inflammatory cell infiltration (Figure 3a). Liver histopathology was assessed on H&E-stained liver sections using a validated 1–4+ scale. A blinded review yielded higher scores for bile duct proliferation in aged IFT88-KO mice (Figure 3b). Sirius red staining of liver samples from aged mice revealed an increased incidence of biliary fibrosis in the IFT88-KO group compared to the WT group, as indicated by a denser deposition of collagen around proliferating bile ducts (Figure 3c,d). Using immunofluorescence microscopy, we found that IFT88 deficiency significantly amplified the expression of biliary fibrosis markers, such as α-SMA and vimentin, in liver BECs (Figure 3e–g). Taken together, these results suggest that disruption of ciliary homeostasis in BECs due to the loss of IFT88 may be instrumental in promoting biliary fibrosis.
3.4 Cilium-deficient mice display marked liver injury at advanced age
Fibrosing cholangiopathies are typically characterized by inflammation and fibrosis focused on the biliary epithelium, leading to progressive cholestasis, bile duct proliferation, periductal sclerosis, and hepatic fibrosis, which ultimately result in liver injury (Deng et al., 2018; N. Wu et al., 2022). To assess the impact of ciliary loss on liver damage, we analyzed the levels of various biochemical markers in mice. The results demonstrated significantly increased serum ALP levels, a marker of cholangiocyte injury, in IFT88-KO mice (Figure 4a). Alongside, serum TBIL levels, which serve as an indicator of liver function, were noticeably higher in the IFT88-KO group as compared to the WT group (Figure 4b). Furthermore, serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels, which are biochemical markers of liver injury, were significantly elevated in IFT88-KO mice (Figure 4c,d). The expression of pro-fibrogenic genes and extracellular matrix (ECM) components, namely α-SMA, collagen type 1a1 (Col1a1), Col1a2, and tissue inhibitor of metalloproteinases (TIMP1) were all significantly amplified in aged mice deficient in IFT88 (Figure 4e–h). Likewise, the expression of the cholangiocyte marker Krt19, which encodes CK19, followed a similar trend (Figure 4i). Hepatic expression of the macrophage marker, cluster of differentiation 68 (Cd68), along with tumor necrosis factor-alpha (TNF-α) andmonocyte chemoattractant protein-1 (Mcp-1), was elevated in aged IFT88-KO mice (Figure 4j–l), suggesting the activation of inflammatory pathways. Collectively, these results strongly suggest that the progression of liver disease in IFT88-KO mice is significantly more pronounced than in WT mice.

3.5 High-throughput profiling of liver reveals profibrotic response in cilium-deficient mice
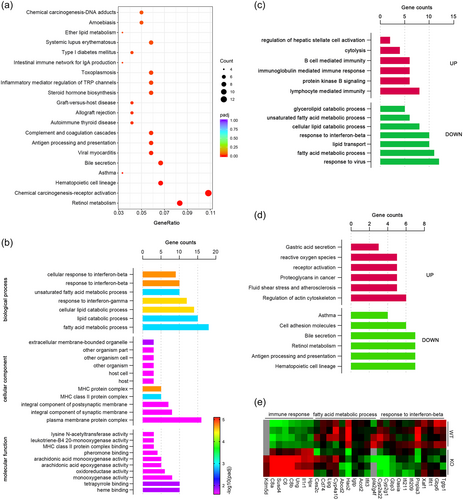
To gain further clues to the regulatory role of cilia in the development of biliary fibrosis, we performed high-throughput RNA sequencing. KEGG analysis showed that DEGs were clustered in pathways related to chemical carcinogenesis-receptor activation, retinol metabolism, and bile secretion (Figure 5a). GO enrichment analysis identified that the DEGs were predominantly involved in fatty acid metabolic processes and responses to interferon (Figure 5b). Specifically, DEGs involved in immune response, reactive oxygen species generation, fluid shear stress, and atherosclerosis processes were found to be upregulated, while DEGs related to lipid catabolic processes, responses to interferon, bile secretion, retinol metabolism, and hematopoietic cell lineage were downregulated (Figure 5c–e). These results further confirm that disruption of cilia homeostasis plays an important role in the pro-fibrotic response.
3.6 Cilium-mediated signaling events underlie age-related biliary fibrosis
To validate the results from the RNA-seq analysis, qRT-PCR was performed to evaluate the expression levels of DEGs in both IFT88-KO and WT mice. The findings indicated a significant decrease in the expression of genes associated with bile secretion processes, including Abcb1a, Abcc4, Adcy1, Aqp8, Slco1a4, and Ugt2b37 (Figure 6a). Similarly, genes associated with fatty acid catabolism, such as Cyp4a12a, Cyp4a10, and Cyp4a12b, were also significantly downregulated (Figure 6b). Moreover, genes in the cAMP pathway, including Abcc4, Sucnr1, Sstr2, Gnai1, Adcy1, Ptger2, Hcn2, and Ppp1r1b, were noted to be downregulated (Figure 6c). However, genes associated with inflammation processes, including Il1r1, Blnk, Bcl2a1d, and Plau, were significantly upregulated (Figure 6d).
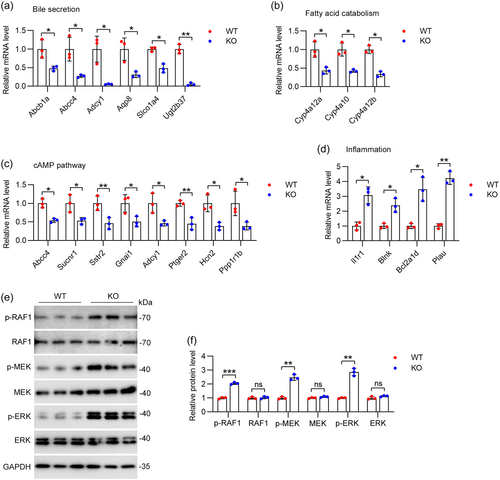
The RAF-MEK-ERK signaling cascade is a well-characterized MAPK pathway involved in cell proliferation and survival (S. Fujii et al., 2022). MEK-ERK activation promotes fibroblast activation and excessive fibrosis, culminating in cardiac dysfunction and heart failure (Umbarkar et al., 2022). Given the important role of the ERK signaling pathway in fibrosis development, we evaluated the phosphorylation levels of key elements in this pathway. Immunoblot analysis showed significant upregulation of phosphorylation of RAF1, MEK, and ERK, which confirmed the activation of the MEK-ERK pathway in aged IFT88-KO mice (Figure 6e,f). Thus, these results suggest that ciliary deficiency-mediated activation of RAF-MEK-ERK signaling may contribute to biliary fibrosis and liver injury in aged mice.
4 DISCUSSION
Age-related biliary fibrosis is a pathologic wound healing process in response to chronic injury of BECs, which may lead to organ dysfunction, biliary cirrhosis, and eventually malignancy. Therefore, understanding the mechanisms that drive biliary fibrosis is important to identify new therapeutic targets. Our study presents substantial evidence that disruption of ciliary homeostasis in BECs by IFT88 depletion leads to significant bile duct proliferation, increased biliary fibrosis, and heightened indicators of liver injury. In addition, ciliary deficiency leads to dysregulation in bile secretion and fatty acid metabolism, which may act through the activation of the RAF-MEK-ERK signaling pathway.
Primary cilia are pivotal in the polarization of BECs and for sensing flow in the bile ducts, and their reduction is observed in biliary atresia BECs (Amarachintha et al., 2022; Lam et al., 2021). Interestingly, we observed that cilium-deficient mice were more susceptible to biliary fibrosis, as reflected by the increase of bile duct proliferation, collagen deposition around proliferating bile ducts, and the enhanced expression of α-SMA and vimentin in liver BECs. These findings indicate a potential correlation between ciliary homeostasis disruption and biliary fibrosis progression. Therefore, the loss of cilia could be a potent driver of biliary fibrosis in aged mice. Additionally, our results demonstrated that cilium-deficient mice displayed marked liver injury as they aged. This was evidenced by increased serum ALP levels, TBIL levels, and ALT and AST levels, all indicative of liver injury and dysfunction. The expression of pro-fibrogenic genes, ECM components, and indicators of inflammation were also elevated in aged cilium-deficient mice, clearly demonstrating an aggravation in liver disease development in the absence of cilia.
Age-related biliary fibrosis is a pathological response to chronic injury in the liver that is characterized by the excessive accumulation of ECM proteins, including collagen, in the bile ducts (Lindor et al., 2019). This process often precedes and contributes to the progression of more serious liver conditions, such as cirrhosis and liver failure (Shen et al., 2019). It's important to note that the bile ducts play a critical role in liver function, including the transport of bile, which is essential for the digestion and absorption of dietary fats (Sampaziotis, 2018). Moreover, fibrosis can also impact the liver's regenerative ability, a remarkable capacity following injury (J. Li et al., 2021). Sustained fibrotic injury can lead to the formation of scar tissue, disrupting the normal architecture of the liver, and ultimately impairing its ability to regenerate (Forbes & Newsome, 2016). In our study, we observed that ciliary deficiency led to a significant increase in biliary fibrosis in aged mice, which could have important implications for hepatic function and could potentially impair the transport of bile, leading to cholestasis, a condition characterized by a decrease in bile flow. This reduction can lead to the build-up of toxic bile acids in the liver, causing further liver damage. Consistently, we found that cilium-deficient mice had significantly higher levels of liver injury indicators, such as serum total TBIL, ALP, ALT, and AST (Ghallab et al., 2022), further underscoring the potential impact of biliary fibrosis on hepatic function. These findings are in line with existing research, which indicates a close relationship between biliary fibrosis and liver function (Corpechot et al., 2022). Therefore, preventing or reversing biliary fibrosis may represent a promising therapeutic strategy for preserving hepatic function and managing liver diseases. However, further research is needed to better understand the mechanisms driving biliary fibrosis and to develop effective interventions for this process.
In exploring the regulatory role of cilia in biliary fibrosis development, our RNA sequencing results demonstrated an activation of the immune response and stress processes in the absence of IFT88. Notably, the genes associated with bile secretion and fatty acid catabolism processes were significantly decreased, while those associated with inflammation were significantly increased. This suggests that ciliary deficiency may promote liver injury and fibrosis by dysregulating immune response and metabolic processes. One of the remarkable findings in this study was the significant upregulation of the ERK signaling pathway, a crucial regulator of fibrosis development, in cilium-deficient mice, pointing to a potential molecular mechanism by which cilia deficiency could drive biliary fibrosis development. The ERK signaling pathway is a crucial cellular cascade involved in regulating various physiological processes including cell proliferation, differentiation, and survival (Liu et al., 2018). Aberrant activation of this pathway has been implicated in various pathological conditions, including fibrotic diseases in various tissues, including the liver (Cai et al., 2020; Widjaja et al., 2019) (Umbarkar et al., 2022; Zhang et al., 2023). In the context of liver fibrosis, it has been demonstrated that ERK stimulates hepatic stellate cells, resulting in their activation and subsequent production and deposition of ECM proteins, leading to the development of liver fibrosis (Mederacke et al., 2022; Popescu et al., 2019). It is also worth noting that the ERK pathway can be activated by various pro-inflammatory and pro-fibrotic cytokines. Therefore, the increased activation of the ERK pathway in cilium-deficient mice might be related to an increased inflammatory response, as evidenced by upregulation of several genes related to inflammation, including Il1r1, Blnk, Bcl2a1d, and Plau. These findings suggest a potential mechanistic link between cilium-deficiency, ERK pathway activation, and biliary fibrosis. However, the exact mechanisms by which ciliary deficiency leads to ERK pathway activation and how this contributes to biliary fibrosis still need to be elucidated in future studies. It also remains to be determined whether targeted inhibition of the ERK pathway could be a potential therapeutic strategy for preventing or treating biliary fibrosis.
In summary, our study significantly enhances the understanding of the role of ciliary homeostasis in BEC physiology and its implications on biliary injury and fibrosis. However, further research is needed to elucidate the precise molecular mechanisms linking primary cilia to age-related biliary fibrosis and liver injury, as well as the potential therapeutic implications these findings could have in managing hepatobiliary diseases.
AUTHOR CONTRIBUTIONS
Min Liu, and Yanjie Tan: designed experiments. Renjie Hong, Xiaoyu Tian, Hongbo Ma, Hua Ni, Jia Yang, and Weiwen Bu: performed experiments. Renjie Hong, Te Li, Song Yang, Dengwen Li, Min Liu, and Yanjie Tan: analyzed data. Min Liu and Yanjie Tan: wrote the manuscript.
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (32170829 and 32000524) and the Shandong Provincial Natural Science Foundation (ZR2020QC076).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.




