Protective effect of dimethyl itaconate against fibroblast–myofibroblast differentiation during pulmonary fibrosis by inhibiting TXNIP
Abstract
Fibroblast–myofibroblast differentiation (FMD) is a critical cellular phenotype during the occurrence and deterioration of pulmonary fibrosis (PF). FMD can increase with an elevated level of reactive oxygen species (ROS) on fibroblasts under oxidative stress. Thioredoxin-interacting protein (TXNIP) is an α-arrestin family protein that regulates the level of intracellular ROS. Nuclear factor erythroid 2-related factor 2 (Nrf2) can protect against FMD in PF. However, the relationship between Nrf2 and TXNIP in FMD remains elusive. Therefore, we established TGF-β1-induced FMD in vitro and bleomycin (BLM)-induced mouse PF model in vivo to explore whether the activation of Nrf2 can inhibit TXNIP-mediated FMD in PF. Dimethyl itaconate (DMI) was selected to activate Nrf2. Our results showed that TXNIP was elevated and FMD was aggravated in mice lung tissues after BLM administration compared with the saline group. Inversely, Nrf2 decreased TXNIP expression and alleviated FMD in PF. In vitro, TXNIP overexpression enhanced FMD and increased the level of ROS. In contrast, TXNIP deficiency by small interfering RNA (siRNA) attenuated TGF-β1-induced FMD and reduced ROS. An increase in ROS by H2O2 can upregulate TXNIP expression. Moreover, Nrf2 also inhibited TGF-β1-induced FMD and the increase of ROS, with reducing expression of TXNIP, and the inhibitory effect was better than TXNIP siRNA. These results suggest that activation of Nrf2 by DMI can protect against PF via inhibiting TXNIP expression. Our study may provide new therapeutic targets and treatment approaches for PF.
Abbreviations
-
- BLM
-
- bleomycin
-
- DMI
-
- dimethyl itaconate
-
- FMD
-
- fibroblast–myofibroblast differentiation
-
- HFL1
-
- human fetal lung fibroblast 1
-
- Nrf2
-
- nuclear factor erythroid 2-related factor 2
-
- PF
-
- pulmonary fibrosis
-
- ROS
-
- reactive oxygen species
-
- TXNIP
-
- thioredoxin-interacting protein
1 INTRODUCTION
Pulmonary fibrosis (PF) is a severe lung disease with interstitial fibrosis and high mortality after diagnosis (Richeldi et al., 2017). It is characterized by abnormal activation and proliferation of myofibroblasts, which contributes to excessive secretion and accumulation of extracellular matrix (ECM) (Loomis-King et al., 2013). Unfortunately, there are limited therapeutic interventions to reverse established PF. Hence, it is urgent to explore the pathological mechanisms and discover effective therapeutic targets. An increasing number of studies supports the view that lung fibroblasts (LFs) with fibroblast–myofibroblast differentiation (FMD) is a crucial cellular phenotype in which LFs are stimulated by a variety of cytokines, especially transforming growth factor (TGF)-β1, and stress induces differentiation into myofibroblasts, eventually leading to PF (El Agha et al., 2017; Hinz et al., 2012; Lederer et al., 2018). During the FMD process, LFs express more myofibroblasts maker like alpha-smooth actin (α-SMA) and produce substantial collagen and reactive oxygen species (ROS) (Saito et al., 2019). Therefore, FMD is an important target of pulmonary fibrosis.
Oxidative stress, the imbalance of oxidation and anti-oxidation effects, increases the production of ROS and has a crucial effect on the pathogenesis of PF (Duecker et al., 2018; Wuyts et al., 2013). Meanwhile, thioredoxin-interacting protein (TXNIP) is an α-arrestin family protein that could be upregulated by the accumulation of intracellular ROS (DeBalsi et al., 2014; Han et al., 2018). Recent studies have found that TXNIP expression was increased in the bleomycin (BLM)-induced PF model (Kseibati et al., 2020). However, the relevant mechanism and whether it participated in FMD are unclear. Recent studies have highlighted the protective role of nuclear factor erythroid 2-related factor 2 (Nrf2) in PF (Qu et al., 2018). Our previous research showed that Nrf2 suppressed EMT progression, which is another target as important as FMD in PF (Z. Zhang et al., 2018). Here, we further verify that the activation of Nrf2 could also inhibit TGF-β1-induced FMD progression and BLM-induced PF. Meanwhile, activation of Nrf2 can prevent oxidative stress-driven hepatotoxicity by inhibiting TXNIP (Lv et al., 2020). Therefore, this study was conducted to explore whether ROS/TXNIP signaling pathway is involved in FMD and could it be inhibited by Nrf2 antioxidant protein. Among the reported activators, dimethyl itaconate (DMI), a cell-permeable itaconate derivative synthesized in vitro, is expected to become a novel activator of Nrf2 due to its promising safety profile (Bambouskova et al., 2018; Mills et al., 2018). Initial studies on itaconate have concentrated on its anti-inflammatory activity as an endogenous metabolite of macrophages (Lampropoulou et al., 2016; Strelko et al., 2011). Hence, the protective effect of DMI against FMD-involved PF was observed in this study.
Here, we constructed the BLM-induced PF model in vivo and TGF-β1-induced FMD in vitro to investigate whether the activation of Nrf2 can inhibit FMD during PF by inhibiting TXNIP expression.
2 MATERIALS AND METHODS
2.1 Animal model
C57BL/6J male mice (8–10 week old) were purchased from the Institute of Genome Engineered Animal Models for Human disease of Dalian Medical University (Dong & Ma, 2019). All experiments performed on the mice conform to the guidelines from the National Institutes of Health and were approved by Dalian Medical University Animal Care Committee and Use Committee (ethical approval code number: AEE19013). Mice were randomly divided into saline group, BLM group, DMI (50 mg/kg) + BLM group, DMI (100 mg/kg) + BLM group, and DMI (200 mg/kg) + BLM group (n = 15 per group). Intratracheal injection of 4.5 mg/kg BLM to establish mouse PF model. Mice in the saline group were intratracheally injected with saline in the same condition and DMI groups were further intraperitoneally injected with different doses of DMI every day after BLM administration (C. Zhao et al., 2019). Mice lung tissues were removed on 14 and 28 days for further detection.
2.2 Histopathological staining and immunohistochemistry
In brief, mice lung tissues were transferred to 50% alcohol after fixing with 4% paraformaldehyde solutions for 24 h and transferred to alcohol after 2 h. The lung tissues were then embedded in paraffin and cut into 5 µm sections on a microtome. After that, tissue slices were stained with hematoxylin and eosin (H&E) and Masson's trichrome for observing the tissue structure and collagen content. The expression level of collagen I (Col-I) (anti-Col-I; bs-10423R; Bioss, 1:200) was determined by immunohistochemistry analysis (IHC) (Z. Zhang et al., 2018).
2.3 Cell lines and culture
Human fetal lung fibroblast 1 cells (HFL1) were obtained from the Chinese Academy of Sciences Cell Bank (Shanghai, China) and cultured in Ham's F-12K (Procell, Wuhan, China) medium supplemented with 10% fetal bovine serum (FBS; Gibco) in a humidified atmosphere of 5% CO2 at 37°C.
2.4 Transfection of TXNIP-overexpressing plasmid and TXNIP siRNA
HFL1 cells that reached 60%–70% confluence on 6-well plates were transfected with TXNIP small interfering RNA (synthesized by GenePharma) or with TXNIP-overexpressing plasmid (synthesized by GenePharma) mixed with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. HFL1 cells were transfected with TXNIP-overexpressing plasmid for 48 h before TGF-β1 stimulation.
2.5 Western blot analysis
Mice lung tissues and cells were lysed with radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology) containing protease and phosphatase inhibitors (Beyotime Institute of Biotechnology), then protein was quantified by BCA Protein assay kit (Beyotime Institute of Biotechnology). Protein samples were mixed with loading buffer separated on 8%–12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then were transferred to polyvinylidene difluoride membranes (IPVH00010; Millipore). After being blocked with 5% skim milk, the membranes were incubated overnight at 4°C with primary antibody, including anti-Nrf2 (ab31163; Abcam, 1:900), anti-TXNIP (14715; CST, 1:800), anti-α-SMA (ab5694; Abcam, 1:1000), or anti-GAPDH (2118; CST, 1:1500). On the second day, after being combined with antimouse IgG (H + L) (Thermo Fisher Scientific, Invitrogen) or antirabbit IgG (H + L) (Thermo Fisher Scientific, Invitrogen), the protein bands on the membranes were detected by using the Oddessy Clx. GAPDH was used for normalizing the expressed level of relative protein.
2.6 Immunofluorescence analysis
Immunofluorescence staining of Nrf2, TXNIP, Col-I, and α-SMA on HFL1 cells or mice lung tissues was incubated with primary antibody respectively overnight at 4°C and then combined with TRITC-conjugated goat antimouse IgG (ZF-0313; 1:100) or FITC-conjugated goat antirabbit IgG (ZF-0311; 1:100). 4ʹ,6ʹ-diamidino-2-phenylindole (C1005; Beyotime Institute of Biotechnology, Shanghai) was used to stain the nucleus. The images were obtained by Olympus UTBI90 Fluorescence microscope at ×100 magnification.
2.7 Assessment of oxidative stress
Intracellular ROS were detected by using dihydroethidium (DHE) and ROS assay kit separately according to the manufacturer's instructions. In brief, DHE stain: HFL1 cells were incubated with 5 µM DHE at 37°C for 30 min; ROS stain: HFL1 cells were cultured with 10 µM DCFH-DA at 37°C for 20 min. The ROS images were observed by Olympus UTBI90 Fluorescence microscope at ×50 magnification.
2.8 Cytotoxicity assays
Cell Counting Kit-8 (CCK-8; Meilunbio) was performed to test cytotoxicity according to the manufacturer's instructions. In brief, the cells were incubated with a CCK-8 working solution for 4 h to detect the optical density (OD) value at 450 nm.
2.9 Statistical analysis
The data were presented in this study as mean ± standard deviation (SD), two groups of data were determined by t-test, and groups containing multiple were analyzed by one-way analysis of variance. Statistical analyses were performed by social science statistical software package (SPSS) software and column graphs used GraphPad prism6 software. p < .01 or p < .05 was considered statistically significant.
2.10 Chemicals and materials
DMI was purchased from Sigma company. Recombinant TGF-β1 was purchased from PeproTech (100-21C). BLM was purchased from Hanhui Pharmaceuticals.
3 RESULTS
3.1 The expression level of TXNIP was enhanced in FMD and PF
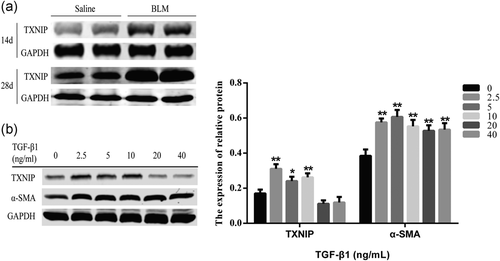
The potential role of TXNIP on HFL1 cells with TGF-β1 and mice with BLM was explored in this study. As shown in Figure 1a, the expression level of TXNIP was remarkably increased on Days 14 and 28 in BLM-treated mice groups compared with the saline group. In vitro, HFL1 cells were treated with different concentrations of TGF-β1 for 72 h. Treatment with 2.5, 5, and 10 ng/mL TGF-β1 significantly upregulated the expression of TXNIP on HFL1 cells, thereby upregulating myofibroblast marker α-SMA (Figure 1b). These results suggest that TXNIP has a significant expression level change in FMD and PF.
3.2 ROS/TXNIP signaling pathway was activated by TGF-β1 on HFL1 cells with FMD
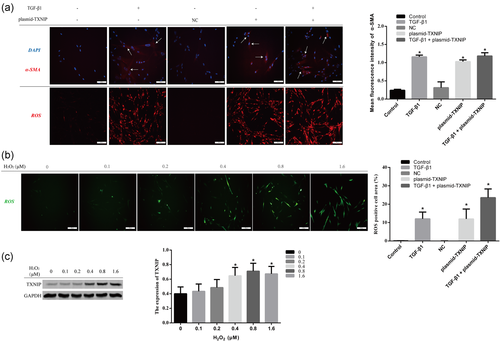
As shown in Figure 2a, FMD was accelerated in HLF1 cells with TGF-β1 or TXNIP overexpression compared with the control group, accompanied by a significant increase in the ROS level. Co-treatment of TGF-β1 and TXNIP-overexpressing plasmid on HFL1 cells can promote more ROS generation compared with the TGF-β1 group or TXNIP overexpression group. Simultaneously, as shown in Figure 2b,c, HFL1 cells were treated with different concentrations of H2O2 (ROS inducer) for 24 h; ROS production increased by enhancing the expression level of TXNIP. These results suggest that TXNIP may increase ROS level and promote FMD in HFL1 cells, and ROS increased by H2O2 can upregulate the TXNIP expression level.
3.3 DMI inhibited TGF-β1-induced FMD by activating Nrf2 signaling in HFL1 cells
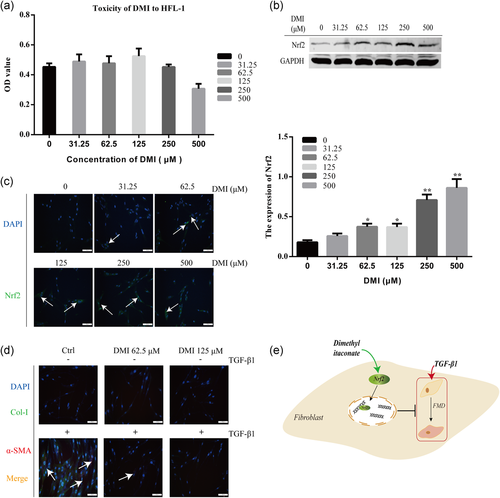
Nrf2 is a key antioxidative transcription factor and plays a protective role in FMD. Here, we explored whether the DMI could decrease oxidative stress by activating Nrf2 on HFL1 cells. First, we performed a CCK8 assay to examine the effect of various concentrations of DMI on the cell viability of HFL1 cells. The results showed DMI had no effect on the cell viability at concentrations of 0–500 μM (Figure 3a). As shown in Figure 3b, DMI significantly enhanced the expression level of Nrf2 in a dose-dependent manner in HFL1 cells. At the same time, the nuclear accumulation of Nrf2 increased after DMI treatment by immunofluorescence detection (Figure 3c). As shown in Figure 3d, activation of Nrf2 by DMI inhibited the expression level of Col-I and α-SMA. These results reveal that DMI could inhibit FMD by upregulating the Nrf2 level in HFL1 cells (Figure 3e).
3.4 The activation of Nrf2 protected against TGF-β1-induced FMD via ROS/TXNIP signaling pathway in HFL1 cells
The results in Figure 4a showed that the upregulation of TXNIP in TGF-β1-induced FMD was reversed by Nrf2 activated by DMI. Meanwhile, DMI reduced the accumulation of ROS in HFL1 cells stimulated by TGF-β1 (Figure 4b). In addition, the expression level of FMD maker was also decreased in TGF-β1-induced FMD on HFL1 cells transfected by siTXNIP, and siTXNIP treatment reduced the level of ROS in HFL1 cells (Figure 4c,d). However, DMI + TGF-β1 group has a better effect in inhibiting FMD than the siTXNIP group. The above results indicate that activated Nrf2 by DMI may attenuate TXNIP-mediated FMD by decreasing ROS accumulation.

3.5 DMI attenuated BLM-induced lung fibrosis in mice
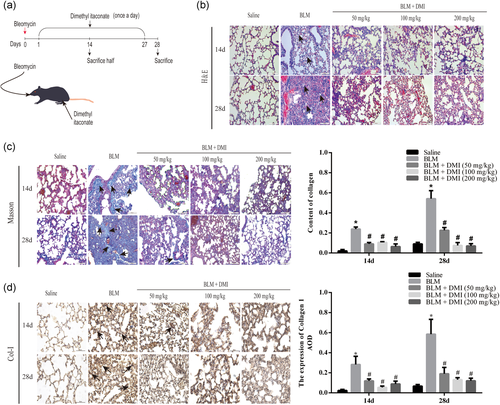
To observe the pathological changes in vivo, mice were treated with saline or BLM. Then parts of BLM groups were treated with DMI (Figure 5a). As shown by H&E and Masson trichrome staining (Figure 5b,c), we observed the abnormal pathologic changes after BLM administration, which were relatively attenuated in different doses of the DMI-treated group. These changes covered: the alveolar structures were destroyed and the deposition of collagen. Staining results indicated that the BLM-induced lung fibrosis models were successfully established and DMI could reverse this process. To further confirm the effect of treatment of DMI in PF, we examined the expression level of Col-I in lung tissues by IHC. We found that the level of Col-I in lung tissues was obviously increased after BLM treatment (Figure 5d). However, this effect was faded in the DMI-treated group. These results remind us that DMI could alleviate BLM-induced PF in mice.
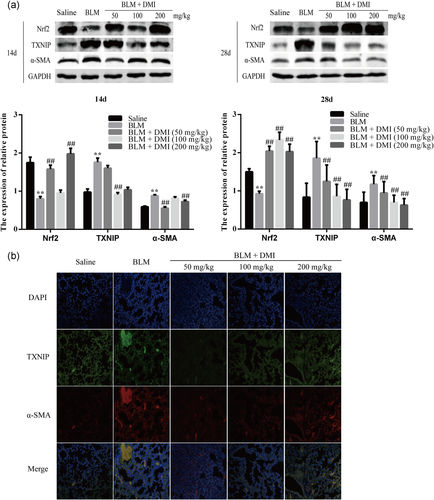
3.6 DMI alleviated FMD in PF by increasing Nrf2 expression level and decreasing TXNIP expression level
As shown in Figure 6a,b, the expression level of α-SMA was increased in mouse PF but decreased in the DMI intervention groups. Moreover, TXNIP was obviously increased in FMD during PF (Figure 6b). In addition, the expression of TXNIP was upregulated and Nrf2 was downregulated in the PF model on days 14 and 28 compared with the saline group, but the expression of TXNIP was further downregulated in DMI-treated groups accompanied by the activation of Nrf2.
4 DISCUSSION
PF is a fatal pulmonary interstitial disease, and currently, there are no effective prevention and treatment approaches clinically. Molecular mechanisms of PF have not been fully elucidated so far. Previous studies showed that BLM-induced PF was formed in 7–14 days (Rangarajan et al., 2018; W. Zhou et al., 2016). Mice lung tissues mainly showed inflammation in 7 days after BLM administration (Nie et al., 2017). Hence, the mice lung tissues under BLM intervention in 14 and 28 days were removed for the experiment. Several studies have found that abnormally proliferated and activated myofibroblasts increased in BLM-induced mouse PF model, and differentiation of inherent fibroblasts into myofibroblasts (FMD, also known as myofibroblast differentiation) is the most important source of myofibroblasts (Thannickal et al., 2004; Tsubouchi et al., 2017). In the TGF-β1-induced FMD process, myofibroblasts can express more α-SMA and secrete large amounts of collagen-like collagen I (Sato et al., 2016). Therefore, interference with the FMD process has been a potential treatment for PF. Although the increasing number of research works have focused on FMD in PF pathogenesis, the mechanism of FMD has not yet been elucidated. Here, we observed that knockdown of TXNIP reduced TGF-β1-induced FMD and TXNIP was upregulated in experimental pulmonary fibrosis. However, the expression level of TXNIP was decreased by a high concentration of TGF-β1, indicating that TXNIP may also be involved in other mechanisms of PF. The results in this study prove that TXNIP is one of the key proteins in FMD during PF, which may provide a novel target for PF in clinical treatment.
The oxidative stress is manifested in the form of the accumulation of ROS in the cells, which plays a decisive role in the pathogenesis of PF (Duecker et al., 2018; Guo et al., 2010). Meanwhile, the massive generation of ROS is an important factor in TGF-β1-induced FMD (Bindu et al., 2017). Previously, TXNIP has been recognized as a key signal of oxidative stress and directly participates in the regulation of ROS generation on bone marrow cells (Jung et al., 2013). An increase in ROS level can also enhance the expression level of TXNIP on human embryonic kidney (HEK293) T cells (R. Zhou et al., 2010). Nonetheless, the relationship of ROS and TXNIP on lung fibroblasts is not clear. The results that increase of ROS generation can up-regulate TXNIP expression in TGF-β1-induced FMD suggest ROS/TXNIP signal pathway was activated in FMD. However, whether ROS plays a critical role in TXNIP-mediated FMD needs further research works.
Itaconate is an endogenous small molecule metabolite that is secreted by mammalian macrophages (Strelko et al., 2011). Recent research found that it can exert anti-inflammatory effects by activating Nrf2. DMI, an active derivative of itaconate, can also activate Nrf2 the same as itaconate (Mills et al., 2018). In addition, another recent study reported that DMI has a good safety profile in vivo (Bambouskova et al., 2018). And increasing evidence shows that DMI has a certain medicinal value as an activator of Nrf2 (Shan et al., 2019; S. Zhang et al., 2020; C. Zhao et al., 2019). Meanwhile, Nrf2 has a protective effect during PF and it can inhibit the FMD process (Yang et al., 2013; H. Zhao et al., 2017). Here, DMI was selected to activated Nrf2 and protect against PF. The preventive effect of DMI on PF was explored in this study. According to our results, we observed that DMI may upregulate Nrf2 in vivo and in vitro, and alleviate BLM-induced mouse PF and TGF-β1-induced FMD. These results confirm that DMI can activate Nrf2 and further attenuate FMD in PF. However, the dose dependence of DMI, which did not appear in vivo, suggests that more pharmacokinetic studies need to be carried out.
Nrf2 is a critical transcription factor that regulates redox balance and can exert antioxidant effects by reducing the accumulation of ROS in cells (Ge et al., 2017). TXNIP and Nrf2 are both involved in the regulation of intracellular ROS levels. Our data show that activation of Nrf2 inhibited ROS/TXNIP signaling pathway and FMD during PF. However, the database predicts that there is no direct interaction between Nrf2 and TXNIP (Chatr-Aryamontri et al., 2017). Hence, more in-depth experiments need to be designed to demonstrate that Nrf2 inhibits TXNIP by attenuating the accumulation of intracellular ROS. Nonetheless, our study shows that the activation of the ROS/TXNIP signaling pathway may accelerate the FMD process, which is inhibited by Nrf2 in FMD-induced PF. However, Nrf2 has a stronger inhibitory effect on FMD than knockdown of TXNIP, indicating that the decrease of TXNIP may not be the specific way in this process. In addition, although Nrf2 can suppress TXNIP via reducing ROS level, there may still be other approaches that have not been explored. Nevertheless, our experimental results in vivo and in vitro indicate the potential therapeutic value of DMI and the importance of TXNIP in the progression of PF.
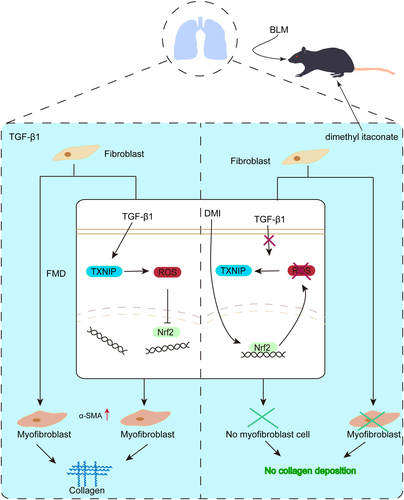
In conclusion, our study proves that DMI protects against PF via activating Nrf2, and activation of Nrf2 can further reduce FMD in PF via inhibiting ROS/TXNIP signaling pathway (Figure 7). Therefore, DMI may be a novel Nrf2 activator and can become a promising medicine for PF. In addition, antagonizing the increase of TXNIP in the lung of patients with PF may have a therapeutic prospect clinically.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (No. 81274172, 81473267, and 81973637).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Yong-Yue Han and Jian Gao designed the experiments. Yong-Yue Han wrote the manuscript. Yong-Yue Han, Xuan Gu, Chong-Yang Yang, Hui-Min Ji, Yu-Qian Bi, Yue-Jiao Lan, Rong Si, and Jiao Qu performed the animal experiments. Yong-Yue Han performed the western blot assays. Ming-Han Cheng and Jian Gao edited the manuscript. Yong-Yue Han generated statistical analysis and handled figure data.




