Transcriptome-based screening of ion channels and transporters in a migratory chondroprogenitor cell line isolated from late-stage osteoarthritic cartilage
Csaba Matta and Rebecca Lewis should be considered as joint first author.
Abstract
Chondrogenic progenitor cells (CPCs) may be used as an alternative source of cells with potentially superior chondrogenic potential compared to mesenchymal stem cells (MSCs), and could be exploited for future regenerative therapies targeting articular cartilage in degenerative diseases such as osteoarthritis (OA). In this study, we hypothesised that CPCs derived from OA cartilage may be characterised by a distinct channelome. First, a global transcriptomic analysis using Affymetrix microarrays was performed. We studied the profiles of those ion channels and transporter families that may be relevant to chondroprogenitor cell physiology. Following validation of the microarray data with quantitative reverse transcription-polymerase chain reaction, we examined the role of calcium-dependent potassium channels in CPCs and observed functional large-conductance calcium-activated potassium (BK) channels involved in the maintenance of the chondroprogenitor phenotype. In line with our very recent results, we found that the KCNMA1 gene was upregulated in CPCs and observed currents that could be attributed to the BK channel. The BK channel inhibitor paxilline significantly inhibited proliferation, increased the expression of the osteogenic transcription factor RUNX2, enhanced the migration parameters, and completely abolished spontaneous Ca2+ events in CPCs. Through characterisation of their channelome we demonstrate that CPCs are a distinct cell population but are highly similar to MSCs in many respects. This study adds key mechanistic data to the in-depth characterisation of CPCs and their phenotype in the context of cartilage regeneration.
Abbreviations
-
- AD-MSC
-
- adipose tissue-derived mesenchymal stem cell
-
- BK
-
- large conductance calcium-activated potassium channel
-
- BM-MSC
-
- bone marrow–derived mesenchymal stem cell
-
- CPC
-
- chondrogenic progenitor cell
-
- DEP
-
- dielectrophoresis
-
- ECM
-
- extracellular matrix
-
- GAG
-
- glycosaminoglycan
-
- IBTX
-
- iberiotoxin
-
- MSC
-
- mesenchymal stem cell
-
- OA
-
- osteoarthritis
-
- PCA
-
- principal component analysis
-
- PG
-
- proteoglycan
-
- RMP
-
- resting membrane potential
-
- SOCE
-
- store-operated Ca2+ entry
-
- TRP
-
- transient receptor potential
1 INTRODUCTION
The prevalence of musculoskeletal conditions is constantly increasing, making age-related and chronic inflammatory joint diseases the major causes of disability in the elderly population (Al Maini et al., 2020). Osteoarthritis (OA) is the most common form of chronic musculoskeletal disorders (Hunter & Bierma-Zeinstra, 2019). Although the primary target of OA is articular cartilage, it also affects other tissues within and around the joint (Loeser et al., 2012). The affected tissues undergo metabolic, structural and functional alterations that contribute to joint pain, disease progression and patient disability (Henrotin et al., 2016).
Chondrocytes are the main cell type in articular cartilage (Archer & Francis-West, 2003), along with a scarce population of cartilage progenitor cells (CPCs) (Nakayama et al., 2020). The resident cells are embedded in a cartilage-specific extracellular matrix (ECM) that consists of collagen type II, large aggregating proteoglycans (PG; e.g., aggrecan), constituent glycosaminoglycans (GAG), hyaluronan, small PGs and other collagenous and noncollagenous proteins (Buckwalter et al., 2005). A high amount of interstitial water (∼80% of the total weight of cartilage) is osmotically drawn to the freely mobile cations (i.e., Na+, K+, Ca2+) balancing the negatively charged GAG side chains of PGs and are therefore present at high local concentrations. Cells in cartilage ECM are thus exposed to a unique ionic microenvironment (Mobasheri et al., 1998; Urban et al., 1993). Cartilage is avascular, and as a consequence of the scarcity of available nutrients and oxygen, it is unique, relatively hypoxic and acidic milieu, as well as low cellularity, it is incapable of mounting a sufficient healing and repair response following injury (Gomoll & Minas, 2014).
The presence of a cartilage-specific CPC population with stem cell properties in the superficial zone of articular normal cartilage is now widely accepted (Dowthwaite et al., 2004). More recently, cells in OA articular cartilage with mesenchymal progenitor cell characteristics have also been observed and characterised (Fellows et al., 2017). These cartilage progenitor/stem cell populations have the potential for chondrogenic induction and trilineage plasticity. CPCs have also been described in late-stage OA cartilage, which is believed to be migrating in response to chemotactic signals from the bone marrow through breaks in the tide mark, as an attempt to regenerate damaged cartilage ECM (Koelling et al., 2009). However, knowledge concerning the specific phenotypic features and regenerative potentials of chondroprogenitor cells is still incomplete. CPCs are considered an ideal source for cell-based cartilage repair, and therefore progress has been made in identifying, understanding and characterising these cells.
In OA, chondrocytes and CPCs exist in a microenvironment dominated by mediators that promote matrix degradation and low-grade inflammation. There is evidence that cartilage ECM undergoes profound alterations during OA in terms of GAG and water content (Mankin & Lippiello, 1970). That in turn alters the osmolality of the matrix and the composition of the unique ionic milieu (Mow et al., 1999). Chondrocytes and other cells in cartilage respond to these changes and maintain their homoeostasis by altering the transport of ions across the cell membrane (Hdud et al., 2014) via transporters and ion channels, collectively referred to as the ‘channelome’ (Asmar et al., 2016; Barrett-Jolley et al., 2010; Mobasheri et al., 2019).
Plasma membrane transporters including voltage-gated sodium, potassium and calcium channels, chloride channels, calcium-activated potassium channels, transient receptor potential (TRP) channels, N-methyl-d-aspartate receptors and purinergic receptors have been described in chondrocytes, which allow them to respond to the local ionic composition of the pericellular matrix by adjusting the resting membrane potential (RMP) (Maleckar et al., 2020), which has been shown to play a crucial role in regulating metabolic activity and synthetic rate of cartilage ECM, as well as proliferation, differentiation or volume regulation (Asmar et al., 2016; Matta & Zakany, 2013; Mobasheri et al., 2019). Although much progress has been made towards characterising the chondrocyte channelome, many open questions remain concerning the composition of the ion channel complement and their function in chondroprogenitor cells. Whilst there is accumulating data suggesting that several genes encoding ion channels which are involved in the regulation of mechanotransduction, cell volume, RMP and apoptosis are differentially expressed in OA chondrocytes (Lewis & Barrett-Jolley, 2015), current understanding concerning the channelome of CPCs, especially with regard to differentially regulated ion channel genes, is incomplete. Addressing this gap in knowledge is a high priority for the identification and targeting of new therapeutic targets for the treatment of OA.
In this study, we hypothesised that CPCs derived from OA cartilage may be characterised by a different assembly of ion channels and transporters that regulate their function and phenotype and maintain communication with the altered ECM. Given that there is some evidence that migratory CPCs are related to mesenchymal stem cells (MSCs) residing in the bone marrow close to the subchondral bone which has migrated to lesioned cartilage through breaks in the tide mark, we used bone marrow–derived MSCs (BM-MSCs) as a reference cell population. We have recently analysed the surfaceome of CPCs using selective cell surface protein labelling followed by quantitative high-throughput mass spectrometry and identified alterations in the composition of the surfaceome compared to MSCs (Matta et al., 2019). However, even that approach was not sensitive enough to detect alterations in very low-abundance ion channels or other transporters. In this study, we attempted to differentiate CPCs from BM-MSCs based on their transcriptome and electrophysiological properties. We first performed a global transcriptomic analysis using Affymetrix microarrays. We studied the profiles of those ion channels and transporter families that are known to be involved in regulating chondrocyte physiology, RMP, volume regulation, calcium signalling, matrix secretion or chondrogenesis, which may have relevance in chondroprogenitor cell physiology (Barrett-Jolley et al., 2010; Matta & Zakany, 2013; Mobasheri et al., 2019; Suzuki et al., 2020). We then employed patch clamping and dielectrophoresis (DEP) to characterise the basic electrophysiological profile (the ‘electrome’; De Loof, 2016) of the two cell types. Following validation of the microarray data using quantitative real-time polymerase chain reaction (RT-qPCR), we examined the role of calcium-dependent potassium channels in the cellular physiology and homoeostasis of migratory CPCs and found that the large-conductance calcium-activated potassium channels (BK) are functionally expressed and are involved in the maintenance of the chondroprogenitor phenotype.
2 MATERIALS AND METHODS
2.1 Cell culture
Experiments were carried out on a human migratory CPC cell line derived from late-stage OA knee articular cartilage, which has been immortalised by viral transfection of the human telomerase reverse transcriptase (hTERT) as previously described (Koelling et al., 2009). CPCs were cultured in monolayers in 75 cm2 cell culture flasks (Nunc; Thermo Fisher Scientific) until approximately 80% confluence in GlutaMax DMEM (1.0 g/L glucose; Gibco; Thermo Fisher Scientific) containing 10% foetal calf serum (FCS; Gibco) and 50 μg/ml gentamycin (Sigma-Aldrich). As a reference cell population, human BM-MSCs (Lonza) were used. The cells were received at passage 2 and were expanded until passage 4 in Lonza hMSC medium at 37°C in a humidified atmosphere of 5% CO2. MSCs were hTERT-immortalised as previously described in (Okamoto et al., 2002) with the modifications detailed in (Saeed et al., 2015). Immortalised MSCs were expanded in monolayers in 75 cm2 cell culture flasks (Nunc) until approximately 80% confluence in GlutaMax DMEM (4.5 g/L glucose; Gibco) containing 10% FCS (Gibco) and 1% P/S (Sigma-Aldrich).
2.2 RNA isolation and reverse transcription
Total RNA was isolated from cells grown in monolayers in 75 cm2 cell culture flasks using the RNeasy kit (Qiagen) as per the instructions of the manufacturer and stored at –80°C. RNA concentration and purity were determined by a NanoDrop 2000 UV-Vis spectrophotometer (Thermo Fisher Scientific). For gene expression analyses, 2 μg of RNA was reverse-transcribed into complementary DNA (cDNA) using the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific), following the protocol supplied by the manufacturer. cDNA was stored at –20°C.
2.3 Affymetrix microarray analysis
RNA integrity was confirmed using an Agilent 2100 Bioanalyzer with the RNA 6000 Nano Kit (Agilent Technologies). The RNA integrity numbers were ≥9.6 for all samples. Whole-genome transcriptome analysis was conducted by hybridising three biological samples of total RNA per cell type to Affymetrix Human Gene 2.1 ST Arrays Strips (Affymetrix). All steps were conducted at the Nottingham Arabidopsis Stock Centre. Gene expression data were analysed using Partek Genomics Suite 6.6 software (Partek Incorporated). The raw CEL files were normalised using the RMA background correction with quantile normalisation, log base 2 transformation and mean probe-set summarisation with adjustment for GC content. Differentially expressed genes (DEG) were identified by a two-way analysis of variance, and p values were adjusted using the false discovery rate (FDR) method to correct for multiple comparisons. DEG was considered significant if p value with FDR was ≤.05. The expression data of genes coding for selected transporter and ion channel subunits are summarised in Tables S1–S4. The data set is published in a MIAME compliant format in the GEO database (http://www.ncbi.nlm.nih.gov/geo/); accession numbers: GSM4885525-GSM4485530 (GSE160886).
2.4 Pathway analysis
CytoScape v3.4 software with ClueGo v2.3.5 application was used for identifying overrepresented gene ontology (GO) terms. A two-sided hypergeometric test with Bonferroni step-down correction was performed using the list of DEG and the GO Biological process database.
2.5 RT-qPCR analyses
Selected ion channel subunit genes were analysed by RT-qPCR using a custom configured TaqMan 96-Well Fast Gene Expression Array plate (see Tables S5 and S6) and then by absolute quantification using the standard curve method. Primer pairs were ordered from Eurogentec. For sequences of primer pairs please see Table S7. First, standard curves had been generated by conventional PCR using the Promega GoTaq Flexi DNA Polymerase kit (Promega) by adding the following components (per 50 μl reaction): 1.25 U GoTaq polymerase, 3 mM MgCl2, 0.2 mM dNTP, 200 nM primers and 10 ng cDNA. Amplification was performed in a Techne Prime Thermal Cycler (Techne; Bibby Scientific Ltd) using the following thermal profile: initial denaturation at 95°C for 5 min, followed by 40 cycles of denaturation at 95°C for 15 s, annealing at 58°C for 20 s, extension at 74°C for 20 s, and then final extension at 74°C for 5 min. PCR products were isolated using a Roche High Pure PCR Product Purification Kit (Roche) according to the instructions of the manufacturer. DNA concentration of purified PCR products was determined using a NanoDrop 2000 UV-Vis spectrophotometer (Thermo Fisher Scientific). Standard curves were prepared by a serial (10-fold) dilution starting from 1 ng/μl.
RT-qPCR reactions were set up using the Promega GoTaq qPCR Master Mix and 20 ng input cDNA per each 10-μl reaction. Reactions were run in a Techne Prime Pro 48 Real-time qPCR machine using the following thermal profile: activation and initial denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 10 s, annealing at 58°C for 30 s, extension at 72°C for 20 s, and then final extension at 72°C for 20 s. Due to spatial limitations in the 48-well qPCR plates, quantification of qPCR products was performed by absolute quantification using the standards prepared in the previous step, followed by normalising the expressional data of genes of interest to those of the most stably expressed reference gene (PPIA), and then the expression levels for the genes were normalised to those in MSCs (set at 1.0). The optimal normalisation gene was selected using the NormFinder algorithm (Andersen et al., 2004). RT-qPCR reactions were performed on three biological replicates (N = 3).
2.6 Cell proliferation and mitochondrial activity assays
To assess the effect of BK channel modulators, cells were plated into 96-well plates at a density of 5000 cells/well. Before the assays, cells were incubated for 48 h in the presence of the selective BK channel blocker iberiotoxin (IBTX) (100 nM; Tocris; Bio-Techne); the potent BK channel inhibitor paxilline (1 μM); the BK channel activator NS1619 (10 μM; Sigma-Andrich); or vehicles at equal volumes (water, dimethyl sulfoxide and ethanol, respectively). The rate of cell proliferation was determined by detecting the amount of incorporated radioactivity from 3H-thymidine. One microcurie per millilitre 3H-thymidine (diluted from methyl-3H-thymidine; 185 GBq/mM; Amersham Biosciences) was added to each well (Wallac, PerkinElmer Life and Analytical Sciences) for 24 h. After washing with phosphate-buffered saline (PBS), proteins were precipitated with ice-cold 5% trichloroacetic acid and rinsed with PBS again. Cells were then air-dried and radioactivity was counted by a liquid scintillation counter (Chameleon; Hidex). Measurements were carried out in seven samples of each experimental group (n = 7) in three independent experiments (N = 3). For mitochondrial activity assays, 10 μl 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent (thiazolyl blue tetrazolium bromide; 5 mg MTT/ml PBS; Sigma-Aldrich) was pipetted into each well. Cells were incubated for 2 h at 37°C, and following the addition of 500 μl MTT solubilizing solution, optical density was measured at 570 nm (Chameleon; Hidex).
2.7 Migration assays
2.7.1 Boyden chemotaxis chamber
CPCs were washed twice in PBS, harvested with 0.25% trypsin (Sigma-Aldrich) and resuspended in a culture medium. The lower wells of a 48-well Boyden chemotaxis chamber (Neuro Probe Inc.) were filled with 1 μl/ml human fibronectin (Sigma-Aldrich) dissolved in PBS and covered with a polycarbonate filter (Neuro Probe Inc.) containing pores with a diameter of 3 μm. Cell suspension (50 μl) at a density of 2 × 105 cells/ml was inoculated into the wells on the top of the membrane and the chamber was incubated for 5 h at 37°C in a humidified atmosphere with or without 1 μM paxilline. Nonmigrated cells were removed from the cis-surface of the membrane and after fixation in methanol, migrated cells were stained with 1% toluidine blue (Sigma-Aldrich) dissolved in water. Membranes were air-dried and mounted with Pertex medium (Sigma-Aldrich). Toluidine blue-stained migratory cells were counted on the trans surface of the membrane by an internally developed MATLAB (Mathworks Inc.) application. Cells were defined by an approximate range of values in the RGB colour space with a particle size of at least 30 pixels. Other structures, primarily membrane pores, cell debris and staining artefacts, were omitted based on their different overall size and their distinct position in the RGB colour space. Cells in six wells were counted in each experimental group and three independent assays (N = 3) were performed.
2.7.2 Time-lapse imaging of live cells
Live-cell imaging was carried out using the CytoSMART 2 imaging system (CytoSMART Technologies). For imaging, cells were seeded in 35-mm Petri dishes at a density of 1 × 104 cells. After allowing the cells to adhere for 120 min, the Petri dishes were transferred onto the device and imaging has started, either with or without 1 μM paxilline. Phase-contrast time-lapse images were obtained automatically at 30-min intervals for 18 h at 37°C and 5% CO2. Time-lapse images were analysed using ImageJ (National Institutes of Health; https://imagej.nih.gov/ij/) and CellTracker (http://celltracker.website/) software programmes. The cells were tracked manually through every frame, and the x and y coordinates of the movements were recorded. We excluded dying, dividing or damaged cells from the analysis. The length of the total path, maximal distance from the origin, as well as average and maximum cell speeds were calculated. To create wind rose plots illustrating the trajectories, the migratory tracks of the individual cells were shifted to a common origin. Three independent experiments (N = 3) were performed.
2.8 Electrophysiology
2.8.1 DEP
Cells were grown to approximately 80% confluence before analysis. Cells were dissociated from the culture plates by trypsinisation. Afterwards, cells were washed twice and resuspended in an iso-osmotic DEP medium with low ionic strength containing 8.5% (wt/vol) sucrose (Sigma-Aldrich), 0.3% (wt/vol) glucose (Sigma-Aldrich), and adjusted to a final conductivity of 10 mS/m using PBS (Sigma-Aldrich). Buffer conductivity was measured with a conductivity metre (RS Components Ltd). Cell counts and viability for each experiment were determined using a haemocytometer and trypan blue, which indicated a 96 ± 2% viability. To reduce the effect of variation in cell numbers in each sample, the final cell concentration was adjusted to 5 × 105 cells/ml.
DEP spectra were obtained using the 3DEP DEP-Well system (DEPtech), as described in more detail earlier (Labeed et al., 2011). Briefly, cells resuspended in DEP media were administered into the wells of DEP-Well chips containing 12 ring-shaped, 17-μm wide, gold-plated copper electrodes around the good circumference with gaps of 75 μm between electrodes, and energised with currents at specific frequencies. The DEP method is based on the principle that the cells are either attracted or repelled from the side of the well by an amount proportional to their polarizability, as described previously (Hoettges et al., 2019).
Changes in light intensity across the wells over time were determined using a 1.3 MegaPixel video camera installed on the microscope and a MATLAB (Mathworks Inc.) script. The change in cell distribution was monitored by recording an image every 3 s for a total of 30 s. The entire well was divided into 10 segments that were monitored separately; however, only segments 7–9 were analysed as previously described (Hoettges et al., 2008). The wells were energised with frequencies ranging from 1 kHz–20 MHz at five points per decade. Spectra were generated using MATLAB (Mathworks Inc.) and presented in values of light intensity versus frequency. Light intensity data were fit to the single-shell model (Broche et al., 2005) and the best-fit model was used to determine the following features: specific membrane capacitance (CSpec), specific membrane conductance (GSpec) and crossover frequency (i.e., the frequency where the DEP force is zero). The best-fit model (highest Pearson correlation coefficient) was established by matching the curve to the measured data and adjusting the dielectric cytoplasmic and membrane parameters until the best match was found. MATLAB scripts were used for all images as well as for signal processing and data analysis as previously described (Hoettges et al., 2008). Given that the key parts of the DEP curve (starting and end values and transition frequencies) can be associated with the membrane capacitance and conductance and cytoplasm conductivity and permittivity, these parameters were determined uniquely by fitting the curve to the data points. Cell diameters were measured in a haemocytometer and the average radius was calculated using ImageJ software version 1.51 (http://imagej.nih.gov/ij/); the mean radii were used for the DEP model. All experiments were repeated three times (N = 3 independent experiments); data are expressed as mean ± SD.
2.8.2 Patch clamp
Whole-cell currents were measured under voltage-clamp conditions as described earlier (Almassy & Begenisich, 2012). Extracellular solution contained (in mM): NaCl, 140; KCl, 5; CaCl2, 2; MgCl2, 1; HEPES, 10; glucose, 5; pH 7.4 with NaOH. Patch pipettes were fabricated from thick-walled borosilicate glass (o.d. 1.5 mm, i.d. 0.85 mm) with the resistance of approximately 5 MΩ when filled with intracellular solution. The pipette solution contained (in mM): d-gluconic acid potassium salt, 115; KCl, 26; MgCl2, 1; EGTA, 5; HEPES, 10; pH 7.2 with KOH. The specific BK channel inhibitor paxilline (Alomone Labs) was used in 5 µM to verify the functional expression of the channels. Vm values were determined following 2 min of stable membrane potential recording with I = 0 on Axon 200 A/B amplifiers (Lewis et al., 2011). Only cells with seal resistance above 10 GΩ and good access (Rs < 20) were used. Whole-cell currents were recorded in n = 27 CPC and 7 MSC cells. For RMP measurements, all values are quoted as mean ± SEM, with sample size (n) = 22 (CPC) and 8 (MSC). All membrane potentials are corrected for liquid junction potentials estimated using JPCalc (Barry & Lynch, 1991).
2.9 Measurement of intracellular Ca2+ oscillations in CPCs
Cells were loaded with 10 μM Fura-2-AM for 50 min at 37°C in a CO2 incubator. After loading, cells were kept in Tyrode's solution and placed on the stage of a ZEISS Axiovert 200M inverted microscope (Zeiss) equipped with a Plan-Neofluar ×20 objective (NA = 0.5). Fura-2 was excited with a CoolLED pE-340fura light source (CoolLED Ltd.). Illumination was alternating between 340 and 380 nm wavelength and the emitted fluorescent light was measured through a band-pass filter (505–570 nm) and digitised at 16 bit with an AXIOCam MR3 monochrome camera (Zeiss) at a frequency of 12 frames/min. Image acquisition and postprocessing were carried out with the AxioVision (rel. 4.8) software (Zeiss). The cells were examined in resting conditions for 15 min at room temperature. At the end of the experiments, 180 μM ATP was added to the bath to check the viability of the cells. Cells not responding to the ATP challenge were excluded from the analysis. Intracellular Ca2+ concentration ([Ca2+]i) was calculated from the ratio of the images taken at 340 and 380 nm after background correction with constants determined during in vitro calibration of the setup. The cells were classified as active if the increase of [Ca2+]i was more than 10% of the resting level. n = 332 cells were analysed.
2.10 Statistical analysis
All data are representative of at least three independent experiments (biological replicates). RT-qPCR, cell proliferation and mitochondrial activity, as well as cell migration data, are expressed as mean ± SEM, DEP data are expressed as mean ± SD. Statistical analysis was performed using Student's unpaired two-tailed t-test (*p < .05; **p < .01; ***p < .001).
3 RESULTS
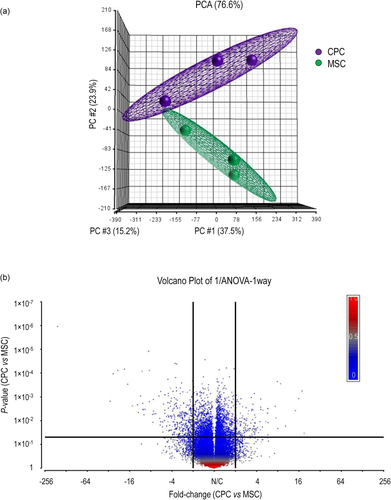
3.1 Affymetrix analysis reveals differentially expressed ion channel genes in migratory CPCs
We performed a principal component analysis of the Affymetrix data on normalised samples to explore their interrelationships (Figure 1). Biological replicates within the CPC/MSC groups were separated into two distinct clusters, showing that the pattern of gene expression was different in CPCs and MSCs. In total, 3214 genes showed significantly different expression levels between CPC and MSC cells, with 2379 genes being downregulated and 835 genes upregulated. Using CytoScape software with the ClueGo application we identified those GO Biological process terms which were overrepresented in our gene list. When we analysed the full gene list (up- and downregulated genes together), and the list of downregulated genes, we found that mainly cell cycle-related categories such as cell cycle, regulation of cell cycle, cell cycle checkpoint and mitotic/meiotic cell cycle processes were enriched. Furthermore, DNA/nucleic acid metabolism-related pathways were also overrepresented. Separate analyses of upregulated genes indicated the enrichment of different types of tissue development and cell proliferation processes, such as skin development and mesenchymal cell proliferation (Figure S1 in the File S1; see also File S2).
To characterise the expression profiles of genes encoding ion channel subunits in CPCs and to identify differences in expression levels compared to MSCs, we further explored the microarray analysis data. We assessed genes encoding ion channels and transporters commonly involved in regulating potassium, sodium and chloride transport and calcium homoeostasis in chondrocytes (Figure 2; see also Tables S1–S4). The expression levels for some of the genes studied were different in migratory CPCs versus MSCs. Genes encoding members of the calcium-activated potassium channels (KCa) showed the most prominent expression changes; KCNMA1 and KCNN4, the genes coding for the pore-forming alpha subunit of the large-conductance calcium-activated potassium channel (KCa1.1), and the intermediate/small conductance calcium-activated potassium channel (KCa3.1), respectively. In both cases, there was a more than 1.5-fold upregulation in CPCs. In contrast, the small conductance calcium-activated potassium channel (KCa2.3) encoded by the KCNN3 gene was unchanged. The β4 regulatory subunit of the large-conductance calcium-activated potassium channel (KCNMB4) was significantly downregulated in CPCs. KCNB1 (voltage-gated potassium channel subunit Kv2.1); KCND2 (Kv4.2) and KCNH6 (Kv11.2) were also downregulated in CPCs (Table S1).
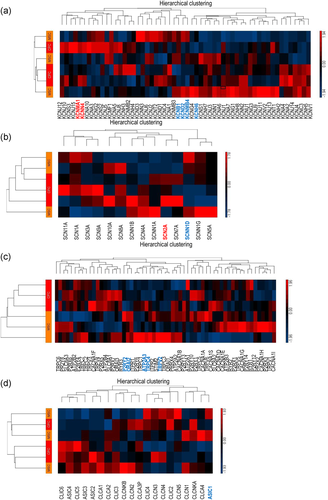
Whilst most of the genes coding for the alpha subunits of voltage-gated sodium channels were upregulated in CPCs, with SCN2A encoding the NaV1.2 sodium channel showing a 1.3-fold upregulation, all epithelial sodium channel subunit genes (SCNN1A, SCNN1B, SCNN1D, SCNN1G) were found to show a trend towards downregulation (Table S2), suggesting an altered K+/Na+ ion handling in CPCs. The only gene showing a significantly lower expression in CPCs was SCNN1D. We also checked the expression patterns of genes encoding the alpha and beta subunits of the Na+/K+-ATPase and found that the ATP1A1 gene was significantly downregulated in CPCs. Genes coding for the rest of the subunits were unchanged.
Interesting differences were found also in the expression patterns of genes coding for proteins involved in global calcium handling. The genes coding for molecules that regulate store-operated Ca2+ entry (SOCE); inositol 1,4,5-trisphosphate receptors (IP3Rs) that mediate calcium release from the intracellular calcium stores; as well as the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA) that are responsible for calcium sequestration, were showing a trend for downregulation in CPCs. Transcript levels of both ORAI2 and ATP2A3 (SERCA3) displayed a significant reduction in CPCs. In contrast, genes coding for voltage-gated calcium channel subunits were unchanged. In a similar way, the ATPases and exchangers that mediate calcium extrusion (PMCA2-4, as well as NCX1-2); as well as the metabotropic purinergic receptors were also unchanged with a trend towards upregulation, with the exception of P2RY2, which was significantly downregulated in CPCs. The genes coding for nonselective cation channels, including members of the ionotropic P2X purinergic receptors, as well as the TRP cation channel families TRPC and TRPV, were showing a general trend towards downregulation, in particular, TRPC4 and TRPV2 (Table S3).
We have also looked at other ion channel expression patterns and found that the only gene which had a significantly different expression (downregulation in CPCs) between the two cell types was ASIC1 coding for the acid-sensitive cation channel subunit 1. None of the other transcripts showed significantly different expression levels between CPCs and MSCs (Table S4).
3.2 Validating microarray data by RT-qPCR confirmed the differential expression of ion channel genes in CPCs
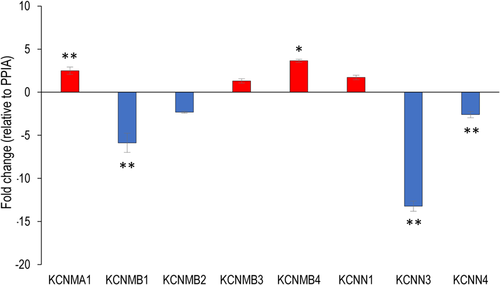
The messenger RNA (mRNA) expression profiles of selected candidate genes encoding various ion channel subunits were first validated using custom-configured TaqMan 96-Well Fast Gene Expression Array plates (see Tables S5 and S6). Given their role in chondroprogenitor and chondrocyte physiology, we were particularly interested in studying the roles of the calcium-activated potassium channels, and therefore we looked at the expression patterns of the genes encoding the various subunits of these channels in details. To confirm the expression patterns of these genes, we employed custom-designed primers and performed individual qPCR reactions. The genes coding for the α and β subunits of the large-conductance calcium-activated potassium channel (BK) were all detected; KCNMA1 and KCNMB4 were upregulated; KCNMB1 was downregulated in CPCs; KCNMB2 and KCNMB3 were unchanged. Of the genes encoding the small conductance calcium-activated potassium channel proteins 1–3 (KCNN1–3), KCNN1 was unchanged, whereas KCNN3 was massively downregulated in CPCs. The gene for the intermediate conductance calcium-activated potassium channel protein 4 (KCNN4) was showing a significant downregulation in CPCs (Figure 3). Differences were detected between our qRT-PCR and microarray data. This has been observed before and is largely attributed to the differences in probe sequences (Canales et al., 2006); however, the observed differences could also be attributable to different methodological aspects of the two approaches, such as the fundamentally different data normalisation strategies (Morey et al., 2006).
3.3 Modulation of the BK potassium channel alters cell proliferation, marker gene expression and migration of CPCs
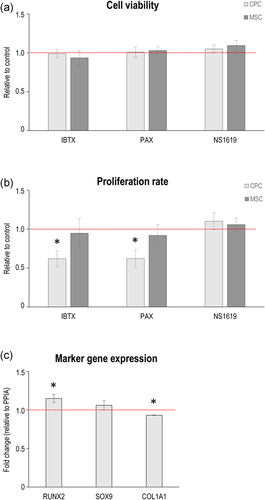
To assess the role of large-conductance calcium-activated potassium channels in CPCs and MSCs on cell proliferation and viability (mitochondrial activity), cells were incubated in the presence of the selective BK channel inhibitors IBTX (100 nM) and paxilline (1 μM); or the BK channel activator NS1619 (10 μM) before assays. Whilst none of the tested compounds interfered with the mitochondrial activity of the cells (i.e., did not have adverse effects on general cell physiology; Figure 4a), both IBTX and paxilline significantly reduced the proliferation rate in CPCs, but had no significant effect in MSCs (Figures 4b and S2). When CPCs were cultured in the presence of 1 μM paxilline, the osteogenic transcription factor RUNX2 was significantly upregulated compared to the control, whereas the expression profile of SOX9, the chondrogenic master regulator, remained unchanged. COL1A1, on the other hand, was upregulated following paxilline treatment (Figure 4c).
Given that the CPCs employed in this study have previously been demonstrated in vitro and in vivo migratory features (Koelling et al., 2009), we performed two different migration assays with or without the BK channel inhibitor paxilline. Fibronectin-guided migration of CPCs in a Boyden chemotaxis chamber was significantly increased in the presence of 1 μM paxilline (Figure 5a). The effect of paxilline was also examined in random cell migration assays. Paxilline at 1 μM enhanced the migratory parameters of chondroprogenitor cells, significantly increased the total path of migration, the maximum distance from the origin; furthermore, the average cell speed was also significantly greater compared to the control (Figure 5b). Next, we shifted the migratory tracks (total paths) of the individual cells to a common origin to generate static wind rose plots (Figure 5c). The bigger diameter of the wind rose plot in paxilline-treated cells indicates the increased motility of these cells.
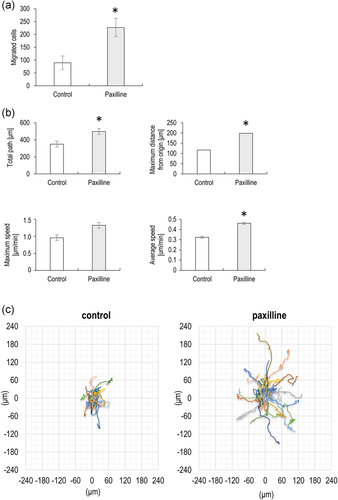
3.4 Electrophysiological properties and intracellular calcium oscillations of chondroprogenitor cells
CPC and MSC cell lines with a characteristic mesenchymal morphology were grown under standard culturing conditions before preparation for DEP analysis (Figure 6a). The measured DEP spectra of the cell lines were used to determine specific membrane capacitance (CSpec) and conductance (GSpec) values; as these calculations take cell size into account (the values of capacitance and conductance are cell size-independent), the cell radii were determined after trypsinisation, which was statistically different. The electrophysiological properties of CPC and MSC cells are shown in Figure 6b, and summarised in Table 1. Effective membrane capacitance (CEff) is a measure of the ability of the membrane to store charge and generate a dipole in a frequency-dependent manner in DEP. The CEff values of CPC and MSC cells were not significantly different from each other. The membrane capacitance per cell area values were also very similar between CPC and MSC. The intracellular conductivities of CPC and MSC were also almost identical between the two cell types. The greatest difference between the two cell types was seen with regard to the specific membrane conductance (GSpec) parameter: CPCs were characterised by a significantly higher GSpec value than MSCs, which equals to a 32.8% difference. Representative DEP spectra for CPC and MSC cells are shown in Figure 6c.
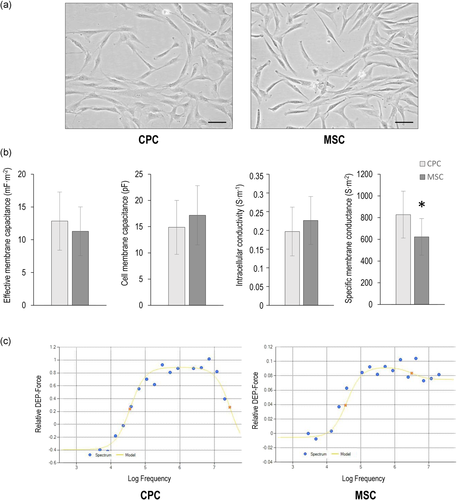
| CPC | MSC | p Value | |
|---|---|---|---|
| Cell radius (µm) | 9.6 (±1.43) | 11 (±1.37) | <.001 |
| Effective membrane capacitance (mF/m2) | 12.84 (±4.44) | 11.29 (±3.71) | .298 |
| Membrane capacitance per cell (pF) | 14.86 (±5.13) | 17.16 (±5.65) | .231 |
| Intracellular conductivity (S/m) | 0.19 (±0.06) | 0.22 (±0.06) | .203 |
| Specific membrane conductance (S/m2) | 827.48 (±215.64) | 623.15 (±168.56) | .006 |
- Note: Values shown were averaged over three repeats of separate populations with ±SD given in brackets.
- Abbreviations: CPC, chondrogenic progenitor cell; DEP, dielectrophoresis; MSC, mesenchymal stem cell.
As RT-qPCR analysis revealed changes in the expression of several ion channels at the mRNA level, whole-cell currents were examined. Both CPC and MSC cells were heterogeneous in respect to the ionic current expression. The significant current was recorded in 6 of 27 CPC cells. The voltage-dependent features of these outward currents were reminiscent of that of BK channels. In addition, the time-dependent component was inhibited by paxilline, indicating that the current was partially carried by BK channels. The linear component of the current was not specified further. In a similar way, the paxilline-sensitive composite current was observed in four of seven MSC cells (Figure 7a,b). The RMP of CPCs did not differ significantly from that of MSCs was determined by whole-cell patch-clamp measurements (–24.1 vs. –21.3 mV) (Figure 7c).
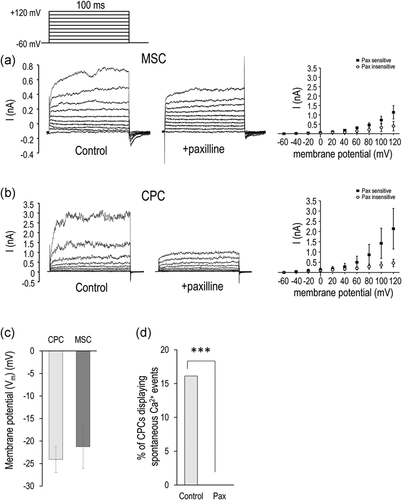
Since CPCs have been previously described to display oscillations in cytosolic calcium levels, we also looked at whether paxilline interfered with these spontaneous calcium events. In control cultures, 16% of CPCs exhibited periodic changes in cytosolic Ca2+ concentration (n = 112), but 1 μM paxilline completely abolished such events (n = 83) (Figure 7d).
4 DISCUSSION
OA is a multifaceted and highly heterogenous whole-joint disease without a common pathophysiological pathway. Therefore, it is unlikely that a single therapeutic target can change the course of disease progression. There are currently no therapeutic strategies able to halt or significantly delay OA progression, and the existing pharmacological treatments are unable to sustain effective and long-lasting symptomatic relief. At present, joint replacement with an artificial prosthesis is the single most effective measure to improve patient quality of life, but of course not all OA patients will progress to this stage (Conaghan et al., 2019). To develop novel therapeutic approaches targeting OA, a more profound understanding of the molecular mechanisms of the disease is required. A broad spectrum of ongoing trials and treatment options target various aspects of the disease, including cartilage and bone regeneration or repair, inflammatory and pain processes, altered metabolic pathways and senescence (Grassel & Muschter, 2020).
Certain stem cell-based cartilage regenerative approaches are already in the phase I clinical study stage. BM-MSCs and adipose tissue-derived MSCs (AD-MSCs) are currently the preferred cell types for regenerative strategies (Grassel & Muschter, 2020). However, whether MSCs are really the optimal cell population for cartilage regenerative therapy is still controversial. In this study, we turned our attention to alternative cell sources with potentially superior chondrogenic potential compared to BM-MSCs, which could be exploited for future cartilage regenerative therapies. Migratory CPCs have been partially characterised; they are known to exhibit a distinct transcriptomic signature compared to osteoblasts, chondrocytes and immortalised foetal chondrocytes (T/C-28 cells) (Koelling et al., 2009). However, the specific cellular identity and detailed molecular phenotype of CPCs is still elusive. Therefore, the aim of this study was to elucidate the biology and phenotype of CPCs by comparing their transcriptomic profile with BM-MSCs. Given the unique ionic composition of the CPC niche within the ECM of diseased cartilage, we were especially interested in differences in the channelome of CPCs, focusing on K+ and Ca2+ transporters potentially involved in maintaining the progenitor phenotype under inflammatory conditions. We also mapped the electrophysiological profile of CPCs using patch-clamp and DEP.
We have recently analysed the surfaceome of CPCs using selective cell surface protein labelling followed by high-throughput mass spectrometry and identified alterations in the composition of the surfaceome compared to BM-MSCs (Matta et al., 2019). However, even the high-throughput mass spectrometry-based approach that we employed was not sensitive enough to detect alterations in very low-abundance ion channels and transporters. Here, we performed microarray analysis and compared the global gene expression signatures of CPCs to BM-MSCs. CPCs harboured a distinct transcriptomic profile and mRNA expression pattern that was different to that of MSCs. Pathway analysis confirmed that mainly cell cycle-related and DNA/nucleic acid metabolism-related GO categories were overrepresented in the list of genes with significantly different expression levels, which was clearly reflected by the lower proliferation rate of CPCs compared to MSCs. There was a 64% correlation with the direction of fold changes of differentially expressed transporter genes when we compared their pattern to the data generated by quantitative mass spectrometric analysis on the surfaceome of CPC and MSC cells (Table S8; see also File S2) (Matta et al., 2019).
4.1 Differential K+ transporter gene expression profiles in CPCs
We chose to study the expression profiles of those ion channels and transporter families that may have relevance in chondroprogenitor cell physiology (Barrett-Jolley et al., 2010; Matta & Zakany, 2013; Mobasheri et al., 2019). The most widely reported ion channels in chondrocytes and MSCs are potassium channels (Mobasheri et al., 2012; Pchelintseva & Djamgoz, 2018). The human genome contains around 70 different potassium channel genes, which makes them the largest family of membrane ion channels (Mobasheri et al., 2012). The α-subunit (KCNMA1) of the large-conductance Ca2+-activated potassium channel (BK, BKCa, MaxiK), as well as the intermediate Ca2+-activated potassium channel (IK, KCNN4, SK4, KCa3.1) transcripts, displayed the largest fold changes, with a 50% upregulation in CPCs. BK channels have been detected in undifferentiated MSCs both at the mRNA level and by single-channel recordings (Kawano et al., 2003), and also in mature chondrocytes (Mobasheri et al., 2010). BK channels may play various roles in chondrocyte physiology including volume regulation, oxygen sensing and mechanotransduction (Mobasheri et al., 2012). Since BK channels have been implicated in driving MSC differentiation (Pchelintseva & Djamgoz, 2018), perhaps the fact that CPCs are more committed to the chondrogenic lineage than undifferentiated MSCs may explain the higher levels of KCNMA1 both at the transcript and at the protein level (Matta et al., 2019).
BK channels may also be potential drug targets to protect against joint degeneration in OA (Haidar et al., 2020). In an in vitro model of synovial inflammation, KCNMA1 was found to be upregulated following cytokine treatment in primary synovial fibroblasts (Haidar et al., 2020). BK channel expression was also found to be upregulated in human OA cartilage (Lewis et al., 2013). Given that the migratory CPCs used in this study had been isolated from late-stage OA, the increased KCNMA1 expression may also be a result of their original inflammatory niche.
To further characterise the contribution of BK channels to the chondroprogenitor phenotype, we first looked at whether BK channel function was required for cell division. The role of BK channels in mediating proliferation has been controversial. BK channels are known to stimulate proliferation in BM-MSCs (Zhang et al., 2014); however, their activation may also lead to antiproliferative effects in human-induced pluripotent stem cell-derived MSCs (Zhao et al., 2013). The BK channel inhibitors paxilline and IBTX have significantly lowered the proliferation rate of CPCs, but not in MSCs. This may be attributed to the different sensitivity of the two cell types to BK channel inhibition, and the dose-dependent effect of paxilline on proliferation. Whilst 1 μM paxilline caused approximately 10% reduction in DNA synthesis in BM-MSCs, at 3 μM it resulted in approximately 80% inhibition (Zhang et al., 2014). On the other hand, in line with our data, IBTX did not alter [3H]-thymidine incorporation levels in rat BM-MSCs (Deng et al., 2007).
The increased abundance of KCNN4 transcripts in CPCs may reflect their inherently enhanced cell motility as IK channels have a confirmed role in migration (Pchelintseva & Djamgoz, 2018). In contrast, BK channels have a rather controversial role in cell motility (Catacuzzeno et al., 2015). In migratory CPCs, BK channels may play an inhibitory role in the motility of the cells as paxilline treatment increased every parameter of migration. These findings are rather unexpected as the BK channel inhibitor IBTX was reported to block the platelet lysate-induced migration of MSCs (Echeverry et al., 2020). In glioma cells, however, BK channel openers inhibited migration (Kraft et al., 2003). Given that CPCs express both SOX9 and RUNX2 and that there is a degree of crosstalk between these transcription factors (Koelling et al., 2009), perhaps BK blockade influenced CPC behaviour in such a way that it attenuated their chondrogenic phenotype, which is also reflected in the upregulation of the osteogenic transcription factor RUNX2.
4.2 Ca2+ homoeostasis in chondroprogenitor cells
Given that BK channel function depends on both RMP and [Ca2+]i, we undertook to look at the transporters mediating the Ca2+ homoeostasis of the chondroprogenitor cells. Calcium plays central roles in cell physiology in nonexcitable cells such as chondrocytes (Suzuki et al., 2020). Dynamic changes in calcium signalling have been shown to be paramount to chondrogenesis (Matta & Zakany, 2013), and we have described earlier the calcium handling in CPCs (Matta et al., 2015). SOCE, one of the main sources of Ca2+ influx, is mediated by Ca2+ release-activated Ca2+ channels formed of ORAI1, ORAI2 and ORAI3 proteins. We found that ORAI2 was present in lower abundance in CPCs; ORAI2 has been reported to modulate the magnitude of SOCE (Inayama et al., 2015; Vaeth et al., 2017), which further pinpoints the important role of SOCE in CPC homoeostasis. Given that purinergic signalling regulates intracellular Ca2+ oscillations in CPCs and MSCs (Jiang et al., 2017; Matta et al., 2015), we also looked at differences in purinergic receptor transcript levels. The only gene with a significantly altered expression was P2RY2, which codes for a metabotropic receptor involved in the negative regulation of the osteogenic differentiation of BM-MSCs (Li et al., 2016).
BK channel function was reported to modulate [Ca2+]i oscillations in various cell types (Mizutani et al., 2016; Wakle-Prabagaran et al., 2016). Both CPCs (Matta et al., 2015) and MSCs (Kawano et al., 2003) are known to exhibit periodic fluctuations in resting cytosolic Ca2+ levels. We confirmed that BK channels play a central role in mediating the Ca2+ homoeostasis in CPCs since the BK channel inhibitor paxilline completely abolished these [Ca2+]i oscillations. This is the first study to report a mechanistic link between the big conductance calcium-sensitive potassium channels and periodic [Ca2+]i oscillations in chondroprogenitor cells.
4.3 Electrophysiological profiling of CPCs
Having established the channelome of CPCs at the transcript level, we then looked at whether the global electrophysiological profile (electrome) of the progenitor cells was different from that of BM-MSCs. In addition to conventional patch clamping, we also employed DEP, which has been shown to be an efficient quantitative method of differentiating between closely related cell types, for example, in the bone marrow (Ismail et al., 2015). DEP can be used for both assessing the passive electrical properties of cellular components and as the basis for a separation method (Mahabadi et al., 2018). This could be especially relevant for CPCs present at very low abundance in arthritic cartilage, given that a truly reliable cell surface marker has still not been identified. Inherent cell properties that do not require the use of specific labelling for detection would provide a unique means to identify progenitors committed to particular cell fates. Whilst the effective membrane capacitance and the intracellular conductivity values did not differ, we report membrane conductance as a specific electrophysiological property that reflects the differentiation stage of human CPCs and MSCs. Membrane conductivity is a parameter that describes the potential of the membrane to transmit charge; and is indicative of ionic flux (Henslee et al., 2017).
We employed patch clamping to establish the RMP of CPCs, which was not statistically different from that of MSCs. The Vm value of MSCs detected in our study (approximately –20 mV) was similar to what has been observed earlier (–10 mV) (Kawano et al., 2003). The RMP of mature chondrocytes is dependent on the coordinated function of different types of ion channels including nonselective cation channels (Lewis et al., 2011) and K+ channels (Maleckar et al., 2018; Wilson et al., 2004). The majority of these channels did not show statistically different expression between MSCs and CPCs, which probably explains why there is no difference in RMP as observed in this study. As described above, various K+ channels were detected at the transcript level in both cell types; therefore, we studied the outward whole-cell currents in CPC and MSC cells. Both cell populations were heterogeneous with respect to ion current expression, and we found evidence of paxilline-sensitive conductances.
5 SUMMARY AND CONCLUSIONS
Here we have provided evidence, for the first time, that BK channels were functional in undifferentiated CPCs, as the voltage-dependent features of the detected potassium currents were reminiscent of that of BK channels. In addition, the time-dependent component was inhibited by paxilline, indicating that the current was partially carried by BK channels. Functional BK channels were required for maintaining the balanced differentiation state of CPCs as paxilline treatment significantly upregulated the osteogenic transcription factor RUNX2, which favours osteogenic differentiation potentials.
In line with our previous paper describing the Ca2+ homoeostasis of CPCs (Matta et al., 2015), here we provide experimental data supporting the hypothesis that periodic increases in [Ca2+]i may activate BK channels. The ionic fluxes mediated by these channels may alter the RMP, which in turn modulates Ca2+ influx. Such a feedback loop has been recently proposed to exist in chondrocytes (Suzuki et al., 2020); we have now identified the key components of that loop in CPCs in this study. It is plausible to hypothesise that under resting conditions, the [Ca2+]i peaks could activate BK channels to such an extent that the K+ efflux they mediate does not favour the changes in cell volume and shape required for cell migration.
6 PERSPECTIVES
A recent systematic review has analysed the outcome of 17 studies assessing articular cartilage repair after the clinical application of cell populations containing MSCs in human subjects with knee OA (Ha et al., 2019). Significantly better clinical outcomes (improvement of the cartilage state on magnetic resonance imaging or repaired tissue on second-look arthroscopy) were reported in the MSC group in most of the studies. However, there is limited evidence to support the efficacy of intra-articular MSC-based therapy. This highlights opportunities for identifying alternative cell sources. Whilst the preferred cells used were bone, adipose tissue or umbilical cord-derived MSCs, perhaps exploiting the resident cartilage progenitor cell population present in both healthy and OA cartilage may further enhance the efficacy of such novel therapies. We demonstrate here that CPCs are a distinct cell population but are still similar to BM-MSCs in many ways. This study adds key mechanistic and cellular phenotype data to the in-depth characterisation of cartilage progenitor cells; however, further research is necessary to reconstruct the progenitor niche which would promote their hyaline cartilage regenerative potential.
ACKNOWLEDGEMENTS
The authors are thankful to Krisztina Biróné Barna for skilful technical assistance. The authors would also like to acknowledge the contribution of William Kothalawala Jayasekara and Roland Ádám Takács. Csaba Matta was supported by the European Commission through a Marie Skłodowska-Curie Intra-European Fellowship for career development (Project no. 625746; acronym: CHONDRION; FP7-PEOPLE-2013-IEF), the Premium Postdoctoral Research Fellowship of the Eötvös Loránd Research Network (ELKH) and the Young Researcher Excellence Programme (Grant no. FK 134304) of the National Research, Development and Innovation Office, Hungary. Project no. TKP2020-NKA-04 has been implemented with the support provided by the National Research, Development and Innovation Fund of Hungary, financed under the 2020-4.1.1-TKP2020 funding scheme. Ali Mobasheri was the coordinator of the D-BOARD Consortium funded by European Commission Framework 7 programme (EU FP7; HEALTH.2012.2.4.5-2; Project no. 305815; Novel Diagnostics and Biomarkers for Early Identification of Chronic Inflammatory Joint Diseases). Ali Mobasheri has received funding from the Deanship of Scientific Research (DSR), King AbdulAziz University (Grant no. 1-141/1434 HiCi). Ali Mobasheri is a member of the Arthritis Research UK Centre for Sport, Exercise and Osteoarthritis, funded by Arthritis Research UK (Grant Reference no. 20194). Ali Mobasheri wishes to acknowledge financial support from the European Structural and Social Funds (ES Struktūrinės Paramos) through the Research Council of Lithuania (Lietuvos Mokslo Taryba) according to the activity ‘Improvement of researchers’ qualification by implementing world-class R&D projects' of Measure no. 09.3.3-LMT-K-712 (Grant application code: 09.3.3-LMT-K-712-01-0157; Agreement no. DOTSUT-215) and the new funding programme: Attracting Foreign Researchers for Research Implementation (2018–2022), Grant no. 01.2.2-LMT-K-718-02-0022. Aniko Keller-Pinter was supported by the National Research, Development and Innovation Office of Hungary (Grant no. FK 134684), and by the GINOP-2.3.2-15-2016-00040 project.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Conceptualisation: Csaba Matta and Ali Mobasheri. Methodology: Csaba Matta, Rebecca Lewis, Christopher Fellows, Janos Almassy, Sean May, Peter Szentesi, Aniko Keller-Pinter, Michael P. Hughes and Ali Mobasheri. Software: Marcos C. Uribe, Szilard Poliska, Janos Fodor, Aniko Keller-Pinter, Fatima H. Labeed and Michael P. Hughes. Validation: Csaba Matta, Christopher Fellows and Szilard Poliska. Formal analysis: Csaba Matta, Rebecca Lewis, Christopher Fellows, Erin Henslee, Janos Fodor, Janos Almassy and Fatima H. Labeed. Investigation: Csaba Matta, Rebecca Lewis, Christopher Fellows, Gyula Diszhazi, Marcos C. Uribe, Szilard Poliska, Peter Szentesi, Tibor Hajdú and Erin Henslee. Resources: Janos Almassy, Sean May, James Dixon, Nicolai Miosge, Richard Barrett-Jolley, Peter Szentesi, Aniko Keller-Pinter, Michael P. Hughes and Ali Mobasheri. Data curation: Csaba Matta, Rebecca Lewis, Christopher Fellows, Janos Almassy, Janos Fodor, Tibor Hajdú, Marcos C. Uribe and Erin Henslee. Writing – original draft preparation: Csaba Matta and Rebecca Lewis. Writing – review and editing: All authors. Visualisation: Csaba Matta, Marcos C. Uribe, Gyula Diszhazi, Szilard Poliska, Janos Fodor, Aniko Keller-Pinter and Erin Henslee. Supervision: Nicolai Miosge, Richard Barrett-Jolley, Fatima H. Labeed, James Dixon, Sean May, Peter Szentesi, Aniko Keller-Pinter and Ali Mobasheri. Project administration: Csaba Matta and Ali Mobasheri. Funding acquisition: Csaba Matta and Ali Mobasheri.




