Modulating stemness of mesenchymal stem cells from exfoliated deciduous and permanent teeth by IL-17 and bFGF
Abstract
Mesenchymal stem cells (MSCs) have been identified within dental pulp tissues of exfoliated deciduous (SHEDs) and permanent (DPSCs) teeth. Although differences in their proliferative and differentiation properties were revealed, variability in SHEDs and DPSCs responsiveness to growth factors and cytokines have not been studied before. Here, we investigated the influence of interleukin-17 (IL-17) and basic fibroblast growth factor (bFGF) on stemness features of SHEDs and DPSCs by analyzing their proliferation, clonogenicity, cell cycle progression, pluripotency markers expression and differentiation after 7-day treatment. Results indicated that IL-17 and bFGF differently affected SHEDs and DPSCs proliferation and clonogenicity, since bFGF increased proliferative and clonogenic potential of both cell types, while IL-17 similarly affected SHEDs, exerting no effects on adult counterparts DPSCs. In addition, both factors stimulated NANOG, OCT4, and SOX2 pluripotency markers expression in SHEDs and DPSCs showing diverse intracellular expression patterns dependent on MSCs type. As for the differentiation capacity, both factors displayed comparable effects on SHEDs and DPSCs, including stimulatory effect of IL-17 on early osteogenesis in contrast to the strong inhibitory effect showed for bFGF, while having no impact on SHEDs and DPSCs chondrogenesis. Moreover, bFGF combined with IL-17 reduced CD90 and stimulated CD73 expression on both types of MSCs, whereas each factor induced IL-6 expression indicating its' role in IL-17/bFGF-modulated properties of SHEDs and DPSCs. All these data demonstrated that dental pulp MSCs from primary and permanent teeth exert intrinsic features, providing novel evidence on how IL-17 and bFGF affect stem cell properties important for regeneration of dental pulp at different ages.
1 INTRODUCTION
Dental pulp (DP) represents highly organized connective tissue enclosed within the root canal of the tooth that plays indispensable role in nutrition, protection, and sensory perception, contributing to tooth homeostasis and longevity (Yang et al., 2016). This special tissue entailing collagenous extracellular matrix infiltrated with blood vessels and nerve fibers contains various cell types, including odontoblasts, stromal fibroblasts and undifferentiated mesenchymal stem cell (MSC) populations. Several stem cell populations have been identified within the human DP, including dental pulp stem cells (DPSCs) from permanent teeth (Gronthos et al., 2000), stem cells from human exfoliated deciduous teeth (SHEDs) (Miura et al., 2003) and stem cells from apical papilla (Sonoyama et al., 2008). All of them possess the ability to replace odontoblasts during dentin repair or form dentine-like structures when transplanted in immunocompromised mice (Xiao & Nasu, 2014). Considering their important roles in pulp homeostasis, MSCs residing in the DP niche have been recognized as potentially important for revitalization procedures and the development of new regenerative endodontic therapies. In that context, an innovative approach based on curcumin loaded liposome treatment was found beneficial in the potentiation of anti-inflammatory activities of DPSCs and restoration of pulp homeostasis (Sinjari et al., 2019). Moreover, the use of dental MSCs and their derivatives, such as extracellular vesicles (EVs), combined with 3D biomaterial scaffolds, has been considered the promising therapeutic tool for bone tissue repair in general (Trubiani et al., 2019).
Both DPSCs from permanent and SHEDs teeth are populations of multipotent cells capable of differentiating into osteoblasts, odontoblasts, stromal fibroblast, chondrocytes, and adipocytes (Kerkis & Caplan, 2012; Miura et al., 2003; Renvoisé & Michon, 2014). Besides, they both possess the unique potential for neurogenesis and angiogenesis which has been related to their origin from neural crest or glial cells, as well as predominant localization within pulp perivascular niche (Ratajczak et al., 2016). However, functional differences attributable to tooth developmental stage have been reported between SHEDs and DPSCs, since higher clonogenic, proliferative, odontogenic and osteogenic capacity was demonstrated for SHEDs in comparison to their adult counterparts DPSCs (H. Wang et al., 2018; X. Wang et al., 2012) or bone marrow MSCs (Isobe et al., 2016; Kunimatsu et al., 2018). Yet, differences in SHEDs and DPSCs response to various environmental stimuli, including certain growth factors and cytokines have not been studied so far.
Maintenance of DP tissue at each developmental stage involves interactions of MSCs with other cells, extracellular matrix proteins (Arakaki et al., 2012), and plenty of growth factors and cytokines delivered by very efficient blood stream (Berggreen et al., 2007). Namely, fibroblast growth factor (FGF) is implicated in tooth development and incisor stem cell renewal (Du et al., 2018) supporting the maintenance of dental epithelial stem cells in particular (Chang et al., 2013). Acting through four receptors in human cells, FGF has been suggested as a regulator of stem cell niche in dental tissue (Renvoisé & Michon, 2014). Although the presence of basic FGF (bFGF) is related to expansion protocol for various MSCs, including SHEDs (J. C. Kim, Park, Kim, et al., 2014; Sukarawan et al., 2014) and DPSCs (Bonnamain et al., 2013), various data were reported regarding its effects on different cell functions depending on dose and treatment duration. Moreover, bFGF roles in tissue homeostasis and repair have been related to cooperation with other cytokines. One of the potentially collaborative cytokines could be interleukin 17 (IL-17), the proinflammatory cytokine that dually affects osteogenesis, contributing to bone-destructive events in arthritis, periodontal disease (Amatya et al., 2017) or pulpitis (Xiong et al., 2015), while also showing protective effects in pulpal infection. Inflammation, as protective immunological response to tissue damage, represents the initial phase of tissue repair followed by proliferation and tissue remodeling. Each of these overlapping phases contribute to tissue regeneration through the collaboration of many cell types and their secreted factors. In this study, we hypothesize that IL-17 and bFGF can cooperate as mediators of inflammatory and regenerative pulp microenvironment, inducing various functional and molecular responses in DPSC and SHED population. To address how 7-day exposure of SHEDs and DPSCs to IL-17 and/or bFGF alters their stem cell features, we investigated proliferation, clonogenicity, cell cycle, pluripotency markers expression, as well as multipotent differentiation capacity of these cells.
2 MATERIALS AND METHODS
2.1 Isolation and cultivation of SHEDs and DPSCs
Human SHEDs and DPSCs were isolated as described previously (Spath et al., 2010) by tissue explant techniques from pulp tissues extracted from exfoliated deciduous and permanent teeth of healthy patients without clinical signs of dental caries. Exfoliated deciduous incisors were collected from children (6–7 years old) at the Department of Pediatric and Preventive Dentistry, while impacted third molars were obtained from adults (20–26 years old) at the Department of Oral Surgery of the Faculty of Dental Medicine, University of Belgrade, in accordance with the local ethical committee standards and upon providing informed consent of donors or donor parents. DP tissues were gently separated from the tooth crown, rinsed in phosphate-buffered saline (PBS) (Capricorn) and minced into small fragments which were subsequently cultured at 37°C in a humidified atmosphere containing 5% CO2 in growth medium (GM) consisting of Dulbecco's modified Eagle's medium (DME) (Sigma-Aldrich) with 10% fetal bovine serum (FBS) (Capricorn Scientific), 100 U/mL penicillin and 100 µg/mL streptomycin (Gibco; Thermo Fisher Scientific). GM was replaced every 2–3 day. After reaching 80-90% confluence, cells were detached using 0.25% trypsin/EDTA (Capricorn Scientific) for further passaging. All experiments were performed using SHEDs and DPSCs from passages 3 to 6.
2.2 Flow cytometry
Isolated SHEDs and DPSCs were subjected to surface marker expression analysis by flow cytometry. To this end, cells cultured under standard conditions were harvested by 1 mM EDTA and washed in cold PBS with 0.5% bovine serum albumine (BSA) (Sigma-Aldrich). Afterwards, each aliqot of 2 × 105 cells was labeled for 30 min in the dark at 4°C with fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated monoclonal antibodies against human antigens CD105 (Invitrogen), CD90 and CD73 (all from R&D Systems) as mesenchymal cell surface markers, and CD45, CD235a (both from R&D Systems) and HLA-DR (Invitrogen) as hematopoetic markers. Moreover, in separate experiments SHEDs and DPSCs were analyzed for CD90 and CD73 surface expression following 7-days treatment with recombinant human IL-17 (100 ng/ml) and/or bFGF (10 ng/ml) (both from R&D Systems). The level of nonspecific binding was determined by using corresponding FITC- and PE-conjugated isotype control antibodies (R&D Systems).
In addition, the percentage of cells positive for intracellular proliferation marker Ki67 was determined by flow cytometry. Following the treatment of SHEDs and DPSCs with IL-17 (100 ng/ml), bFGF (10 ng/ml) or their combination for 7 days, cells were washed with PBS, fixed in 5% formaldehyde and permeabilized in 0.5% BSA/PBS containing 0.1% Triton X-100. Upon the blocking of nonspecific labeling in 0.5% BSA/PBS, cells were incubated for 1 h at room temperature with rabbit anti-Ki67 antibody (Abcam) and subsequently for 30 min with secondary antirabbit antibody FITC (Sigma-Aldrich). The influence of IL-17 and/or bFGF on the cell cycle progression of SHEDs and DPSCs was also analyzed by flow cytometry. To this end, cells were treated with IL-17 (100 ng/ml), bFGF (10 ng/ml) or their combination for 7 days and afterwards deteached with trypsin-EDTA. Aliquots of 2 × 105 cells were washed with PBS, fixed with absolute ice cold ethanol and incubated in a PBS solution containing propidium iodide (PI) (Santa Cruz Biotechnologies), 0.1% Triton X-100 (Serva Electrophoresis GmBh) and 0.1 mg/ml of RNase A (Thermo Fisher Scientific) for 40 min at 37oC. Flow cytometry was performed using Cytomics FC 500 (Beckman Coulter) cytometer, while data were analyzed using WinMDI 2.9 software (J. Trotter; The Scripps Research Institute).
2.3 In vitro multilineage differentiation
To confirm the functional properties of isolated MSCs their differentiation potential was analyzed. SHEDs and DPSCs were seeded in 96-well plates (5000 cells/well) and cultivated in GM in standard conditions until reaching subconfluence when the GM was replaced with differentiation media (DM) to induce the commitment to osteogenic, chondrogenic and adipogenic lineage.
In addition, the influence of IL-17 and/or bFGF pretreatment on the osteogenic and chondrogenic differentiation potential of both MSC types was analyzed. For that purpose, SHEDs and DPSCs were cultivated in GM with IL-17 (100 ng/ml), bFGF (10 ng/ml) or their combination for 7 days and afterwards incubated in the corresponding DM for the appropriate time. Cells cultured in GM were used as controls.
For osteogenesis induction, cells were cultivated in osteogenic medium containing DMEM with 5% FBS, 10 nM dexamethasone (Sigma-Aldrich), 50 μM ascorbic acid-2-phosphate (Sigma-Aldrich), and 10 mM β-glycerophosphate (AppliChem). After 7 days, osteogenesis was detected by staining of alkaline phosphatase (ALP) as an early osteogenic marker, with 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium, (Sigma-Aldrich) and additionally, after 3 weeks by staining of mineralization with alizarin red (Merck).
Adipogenic differentiation was determined by detection of intracellular lipid droplets formation after 28 days of cultivation in adipogenic medium consisting of DMEM supplemented with 5% FBS, 100 μg/ml isobutyl-methylxanthine (Sigma-Aldrich), 1 μM dexamethasone and 10 μg/ml insulin (Sigma-Aldrich). The accumulation of intracellular lipid droplets was confirmed by Oil Red O (Merck Chemicals, Darmstadt, Germany) which stains cholesteryl esters and triacylglycerols.
To induce chondrogenic differentiation cells were cultured in medium containing DMEM with 5% FBS, 2 ng/ml transforming growth factor-β1 (TGF-β1) (R&D Systems), 50 μM ascorbic acid-2-phosphate and 10 nM dexamethasone. After 21 days of cultivation in chondrogenic medium Safranin O (Merck Chemicals) staining was used to confirm cartilage-specific glycosaminoglycans.
Following staining protocols, cells were examined using a light microscope (Olympus) and differentiation level was semi-quantified by densitometry, using NIH-Image J software (LOCI; University of Wisconsin).
2.4 Colony-forming units-fibroblastic (CFU-F) assay
Colony-forming unit-fibroblastic (CFU-F) assay was performed by seeding SHEDs and DPSCs at 200 cells/well in duplicates in six-well plate and culturing in GM under standard conditions. After 14 days in culture, the cells were washed with PBS, fixed with ice-cold methanol and stained with 0.3% crystal violet (Carlo Erba reagents S.A.S.). Visible colonies with more than 50 cells were counted. Colony forming efficiency was determined as the ratio of the colonies number to the number of cells seeded. To analyze effects of the examined factors on SHEDs and DPSCs clonogenic potential, cells pretreated for 7 days with IL-17 (100 ng/ml), bFGF (10 ng/ml) or their combination were assayed for CFU-F number and efficiency as described above.
2.5 β-Galactosidase staining
For β-galactosidase staining, SHEDs and DPSCs of passages P3 to P4 were seeded at concentration 2 × 103 cells/well in 96-well plates and cultivated in standard conditions. After 24 h cells were washed with PBS, fixed and stained using Senescence Cells Histochemical Kit according to the manufacturer's instructions (Sigma Aldrich). The cells stained for β-galactosidase activity were counted under light microscope (Olympus), and the percentage of stained cells was determined for several separated visual fields.
2.6 Cellular proliferation and viability
To compare the population doubling time (PDT) of SHEDs and DPSCs, all the cells were seeded at concentration of 1 × 104 cells/cm2 in tissue culture flasks in GM under standard conditions. After reaching confluency, cells were detached, counted with Trypan blue and reseeded at the initial density. This procedure was repeated at every passage until cells stopped dividing. PDTs were calculated according to the formula PDT = (T-T0) lg2/(lgNt−lgN0), where T0 and T represent the starting and ending time of cell culture, while No and Nt refer to the cell number at the start and at the end of each culture.
For estimation of IL-17 and/or bFGF effects on SHEDs and DPSCs proliferation rate, cells were seeded in 24 well plates at 2 × 104 cells/well in GM. After 24 h cultivation under standard conditions, cells were treated for 7 days with IL-17 (100 ng/ml), bFGF (10 ng/ml) or their combination. Following the treatment, cells were detached and their number counted using Trypan Blue dye exclusion test (Invitrogen, Carlsbad, CA, USA).
Furthermore, to evaluate viability/metabolic activity of SHEDs and DPSCs upon the treatment, 5×103cells/well were seeded in 96 well-plates in GM, cultured for 24 h in standard conditions and subsequently treated with IL-17 (100 ng/ml), bFGF (10 ng/ml) or their combination for 7 days. Afterwards, 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide (MTT) solution (5 mg/ml) (Sigma-Aldrich) was added into cultures and incubated for the next 2 h. The optical density of formazan crystals was measured at 540 nm by the automatic reader for microtiter plates (Labsystems Multiskan PLUS).
2.7 Immunofluorescence assay
For immunofluorescent labeling SHEDs and DPSCs were seeded in 24-well plates over rounded glass coverslips (1 × 103 cells/well) and cultured under appropriate conditions as described in Figure legend. After fixation with 4% formaldehyde in PBS, cells were permeabilized in 0.1% Triton X-100 in PBS, blocked with 1% BSA/PBS, and incubated for 1 h at room temperature with primary antibodies: mouse anti-NANOG, rabbit anti-Oct-4, mouse anti-SOX-2 (all from Cell Signaling Technology) and rabbit anti-IL-6 antibody (Novus Biologicals). Samples incubated in 1% BSA/PBS only were used as negative controls. Afterwards, cells were washed with PBS and the corresponding FITC or AlexaFlour 555-coupled secondary antibodies (Cell Signaling Technology) and 1 ng/ml of nuclear dye 4′,6-diamidino-2-phenylindole (Sigma-Aldrich) were added during 1 h at room temperature. Samples of mounted cells were examined using an epi-fluorescent microscope (Olympus).
2.8 Reverse transcription-polymerase chain reaction (RT-PCR)
SHEDs and DPSCs seeded at concentration 1 × 105 cells/well in six-well plate were cultured under standard conditions and treated upon reaching confluence as described in Figure legends. After treatment, cells were washed with PBS and total RNA was extracted using TRIzol Reagent (Invitrogen). Complementary DNA was generated from 200 ng of total RNA by RevertAidTM H Minus First Strand cDNA Synthesis Kit (Thermo Scientific) using oligo (dT) as a primer. PCR products were obtained after 30 to 35 cycles of amplification with adjusted annealing temperature ranging from 48°C to 55°C. Primer sets (Invitrogen), corresponding annealing temperatures used and the amplified product lengths are all presented in Table 1. Glyceraldehyde 3-phosphate dehydrogenase was amplified as a housekeeping gene control for the amount of complementary DNA present in each sample. Amplicons were resolved in 1.5% agarose gel and stained with ethidium bromide. Intensities of reverse transcription-polymerase chain reaction (RT-PCR) bands were determined by ImageMaster TotalLab v1.11 software (Total Lab; Amersham Biosciences). Values are expressed as relative to the indicated corresponding control.
| Gene | NCBI reference sequence | Forward 5′-3′ | Reverse 3′-5′ | Amplicon (bp) | Annealing temperature (°C) | Cycle number |
|---|---|---|---|---|---|---|
| NANOG | NM_024865.3 | CTCCATGAACATGCAACCTG | CTCGCTGATTAGGCTCCAAC | 209 | 54 | 35 |
| Oct4A | NM_002701.5 | AGTGAGAGGCAACCTGGAGA | GTGAAGTGAGGGCTCCCATA | 270 | 54 | 35 |
| Oct4B | NM_002701.5 | TATGGGAGCCCTCACTTCAC | CAAAAACCCTGGCACAAACT | 194 | 54 | 35 |
| SOX-2 | NM_003106.3 | ATGGGTTCGGTGGTCAAGT | GGCGCCGTGGGAGATACATG | 126 | 50 | 35 |
| ALP | NM_000478.5 | CCCAAAGGCTTCTTCTTG | CTGGTAGTTGTTGTGAGC | 356 | 49 | 33 |
| Runx2 | NM_001015051.3 | ATGCTTCATTCGCCTCACAAAC | CCAAAAGAAGTTTTGCTGACATGG | 261 | 54 | 35 |
| SOX-9 | NM_015869.4 | GAGGAAGTCGGTGAAGAACG | ATCGAAGGTCTCGATGTTGG | 300 | 48 | 36 |
| Col1A | NM_000088.4 | GAGAGCATGACCGATGGATT | CCTTCTTGAGGTTGCCAGTC | 178 | 52 | 33 |
| IL-6 | NM_000600.4 | ATGAACTCCTTCTCCACAAG | AGAGCCCTCAGGCTGGACTG | 626 | 52 | 33 |
| GAPDH | NM_001289746.1 | ACCACAGTCCATGCCATCAC | TCCACCACCCTGTTGCTGTA | 452 | 52 | 33 |
- Abbreviations: NCBI, National Center for Biotechnology Information; PCR, polymerase chain reaction.
2.9 Statistical analysis
All assays were repeated at least three times and the results are presented as mean ± SEM. Differences between groups were tested for statistical significance by Student's two-tailed t test with p values less than .05 considered significant. Data analysis and graphical representations were performed by using GraphPad Prism 7 software (GraphPad).
3 RESULTS
3.1 Comparative analyses of mesenchymal stem/stromal cell features of SHEDs and DPSCs
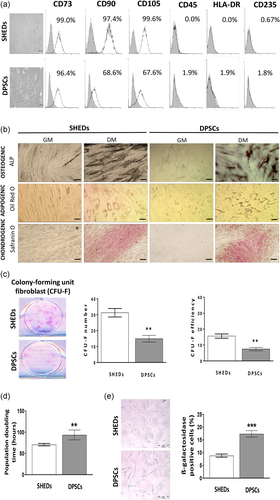
Following isolation and cultivation, both SHEDs and DPSCs exhibited similar fibroblast-like spindle shape morphology. Moreover, analyses of SHEDs and DPSCs immunophenotype evidenced high rates of positive MSCs markers expression, CD73, CD90, and CD105, and low expression or absence of hematopoietic markers CD45, HLA-DR, and CD235 on both types of MSCs (Figure 1a). Results of differentiation potential analyses confirmed MSCs identity of isolated SHEDs and DPSCs demonstrating their osteogenic, adipogenic and chondrogenic differentiation capacity. This was respectively evidenced by detection of early osteogenesis marker ALP, as well as intracellular lipid droplets and cartilage-specific proteoglycans in both MSCs types upon appropriate culture conditions (Figure 1b). Further evaluation of SHEDs and DPSCs clonogenic potential revealed a higher capacity of SHEDs to form CFU-F colonies resulting in two-times higher CFU-F efficiency compared to DPSCs (Figure 1c). Moreover, when the proliferation of different MSC types was determined by cell counting (Figure 1d), the calculated PDT for DPSCs was significantly higher indicating the lower proliferation rate of DPSCs in comparison to SHEDs. In agreement with these observations, the senescence marker β-galactosidase expression showed a higher percentage of β-galactosidase-positive cells in DPSCs population suggesting their higher predisposition toward senescence in comparison to SHEDs.
3.2 The effects of IL-17 and bFGF treatment on SHEDs and DPSCs viability, clonogenicity and cell cycle progression
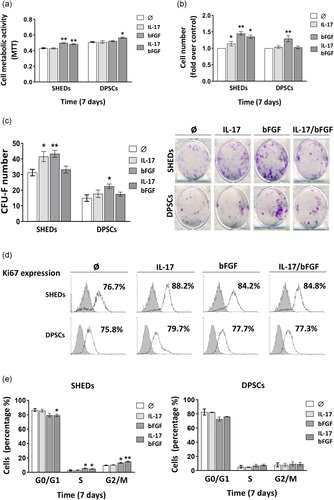
Results obtained by MTT test revealed that after 7 days IL-17 in combination with bFGF stimulates metabolic activity of both SHEDs and DPSCs, while bFGF alone exerts similar effect only in SHEDs (Figure 2a). Similar to the MTT test, 7-day treatment with IL-17 alone or in combination with bFGF increased the number of SHEDs, while bFGF stimulated the growth of both SHEDs and DPSCs (Figure 2b). Moreover, bFGF significantly stimulated CFU-F formation in SHEDs and DPSCs, while IL-17 increased the CFU-F number of only SHEDs (Figure 2c). However, when IL-17 and bFGF were used simultaneously CFU-F number returned to control values for both SHEDs and DPSCs showing no additive effect for the examined factors. Further evaluation of Ki67 expression, as a proliferation marker, demonstrated that both SHEDs and DPSCs are actively proliferative cell populations since in all experimental groups more than 75% of Ki67 positive cells were detected (Figure 2d). Besides, increased abundance of Ki67 positive cells compared to control was recorded for SHEDs upon each treatment, while no changes were detected in DPSCs (Figure 2d). In agreement with Ki67 expression and cell number changes, by using PI staining, we showed that bFGF alone or in combination with IL-17 increased the percentage of SHEDs in S or G2/M cell cycle phase, while bFGF and IL-17 together reduced the percentage of SHEDs in G0/G1 phase. Unlike SHEDs, the cell cycle progression of DPSCs was not changed in the presence of the examined factors (Figure 2e). Overall, obtained data indicated that IL-17 can manifest different effects on SHEDs and DPSCs proliferation and clonogenicity depending on the developmental stage of the DPSC population.
3.3 Influence of IL-17 and bFGF on pluripotency-associated markers expression in SHEDs and DPSCs
To further evaluate the potential of IL-17 and bFGF to influence stemness features of SHEDs and DPSCs, we investigated pluripotency-associated markers expression in tested cells upon 7-days treatment with these bioactive molecules. For that purpose, both MSC types were analyzed for protein and gene expression of NANOG, OCT4, and SOX2, transcription factors that play essential role in the regulation of stem cells pluripotency and self-renewal.
The results of fluorescent immunostaining demonstrated constitutive expression of NANOG, OCT4, and SOX2 in both SHEDs and DPSCs. However, while weak positive staining of NANOG was demonstrated in both cytoplasm and nucleus of untreated SHEDs, a significant increase of NANOG expression was observed in both cellular compartments after treatment with IL-17, bFGF or their combination (Figure 3a). On the other hand, when DPSCs were analyzed for NANOG expression a weak signal was detected only in the cytoplasm, which was markedly increased upon 7-day treatment with IL-17 and/or bFGF particularly when both factors were used in combination (Figure 4a). Likewise, the results of RT-PCR analysis evidenced an increased level of NANOG messenger RNA (mRNA) expression in SHEDs and DPSCs after 7 days incubation in the presence of both examined factors. However, while for SHEDs a statistically significant difference in comparison to untreated control was observed only when IL-17 and bFGF were used simultaneously (Figure 3d), in DPSCs each treatment caused a significant increase of NANOG mRNA expression.

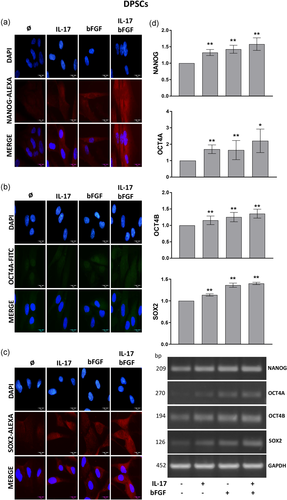
In addition, positive immunoreactivity for OCT4 was found primarily in the nucleus and at the lower level in the cytoplasm of untreated SHEDs (Figure 3b), while 7-day treatment with IL-17, bFGF or their combination induced markedly increased expression of OCT4 protein in the nucleus of SHEDs. On the contrary, although OCT4 protein was also localized mainly within the nucleus of untreated DPSCs, 7-day treatment with IL-17 and/or bFGF increased OCT4 expression predominantly in the cytoplasm of these cells (Figure 4b). These findings were supported by significantly higher OCT4A mRNA expression detected after 7-day treatment with both examined factors in SHEDs and DPSCs (Figures 3d and 4d), as well as by significantly increased OCT4B mRNA expression observed upon each treatment only in DPSCs.
On the other hand, constitutive expression of another pluripotency regulator, SOX2, showed a weak positive signal only in the cytoplasm of both SHEDs and DPSCs (Figures 3c and 4c), while stimulation with IL-17, bFGF or their combination resulted in significant increase of SOX2 expression mainly in the nucleus of SHEDs and only in the cytoplasm of DPSCs. In parallel, SOX2 gene expression analysis confirmed a significantly higher expression of SOX2 mRNA in both SHEDs and DPSCs upon each treatment (Figures 3d and 4d). Although both regulatory factors exerted a more prominent effect on SOX2 mRNA expression in SHEDs, no additive effects between IL-17 and bFGF on SOX2 mRNA expression were observed in both MSCs types.
3.4 Effects of IL-17 and bFGF on osteogenic and chondrogenic commitment of SHEDs and DPSCs
To determine how IL-17 and/or bFGF affect osteogenic and chondrogenic lineage commitment of SHEDs and DPSCs, differentiation of both MSC types was induced upon 7-day pretreatment with the examined factors. Obtained results demonstrated higher activity of early osteogenic marker ALP for both SHEDs and DPSCs pretreated with IL-17, while cells pretreated with bFGF alone or in combination with IL-17 showed significantly lower ALP activity in comparison to untreated controls (Figures 5a,c). In accordance with these findings, increased mRNA expression of osteogenic transcription factor RUNX2 was demonstrated in SHEDs after 7-day treatment with IL-17 (Figure 5b), while IL-17 induced a statistically significant increase of both ALP and RUNX2 mRNA expression in DPSCs (Figure 5d). In addition, a markedly decreased level of ALP mRNA expression was detected when SHEDs and DPSCs were treated with bFGF alone or in combination with IL-17. However, when mineralization levels were analyzed by Alizarin red staining, no significant changes were observed for both MSC types pretreated with the examined factors (Figures 5a,c). Altogether, these findings indicated that 7-day pre-exposure of SHEDs and DPSCs to IL-17 displays a stimulatory effect on early osteogenesis in contrast to strong inhibitory effect of bFGF, while neither IL-17, bFGF nor their combination affected late osteogenesis of pretreated cells.

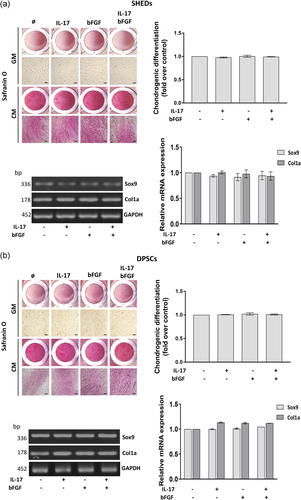
The results of chondrogenic differentiation analyses determined by staining with Safranin O revealed no significant changes of proteoglycan levels in SHEDs and DPSCs treated for 7 days with IL-17 and/or bFGF before differentiation induction (Figures 6a,c). These results were supported with a similar level of mRNA expression of chondrogenic markers SOX9 and COL1 determined in SHEDs and DPSCs following each treatment with the examined factors (Figures 6b,d).
3.5 Effects of IL-17 and bFGF on CD90, CD73 and IL-6 expression profile in SHEDs and DPSCs
Flow cytometry analyses of surface marker expression showed that after 7-day treatment, IL-17 and bFGF did not modulate the high rate of CD90 positive cells within the SHEDs population (Figure 7a). However, data of mean fluorescence intensity (MFI) analyses demonstrated significantly reduced MFI for CD90 expression only on SHEDs treated with bFGF and IL-17 simultaneously. On the other hand, in DPSCs population containing less CD90 + cells (66.8 ± 6.5) than SHEDs (97.4 ± 1%), bFGF alone or in combination with IL-17 induced a significant decrease in their percentage (47.8 ± 4.5 and 43.6 ± 4.4, respectively), while no changes in CD90 MFI were detected upon treatment with the examined factors (Figure 7a). As for the expression of CD73, our results revealed that 7-day treatment with IL-17 and bFGF, either alone or in combination, did not induce any alterations in a high percentage of CD73 + SHEDs and DPSCs (Figure 7b). Nevertheless, increased CD73 MFI was observed in SHEDs population cultivated in the presence of bFGF that reached statistical significance only when used simultaneously with IL-17. Similarly, the combination of both examined factors resulted in a statistically significant increase of CD73 MFI in DPSCs population (Figure 7b). These findings indicated that bFGF, particularly in combination with IL-17, negatively affects CD90 expression, while acting stimulatory on the expression of another MSCs marker, CD73 on both types of MSCs.
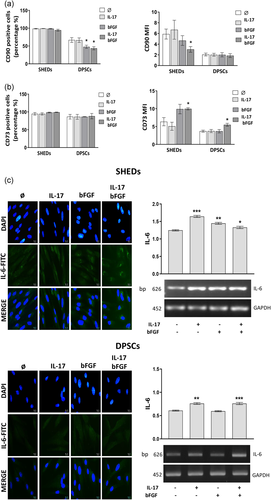
To evaluate possible mechanisms underlying IL-17 and bFGF effects on SHEDs and DPSCs features, we further analyzed their potential to modulate the expression of IL-6, one of the key players in pulpal inflammation also known to modulate MSCs growth and differentiation (J. Liu, Chen, et al., 2017). While immunofluorescence analysis of SHEDs showed weak cytoplasmic and perinuclear IL-6 expression in control cultures, IL-17- and bFGF-treated cells exhibited increased IL-6 staining particularly in the perinuclear region that was the most intense when both factors were used in combination (Figure 7c). These findings were supported by RT-PCR analyses data that revealed significantly increased IL-6 mRNA expression in SHEDs upon 7-day treatment with both examined factors, used either alone or in combination. On the other hand, constitutive IL-6 expression in DPSCs was mainly detected in the cytoplasm of untreated cells, which was notably increased upon 7-day treatment with IL-17, bFGF or their combination (Figure 7c). In addition, increased IL-6 mRNA expression in DPSCs was found when the cells were treated with IL-17, either alone or in combination with bFGF. The data obtained evidenced the potential of both IL-17 and bFGF to stimulate IL-6 expression in SHEDs and DPSCs showing different intracellular localization pattern dependent on MSCs type.
4 DISCUSSION
MSCs residing in the DP niche play important roles in pulp homeostasis and remodeling, contributing to the overall tissue viability. Moreover, accumulating evidence has supported the potential of dental MSCs and their EVs, as promising tools in bone tissue bioengineering (El Moshy et al., 2020; Trubiani et al., 2019). In this respect, getting insights into the dental stem cell niche maintenance and the role of various microenvironmental factors in the regulation of dental MSCs differentiation and self-renewal, could be crucial for the development of new regenerative therapies. In this study, we evaluated how IL-17 and bFGF, as mediators of inflammatory and regenerative pulp microenvironment, modulate main functional features of DP MSCs deriving from the tooth of different developmental stages. For that purpose, proliferation, clonogenicity, pluripotency markers expression and differentiation capacity of SHEDs and DPSCs were analyzed following 7-day treatment with the examined factors.
Our results confirmed that isolated SHEDs and DPSCs both meet minimal criteria for MSCs definition including fibroblast-like morphology, immunophenotype, CFU-F capacity and multilineage differentiation potential being in accordance with previously published results (Isobe et al., 2016; Tatullo et al., 2015). Nevertheless, slight differences between SHEDs and DPSCs behavior were observed, primarily regarding their proliferative capacity. Longer PDT, lower CFU-F capacity, and increased β-galactosidase activity of DPSCs compared to SHEDs were detected, as it was also reported in previous studies (Kunimatsu et al., 2018; H. Wang et al., 2018; X. Wang et al., 2012). In addition, expression of pluripotency markers (NANOG, OCT4, SOX2) was demonstrated in both SHEDs and DPSCs by immunofluorescence staining, confirming higher levels of NANOG, OCT4, and SOX2 expression in comparison to their adult counterparts, DPSCs, as described before (Govindasamy et al., 2010). These findings suggested potential deterministic role of the tooth developmental stage in regulation of dental MSCs proliferation and stemness, implying its potential influence on MSCs response to microenvironment stimuli as well.
Indeed, our results indicated that IL-17 and bFGF differentially affect SHEDs and DPSCs features. Enhanced proliferative and clonogenic capacity demonstrated in our study for both SHEDs and DPSCs upon treatment with bFGF has been supported by previous results (J. C. Kim, Park, Kim, et al., 2014; Morito et al., 2009). On the other hand, we revealed that IL-17 displayed stimulatory effects on proliferation and clonogenic capacity of SHEDs only. Increased proliferation of human BM-MSCs by IL-17 was previously shown (Huang et al., 2009), along with stimulated proliferation and CFU-F efficiency evidenced for IL-17-treated mouse BM-MSCs (Mojsilović et al., 2011). Interestingly, no additive effects for IL-17 and bFGF were observed with respect to proliferative and clonogenic capacity of SHEDs as previously detected for mouse BM-MSCs (Mojsilović et al., 2011). Here, we also demonstrated high expression of proliferation indicator Ki67 (>75%) in both SHEDs and DPSCs, which is consistent with Ki67-percentage detected in adipose tissue MSCs where more than 70% of cells were Ki67-positive (Nawrocka et al., 2017) and also comparable to our previously published results for periodontal ligament MSCs and DPSCs (Kukolj et al., 2019). Moreover, a slight increase in percentage of Ki67-positive cells was detected in presence of IL-17 and/or bFGF for SHEDs, which was followed by the changes in cell cycle progression reflected by higher frequency of dividing cells. On contrary, this trend was not detected in DPSCs. Although these findings implied that at least for SHEDs IL-17 and bFGF can modulate stem cell fate through cell cycle regulation, further studies are needed to confirm these assumptions.
At molecular level, stemness of MSCs is characterized by the expression of pluripotency markers including SOX2, OCT4, or NANOG (Kolf et al., 2007; Niwa, 2007). The role and significance of these markers expression in MSCs has not been clarified so far, and few previous studies showed NANOG and OCT4 can act as regulators of self-renewal and multilineage capacity in BM-MSCs and adipose tissue MSCs (Dudakovic et al., 2014; Tsai et al., 2012). In this study, we evidenced the ability of IL-17 and bFGF to stimulate expression of NANOG, OCT4A, and SOX2 at protein and gene level in both MSC populations investigated. The potential of bFGF to elevate NANOG, OCT4, SOX2, and REX1 expression in human SHEDs and DPSCs was previously demonstrated in parallel with its stimulatory effect on colony forming unit capacity (Osathanon et al., 2011; Sukarawan et al., 2014). Similarly, bFGF was shown to increase pluripotency gene expression levels in stem cells from the apical papilla, which all indicated the important role of bFGF in maintenance of stemness features for dental MSCs (Wu et al., 2012). In addition, stimulatory effect of proinflammatory cytokines, such as IL-1β, tumor necrosis factor α (TNF-α), interferon-γ (INF-γ), on NANOG, OCT4, and SOX2 expression was previously shown in gingival and DP MSCs (Fawzy El-Sayed et al., 2019; Tomasello et al., 2017). Moreover, it has been shown that IL-17B, another member of IL-17 cytokine family, induced the expression of NANOG, OCT4A and SOX2 in human umbilical cord MSCs and gastric cancer-derived MSCs (Bie et al., 2017). However, our findings evidencing stimulatory influence of IL-17 on NANOG, OCT4, and SOX2 expression in both types of DP MSCs brought new data to the scarce literature concerning IL-17s' role in regulation of MSC stemness. Interestingly, in our study, increased expression of OCT4B mRNA was demonstrated only in adult DPSCs upon stimulation with both IL-17 and bFGF. The role of OCT4B in pluripotency has not been clarified and recent study suggested important role of this factor in dental regeneration according to its antiapoptotic activity and increased expression found in DP cells under inflammatory conditions (L. Liu, Huang, et al., 2017). Certainly, the involvement of IL-17- and bFGF-induced NANOG, OCT4 and SOX2 expression in regulation of DP MSCs properties needs to be additionally elucidated. In this context, our findings of different intracellular expression pattern for constitutive, as well as IL-17- and bFGF-stimulated pluripotency markers expression in SHEDs and DPSCs could be of significant importance implying to specific functionality of pluripotency factors in correlation to their cellular localization and developmental stage of dental MSCs population. Indeed, beside few studies indicating that intracellular localization of pluripotency markers might correlate with cancer development and cells growth (van Schaijik, et al., 2018), potential implications of NANOG, OCT4, and SOX2 cellular compartmentalization on stem cells properties are largely unknown and merit further detailed exploration.
In addition to self-renewal capacity, MSCs are able to differentiate into various cell lineages under in vitro conditions (Han et al., 2017). Our study showed that pre-exposure of SHEDs and DPSCs to IL-17 displayed stimulatory effect on early osteogenesis in contrast to strong inhibitory effect of bFGF, while neither IL-17, bFGF nor their combination affected potential for mineralization of pretreated cells. Stimulatory effects of IL-17 on SHEDs and DPSCs osteogenic differentiation demonstrated through increased ALP activity in SHEDs and DPSCs are in line with previous reports showing stimulatory effects of IL-17 on early osteogenic differentiation, mineralization and osteogenic gene marker expression in DPSCs and SHEDs (Sebastian et al., 2018; Yu et al., 2016), as well as markedly enhanced BM-MSCs osteogenic differentiation demonstrated upon treatment with IL-17 (Osta et al., 2014). In contrast, few studies reported inhibitory effect of IL-17 on osteogenic differentiation of periodontal ligament MSCs (PDLSCs) (Okić-Đorđević et al., 2016) and rat BM-MSCs (Y. G. Kim, Park, Lee, et al., 2014) implying to the importance of specific native microenvironment conditions and the tissue origin of progenitor cells on the outcome of IL-17 effects. Inhibition of SHEDs and DPSCs osteogenic differentiation capacity observed in cells pretreated with bFGF confirmed previous findings showing that bFGF decreases ALP gene expression, its enzymatic activity and mineralization in different dental tissue MSCs, including DPSCs, SHEDs, and PDLSCs (Lee et al., 2015; Osathanon et al., 2011, 2013). Indeed, the key role of endogenous bFGF in control of SHED stemness and osteogenic differentiation was demonstrated according to the significant decrease of cell proliferation and colony formation, as well as increase of ALP activity and mineral deposition observed upon bFGF inhibition conducted using shRNA or specific receptor inhibitor (Nowwarote et al., 2015). It is noteworthy that IL-17, which alone stimulated ALP expression and activity, could not overcome the inhibitory effect of bFGF on SHEDs and DPSCs osteogenic differentiation. However, neither bFGF nor IL-17 pretreatment had significant influence on calcium deposition after culturing SHED and DPSC in osteogenic medium for three weeks. In light of the previous data showing that bFGF can differently affect DPSCs osteogenic differentiation depending on the treatment conditions (Qian et al., 2015), as well as that it can reversibly inhibit DPSCs differentiation (Shimabukuro et al., 2009), it is possible that one week of preconditioning was not enough to affect the late osteogenesis. Unlike osteogenic differentiation, IL-17 or bFGF pretreatment did not affect the chondrogenic capacity of DPSCs and SHEDs, although previous studies reported enhanced chondrogenic differentiation of bFGF-treated BM-MSCs (Correa et al., 2015; Ito et al., 2008) and adipose tissue MSCs (Chiou et al., 2006; Kabiri et al., 2012). Moreover, it has been revealed that chondrogenic potential of inflamed SHEDs can be stimulated by bFGF (J. C. Kim, Park, Kim, et al., 2014), while IL-17 was shown to inhibit chondrogenic differentiation of BM-MSCs (Kondo et al., 2013). Nevertheless, despite these discrepancies, it will be taken into consideration that different effects of IL-17 and bFGF on MSCs differentiation capacity could be manifested depending on treatment conditions, as well as on MSCs tissue source.
Interestingly, the effects of IL-17 and bFGF on SHEDs and DPSCs proliferation, stemness and differentiation were accompanied by changes in CD90 and CD73 markers expression. Namely, our flow cytometry analyses showed that bFGF, particularly in combination with IL-17, adversely affected CD90 expression on both SHEDs and DPSCs, while acted stimulatory on expression of CD73 on both types of MSCs. CD90, also known as Thy-1, is a glycosylphosphatidylinositol-anchored protein shown to participate in different processes, including inflammation, wound healing, fibrosis and tumor development (Jósvay et al., 2014; Rege & Hagood, 2006; Schmidt et al., 2015). Since CD90 deficiency in mice was related to decreased bone mass and elevated bone marrow fat, critical role of CD90 in regulation of MSCs differentiation was proposed indicating its stimulatory effect in osteogenesis and bone formation (Picke, Campbell, Blüher, et al., 2018; Picke, Campbell, Schmidt, et al., 2018; Woeller et al., 2015). However, another study demonstrated that CD90 downregulation increased osteogenic differentiation of DP, adipose tissue and amniotic fluid MSCs (Moraes et al., 2016), which all pointed to the tissue source-dependent and intricate correlation between CD90 expression and MSCs differentiation (Hagood, 2019). Whether decreased CD90 in response to bFGF could be associated with bFGF-inhibited osteogenesis of SHEDs and DPSCs and how it might interfere with their proliferation and stemness remains to be elucidated. Changes of CD73 expression observed in this study corroborated previous literature data evidencing the potential of various cytokines, such as INF-γ, TNF-α, and IL-1β, to regulate expression of this ecto-5'-nucleotidase in the cell type-dependent manner (Colgan et al., 2006). However, little is known about the function of CD73 in regulation of MSCs properties and according to lower bone mineral content found in mice lacking this ectoenzyme, its involvement in MSCs osteogenic differentiation has been proposed (Takedachi et al., 2012). In other studies, stimulated osteogenic differentiation of MSCs mediated by increased CD73 enzyme activity and extracellular adenosine production was demonstrated under cycle-compressive loading, as well as upon TGF-β treatment (Hau et al., 2017; Ode et al., 2013). Since SHEDs and DPSCs showed inhibited osteogenic differentiation upon stimulation with combination of bFGF and IL-17, increased CD73 expression intensity evident under same conditions could be rather related to the involvement of this ectoenzyme in regulation of another MSCs functional responses. Therefore, in light of previous data showing that CD73-generated adenosine stimulates MSCs proliferation rate, as well as their immunomodulatory/regenerative phenotype (Roszek & Wujak, 2018), intensive further research is required.
To evaluate possible mechanisms underlying IL-17 and bFGF effects on functional activity of SHEDs and DPSCs, we also investigated their effects on expression of inflammatory cytokine IL-6. The data obtained demonstrated that IL-17 and bFGF, either alone or combined, increased the level of IL-6 expression on gene and protein level in both SHEDs and DPSCs showing different intracellular protein localization pattern dependent on MSCs type. While bFGF-induced IL-6 expression in SHEDs is in accordance with recently published data (Nowwarote et al., 2017), similar data for IL-17-stimulated expression of IL-6 were reported for human DP fibroblasts (Xiong et al., 2015). Importantly, according to the report of Pricola et al evidencing that IL-6 maintained BM-MSCs stemness via extracellular signal-regulated kinase signaling, it has been suggested that IL-6 could be associated with maintenance of both embryonic and adult stem cells (Pricola et al., 2009). Recent study also demonstrated that IL-6 induced significant increase of NANOG and SOX2 mRNA expression as well as osteogenic differentiation of SHEDs (Nowwarote et al., 2018), which indicated that one of the possible mechanism by which IL-17 and bFGF exert their effects on stemness of the investigated cells could involve IL-6. However, the correlation of IL-17- and bFGF-induced IL-6 expression to modified proliferation, stemness markers expression and/or osteogenic differentiation of SHEDs and DPSCs needs to be further elucidated.
5 CONCLUSION
Overall results of this study indicated that IL-17 and bFGF manifest different effects on proliferative and clonogenic potential of SHEDs and DPSCs. While bFGF increased proliferation and clonogenicity of both SHEDs and DPSCs, IL-17 similarly affected SHEDs, exerting no effects on adult counterparts DPSCs. Moreover, both factors stimulated NANOG, OCT4, and SOX2 pluripotency markers expression in SHEDs and DPSCs revealing different intracellular expression pattern dependent on MSC type. Regarding the differentiation capacity, the examined factors showed comparable effects on both SHEDs and DPSCs including stimulatory effect of IL-17 on early osteogenesis in contrast to strong inhibitory effect of bFGF. In addition, both factors induced IL-6 expression indicating its relevance in regulation of IL-17/bFGF-modulated properties of SHEDs and DPSCs. All these data provide novel evidence on IL-17 and bFGF potential to modulate stem cell properties important for regeneration of DP at different ages, that should be considered particularly in the context of potential SHEDs/DPSCs therapeutic use.
ACKNOWLEDGMENTS
The authors are grateful to Mrs Snežana Marković for the excellent technical assistance. This study was supported by the Ministry of Education, Science and Technological Development, Republic of Serbia (contract number 451-03-68/2020-14/200015). Ministry of Education, Science and Technological Development, Government of Republic of Serbia; grant number: 451-03-68/2020-14/200015.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Aleksandra Jauković and Diana Bugarski conceived and designed the study. Aleksandra Jauković, Tamara Kukolj, Drenka Trivanović, Ivana Okić-Đorđević, Hristina Obradović, and Slavko Mojsilović performed the experiments and data analyses. Maja Miletić and Vanja Petrović collected dental pulp tissue samples and participated in data interpretation. Aleksandra Jauković, Tamara Kukolj, and Drenka Trivanović wrote this manuscript. All authors participated in literature search, editing the manuscript, and gave the final approval.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




