IκB kinase inhibition remodeled connexins, pannexin-1, and excitatory amino-acid transporters expressions to promote neuroprotection of galantamine and morphine
Shimaa Magdy and Maha Gamal equally contributed to the work
Abstract
Inflammatory pathway and disruption in glutamate homeostasis join at the level of the glia, resulting in various neurological disorders. In vitro studies have provided evidence that membrane proteins connexions (Cxs) are involved in glutamate release, meanwhile, excitatory amino-acid transporters (EAATs) are crucial for glutamate reuptake (clearance). Moreover, pannexin-1 (Panx-1) activation is more detrimental to neurons. Their expression patterns during inflammation and the impacts of IκB kinase (IKK) inhibition, morphine, and galantamine on the inflammatory-associated glutamate imbalance remain elusive.
To investigate this, rats were injected with saline or lipopolysaccharide. Thereafter, vehicles, morphine, galantamine, and BAY-117082 were administered in different groups of animals. Subsequently, electroencephalography, enzyme-linked immunosorbent assay, western blot, and histopathological examinations were carried out and various indicators of inflammation and glutamate level were determined. Parallel analysis of Cxs, Panx-1, and EAAts in the brain was performed. Our findings strengthen the concept that unregulated expressions of Cxs, Panx-1, and EAATs contribute to glutamate accumulation and neuronal cell loss. Nuclear factor-kB (NF-κB) pathway can significantly contribute to glutamate homeostasis via modulating Cxs, Panx-1, and EAATs expressions. BAY-117082, via inhibition of IkK, promoted the anti-inflammatory effects of morphine as well as galantamine. We concluded that NF-κB is an important component of reshaping the expressions of Cxs, panx-1, and EAATs and the development of glutamate-induced neuronal degeneration.
1 INTRODUCTION
The effect of systemic inflammation on the pathogenesis of neurodegenerative diseases, is a current and challenging topic of research. Systemic inflammation due to aging or disease states (such as obesity, cardiovascular disease, sepsis and infection) can directly impact functioning within the brain. (Batista et al., 2019; Gamal et al., 2015, 2018; Srinivasan & Lahiri, 2015; Walker, 2019). Exaggerated inflammatory pathway and disruption in glutamate homeostasis join at the level of the glia, resulting in various neurological disorders (Badshah et al., 2016; Haroon et al., 2017). Glutamate is considered neurotoxic at high levels through the activation of extrasynaptic N-methyl-d-aspartate (NMDA) receptors that leading to loss of neuron and synaptic integrity. Thus, tight regulation of glutamate is essential for maintaining the brain integrity (Boyko et al., 2014). In recent years, there has been great interest in understanding the mechanisms by which glutamate homeostasis is altered during inflammation.
Systemic cytokines initiate and maintain the inflammatory response within the brain through interacting with the blood brain barrier, vagal nerve and cerebral endothelium. A main consequence of increased inflammatory signaling is the activation of nuclear factor-kappa B (NF-κB), which has been described as the point of convergence of numerous pathways involved in microglia-mediated inflammation (Cunningham, 2013). In inflammation, astrocytes and microglia essentially contribute to the imbalance between glutamate release and uptake (Bachtell et al., 2017; Haroon et al., 2017; Jha et al., 2019). Excitatory amino-acid transporters (EAATs), connexions (Cxs), and pannexins (Panx) are membrane proteins that are recently identified on astrocytes and microglia. Evidence from in vitro studies revealed that Cxs are involved mainly in glutamate release from glial cells (Abudara et al., 2015; Jiang et al., 2011; Orellana et al., 2011), meanwhile, EAATs are crucial to prevent extrasynaptic glutamate spillover (Bajrektarevic & Nistri, 2017; ODonovan et al., 2017). Furthermore, dysregulated pannexin activity is pivotal for facilitating NMDA-induced neurotoxicity (Dvoriantchikova et al., 2018; Shestopalov & Slepak, 2014a). The significance and regulation of these membrane proteins in neuroinflammatory disorder remain unknown.
The principal pathway that arbitrates the response of glial cells to injurious stimuli is the NF-κB pathway. It controls the transcription of numerous genes, often encoding for proteins involved in immune regulation and survival of neuronal cells. NF-κB activation is tightly regulated mainly through its localization in the cytoplasm by inhibitory IκBα proteins (Kaltschmidt et al., 2005; Rauert-Wunderlich et al., 2013). The NFKB signaling pathway was found to be important for the microglia proliferation and differentiation (Dresselhaus & Meffert, 2019; Popiolek-Barczyk & Mika, 2016), but its effects on Cxs, Panx-1, and EAATs, and thus glutamate homeostasis have not been reported to date.
Glial cells express opioid receptors as well as nicotinic acetylcholine receptors (nAChRs). Opioids may have an impact on the cytokines levels in the brain. One study using a N9 murine's microglial cell showed that treatment with morphine alleviates Tat induced cytokines secretion (Turchan-Cholewo et al., 2009), while another using human's microglia demonstrated that exposure to morphine does not significantly influence tumour necrosis factor α (TNF-α) secretion (El-Hage et al., 2015).
nAChRs are a family of ligand-gated ion channels that are detected in neuronal and non-neuronal cells in the brain. Activation of α7 nAChR regulates the cholinergic anti-inflammatory pathway (Parada et al., 2013). An increase in the acetylcholine (Ach) level following galantamine treatment, a centrally acting acetylcholinesterase (AChE) inhibitor, was found to inhibit glial cells and suppress cytokines release (Giunta et al., 2004; Liu et al., 2018). Evidence derived from pancreatic tissue (Klee et al., 2016), endothelial cells (Duerrschmidt et al., 2012), and skeletal muscles (Cisterna et al., 2014) suggests that nicotinic receptors participate in the regulation of Cxs. However, a potential role of galantamine and morphine modifying Cxs, Panx-1 as well as EAATs has yet to be elaborated.
Using the experimental model of systemic lipopolysaccharide (LPS) to imitate a systemic inflammation that activate glia cells, we aimed to characterize the expression patterns of Cx43, Cx32, Panx-1, and EAATs and their relations to glutamate-induced neuronal dysfunction, and to study the impacts of IκB Kinase (IKK) inhibition and various treatments.
2 MATERIALS AND METHODS
2.1 The experimental animals
The present study was conducted following the guidelines for experimental animal use and was approved by the Institutional Animal Care Committee (CU Ш F 55 17). Seventy Sprague–Dawley male rats weighing (200–250) were purchased and kept in the animal house of Kasr El Aini; Faculty of Medicine, Cairo University. They were housed in chip bedded cages (27 × 38 × 17 cm) under normal day–night cycle, controlled humidity and temperature conditions. Both food and water were provided ad libitum.
2.2 General protocol
Rats were divided into two main groups. The first group (LPS group) were injected intraperitoneally (i.p.) with 10 mg/kg bacterial (LPS) (Escherichia Coli, serotype 026:B6; Sigma) dissolved in sterile saline, and the same volume of saline was used in the second group which served as a control group. In the LPS group, rats were randomly divided into 6 subgroups (10 rats per group) according to a random number table, including untreated LPS, LPS-BAY-117082 (a specific inhibitor of IKK) (1 mg/kg i.p.; Sigma-Aldrich; Wang et al., 2016), LPS-morphine (15 mg/kg, i.p.; Misr Pharmaceuticals Co.; Ferger & Kuschinsky, 1995), LPS-morphine-BAY-117082, LPS-galantamine (4 mg/kg, i.p.; Janssen-Cilag Pharmaceuticals, Johnson & Johnson; Pavlov et al., 2009) and LPS-galantamine-BAY-117082 groups. The rats received vehicle or drug 30 min after LPS.
Rats were sacrificed by cervical dislocation 24 h after LPS injection, and blood and brain samples were collected at the same time points and processed as described below.
2.3 Electrodes implantaion for electroencephalogram (EEG) recordings
The EEG power spectrum is a method of EEG signal analysis that provides more accurate information for determining brain functions than traditional EEG techniques (H. Chen et al., 2012). Rats were anesthetized with ketamine–xylazine (80 and 10 mg/kg, respectively) and were positioned in the stereotaxic device (David Kopf Instruments). Three stainless steel electrodes, of 1 mm diameter, were implanted above the frontoparietal cortex (coordinates in mm: 2 mm lateral to bregma, 3.9 anterior to the bregma, 6.4 posterior to the bregma in both hemispheres; active electrodes) along with a reference electrode positioned over the cerebellum implanted 1 mm posterior to the lambda on the midline, as previously described (Mohammed et al., 2020). The electrodes were held in place and isolated by a layer of dental cement (zinc polycarboxylate, Spofa-Dental-Praha, Czech Republic). At least 7 days were allowed for recovery from surgery before the beginning of the experiment. During this period, rats were housed individually in separate glass cages and were habituated to the EEG recording setup.
EEG recordings were performed at the light phase and rats were kept awake and freely moving in an electrically shielded faraday cage (25 × 25 × 30 cm). All recordings were done at the same time of the day (between 9 and 11 a.m.) to avoid the circadian variation of the EEG signals. In all animals, recordings began daily during baseline periods, and all rats were recorded 24 h after LPS or saline injection for 30 min. For EEG monitoring, rats were connected, by a flexible cable, to the system consisting of bioamplifier (Animal BioAmp ML136; ADInstruments) and analog-digital converters (PowerLab 4/30 ML870; ADInstruments). The data were recorded and analyzed with the LabChart 7.1 software (ADInstruments; sampling rate 200 Hz; time constant 0.1 s; low pass filter 60 Hz). The Fast Fourier Transform and spike histogram were performed to obtain power spectra. Power spectral density maps were generated within LabChart. Spectra were segmented into five frequency bands; delta (0.1–4 Hz); theta (4.1–8 Hz); alpha (8.1–13 Hz); beta (13.1–30 Hz); gamma (30–40). The power of each frequency band was relative (percentage) to the total power (0.1–40 Hz) of the bands for comparison purposes.
2.4 Enzyme-linked immunosorbent assay (ELISA) analysis
TNFα, interleukin (IL)-1, and NF-κB p65 in serum and brain tissues were quantified with a rat IL-1, TNF-α, or NF-κB p65 ELISA kit (RayBiotech) following the manufacturers' protocol. Samples and biotin labeling antibodies were incubated in the wells of microplate and washed out with phosphate-buffered saline (PBS). The microplate was incubated with Avidin-horseradish peroxidase (HRP) conjugate reagent for 1 h. The substrates for color development were added to each well. The optical density (OD) is measured colorimetrically at a wavelength of 450 nm. The concentrations of IL-1B and TNF-α in the samples were calculated by comparing the OD of the samples to the standard curve.
In cerebral cortex lysates, glutamate level was measured using a rat glutamate ELISA kit (MyBioSource). Following incubation and washing, the glutamate was estimated by the reading of OD at 450 on microplate spectrophotometer, as described by the manufacturer.
2.5 Real-time quantitative PCR analysis
According to instructions of manufacture, total RNA was extracted from brain tissue by using Qiagen tissue extraction kit (Qiagen) and reverse-transcribed into complementary DNA (cDNA). Real-time quantitative PCR was performed to amplify the cDNA by using a Hybaid Omn-E Thermocycler (Hybaid Limited). Quantitative PCR analysis was carried out using an Applied Biosystem with software version 3.1 (StepOne™). β-Actin was used as an internal reference and the 2−ΔΔCt method was used to estimate the messenger RNA (mRNA) levels of the gene of interest and the internal reference gene. For each sample, the mRNA levels were normalized to Β-actin using the standard curve method. The primer sequences are listed in Table 1.
| Gene | Primer sequences (5′-3′) | |
|---|---|---|
| EAAT1 | Forward | 5′-GGTTGCTGCAAGCACTCATCAC-3′ |
| Reverse | 5′-CACGCCATTGTTCTCTTCCAGG-3′ | |
| EAAT2 | Forward | 5′-TGCCAACAGAGGACATCAGCCT-3′ |
| Reverse | 5′-CAGCTCAGACTTGGAGAGGTGA-3′ | |
| β-Actin | ||
| Forward | 5′-AGGCATCCTCACCCTGAAGTA-3′ | |
| Reverse | 5′-CACACGCAGCTCATTGTAGA-3′ |
- Abbreviation: EAAT, excitatory amino-acid transporter.
2.6 Western blot analysis
The whole brain tissues were homogenized and lysed in the lysis buffer (150 mM NaCl, 0. 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulphate (SDS) supplemented with protease inhibitor buffer (phenylmethylsulfonyl fluoride 0.4 µl/200 µl) and a phosphatase inhibitor buffer (2.5 µl/200 µl; Bio BASIC INC.). Thereafter, tissue lysates were centrifuged at 16,000g, at 4°C for 30 min. The supernatant was collected and boiled with 2X Laemmli loading buffer (4% SDS; 10% 2-mercaptoethanol; 20% glycerol; 0.004% bromophenol blue; 0.125 M Tris HCl; Bio BASIC INC.) at 95°C for 5 min. Then, 20 µg of total protein per well was loaded onto the 10% SDS-polyacrylamide gel electrophoresis. After electrophoresis, the proteins were electrotransferred from the gel to polyvinylidene fluoride membrane. The membrane was blocked in tris-buffered saline with Tween 20 (TEST) buffer and 3% bovine serum albumin at room temperature for 1 h and incubated overnight at 4°C with primary antibody to NF-κB p65 (1:100; Thermo Fisher Scientific), p-IκBα (1:100; Thermo Fisher Scientific), T-p65 (1:100; Thermo Fisher Scientific), P-P65 (1:100; Thermo Fisher Scientific), Iba1 (1:100; Thermo Fisher Scientific), GFAP (1:1000; Thermo Fisher Scientific), Cx43 (1:1000; Thermo Fisher Scientific), cx32 (1:100; Thermo Fisher Scientific), panx-1 (1:1000; Thermo Fisher Scientific), EAAT1 (1:100; Thermo Fisher Scientific), and EAAT2 (1:100; Thermo Fisher Scientific). After being rinsed 3–5 times for 5 min with TBST, the membrane was incubated in the HRP-conjugated secondary antibody solution. Finally, the chemiluminescent substrate (ClarityTM Western ECL substrate—BIO-RAD) was applied to the blot according to the manufacturer's recommendation. The signals were captured and analyzed by ChemiDoc MP analysis software.
2.7 Histomorphometric analysis
Rats were decapitated by cervical dislocation under anaesthesia, and transcardial perfusion with PBS followed by 4% paraformaldehyde was performed. The brain samples were removed and immersed in 10% formalin for fixation. After step-wise dehydration by alcohol, the brain samples were fixed in paraffin. The brain was sectioned by using the microtome at 5 μm. Hematoxylin and eosin stains were applied, and the slides were examined under a light microscope Olympus IX51 by a blind investigator. Clear images were obtained by the microscope-attached a DP72 camera. For each sample, a total of 10 photomicrographs were obtained for counting apoptotic cells by Digital Imaging Solution Cell Software. For immunohistochemistry, sections were deparaffinized in xylene (1 h) and rehydrated in descending grades of alcohol, thereafter, they were incubated in hydrogen peroxide 3% (5 min) to block the activity of endogenous peroxidase. Sections were washed twice in PBS and placed in 0.01 mol/l citrate buffer (pH 6) in a microwave for 5 min for antigen retrieval. The slides were incubated with rabbit polyclonal anti-glial fibrillary acid protein antibody (GFAP [ab7260], 1:1000, IHC-P, Abcam®, mouse brain tissue used as positive control), rabbit polyclonal anti-ionized calcium-binding adapter molecule 1 antibody (IBA1 [ab153696], 1:500, IHC-P, Abcam®, rat brain tissue used as positive control), rabbit polyclonal anti-Connexin 32/GJB1 (CX32 [ab66613], 1:100, IHC-P, Abcam®, human intestinal tissue used as positive control), rabbit polyclonal anti-Connexin 43/GJA1 antibody—Intercellular Junction Marker antibody (CX43 [ab11370], 1:1000, IHC-P, Abcam®, mouse heart tissue used as positive control) and rabbit polyclonal anti-Pannexin 1 antibody (anti-Panx-1, catalog #488100, dilution 1:100, Thermo Fisher Scientific, antibody from Invitrogen) overnight at 4°C. Thereafter, slides were washed with PBS and incubated with goat anti-rabbit immunoglobulin G H&L (HRP) (ab205718) secondary antibody for 20 min at room temperature. After then, they were incubated with diaminobenzidine tetrahydrochloride solution and counterstained with Mayer's hematoxylin for 2 min. Negative controls were processed via the omission of the primary antibodies in the automated staining protocol. The images were captured with an optical microscope, and six optical fields under magnification ×400 were selected to be blindly analyzed by the ImageJ analyzer computer system (J Image Pro Plus6.0, Media Cybernetics).
2.8 Statistical analysis
All analyses were performed using the GraphPad Prism 5 software (GraphPad). The normality of variances was assessed by Kolmogorov–Smirnov and Shapiro–Wilk statistical tests in advance. Statistical differences between saline, LPS untreated, and treated groups were evaluated by one-way analysis of variance with Tukey's post-tests. Correlations were analyzed using Pearson's correlation coefficient. Values were expressed as the means ± SEM.
A two-sided p value of less than .05 was considered statistically significant.
3 RESULT
3.1 IKK inhibition and treatment with morphine or galantamine modulated EEG changes in LPS injected rats
We found that LPS injection had significantly increased (p < .05) the relative power of delta and theta bands with respect to control. However, a tendency decrease was observed in the alpha band with respect to control. In our study, no seizure activity was recorded from all groups. We also examined the acute impact of different treatments on LPS-induced EEG alterations. Compared to the vehicle-treated LPS group, significant changes (p < .05) in the relative power of delta, theta and alpha were observed after treatment with galantamine, BAY-117082 and cotreatments with BAY-117082 and morphine/galanatmine. At the same time, gamma and beta rhythms did not change significantly in all groups (Figure 1).

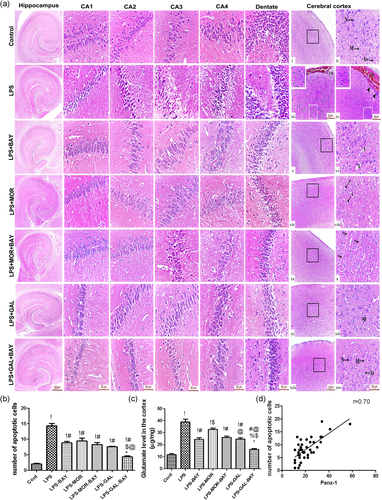
3.2 IKK inhibition and treatment with morphine or galantamine reduced glutamate and neuronal degeneration in LPS injected rats
As shown in Figure 2, the glutamate concentration of the control group was significantly lower (p < .05) than its concentration in the LPS treated groups. However, glutamate concentration of galantamine-treated rats was markedly lower (p < .05) than those in the LPS and LPS-morphine groups. Rats treated with BAY-117082 and exposed to LPS displayed a significantly lower (p < .05) glutamate in comparison with those in the LPS group and LPS-morphine groups. Marked reduction (p < .05) in glutamate level was observed when galantamine/morphine and BAY-117082 were administered concomitantly. Additionally, the number of apoptotic cells in the cortex of LPS-challenged rats without treatment was significantly higher (p < .05) than the control group. The results also showed that different treatments significantly reduced (p < .05) the number of apoptotic cells, but the greatest reduction (p < .05) was observed with combined treatment of galantamine/morphine and BAY-117082 (p < .05) (Figure 2). We found a positive correlation among the number of neuronal apoptotic cells and upregulated PANX-1 in the neuronal cells which could explain the glutamate-induced neurotoxicity (Figure 2).
3.3 Morphine and galantamine altered microglia and astrocyte activities and cytokines level in LPS injected rats
Activated microglia and astrocyte cells release relevant amounts of cytokines that underlie LPS induced neurotoxicity. LPS concomitantly activated microglia and astrocyte significantly, reflected by a significantly increases (p < .05) in Iba 1 and GFAP level and immunoreactivity, when compared to the control group. As expected LPS strongly activated IκBα phosphorylation (p < .05), an upstream pathway for NF-κB translocation, in concomitant to a significant increases (p < .05) in NF-kB activation and increases in IL-1 and TNF-α (p < .05) (Figures 3 and 4).
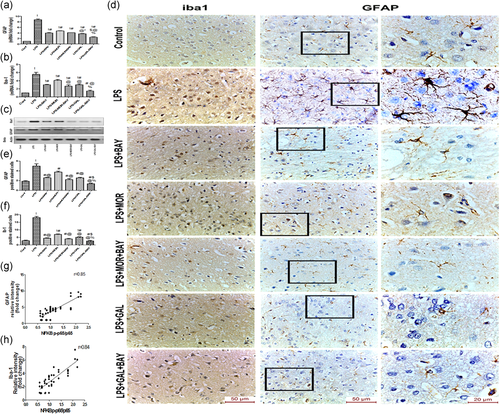
Activation of opioid receptor (using morphine) and nicotinic receptor (using galantamine) significantly ameliorated (p < .05) the activation of microglia and astrocyte. Furthermore, significant suppression (p < .05) of IκBα phosphorylation in parallel with blocking of NF-kB activation (p < .05) and a reduced (p < .05) release of IL-1 and TNF-α were found in morphine and galantamine treated groups, but more obvious effects were observed after galantamine (Figures 3 and 4).
3.4 Inhibition of IKK reversed LPS effects and exacerbated galantamine and morphine effects on glial cells
We investigated the contribution of direct IKK inhibition on microglia and astrocyte activation induced by LPS. Notably, direct IKK inhibition by BAY-117082 significantly reduced (p < .05) microglia and astrocyte activations. We also noticed the significant suppression (p < .05) effects of BAY 11-7082 on IκBα phosphorylation and NF-κB DNA binding activity parallel with a decrease (p < .05) in IL-1 and TNF-α levels. Moreover, strong positive correlations were found between phosphorylated NF-κB p65 and Iba-1 and GFAP expressions (Figures 3 and 4).
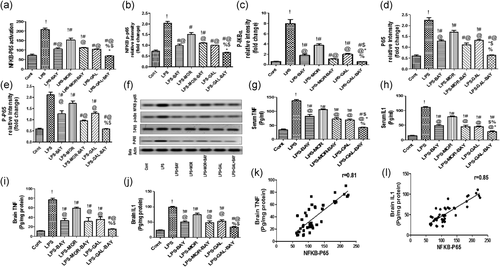
We hypothesized that direct inhibition of IKK might play an important role in optimizing the protective effects of morphine and galantamine. To validate this hypothesis, we combined treatments with morphine/galantamine to the IKK inhibitor BAY-117082. Our data indicated that the block of IKK significantly reinforced (p < .05) the effects of galantamine as well as morphine on the glial cells and cytokine levels (Figures 3 and 4).
3.5 Morphine and galantamine altered EAATs, CXs, and Panx-1 expressions in LPS injected rats
We examined whether LPS injected rats with or without morphine or galantamine could modulate Cx43, Cx32, Panx-1, and EAATs expressions in the brain tissue. As expected, LPS significantly increased (p < .05) Cx43, Cx32, and Panx-1, but significantly decreased (p < .05) EAATs expressions (Figure 5).
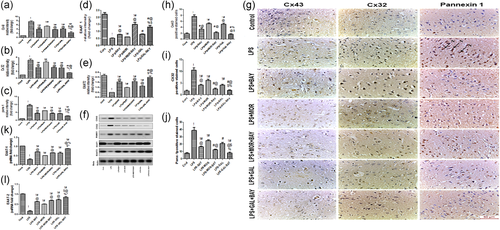
Morphine and galantamine significantly ameliorated (p < .05) the changes in Cx43, Cx32, and Panx-1 expressions induced by LPS. Furthermore, EAATs overexpression (p < .05) was observed after morphine as well as galantamine treatments. In compare to morphine—LPS group, galantamine downregulated Cxs and Panx-1 and upregulated EAAT2 expressions more significantly (p < .05) than morphine alone (Figure 5).
3.6 Inhibition of IKK reversed LPS effects and exacerbated galantamine and morphine effects on EAATs, CXs, and Panx-1 expressions
We found that direct IKK inhibition by BAY-117082 significantly reduced (p < .05) Cx43, Cx32, and Panx-1 expressions, but upregulated (p < .05) EAATs expression in LPS treated rats (Figure 5). Moreover, strong correlations were found between phosphorylated NF-κB p65 and Cx43, Cx32, Panx-1, and EAATs expressions (Figure 6).

The effects of galantamine and morphine on LPS induced CXs, EAATs, and Panx-1 expressions were significantly aggravated (p < .05) after BAY-117082 administeration (Figure 5).
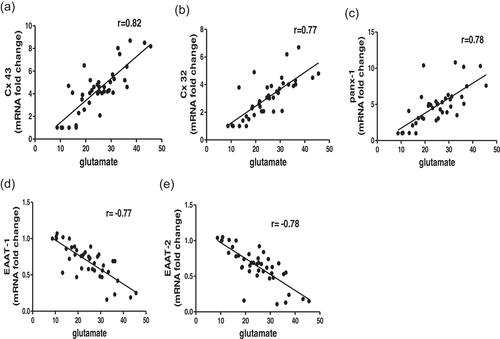
3.7 Glutamate level relation to Cxs, Panx-1, and EAATs expressions
The correlativity among glutamate levels and the expressions of CXs, Panx-1, and EAATs were evaluated by Pearson's correlation coefficient (r). There were significant positive correlations between the glutamate level and both CXs and Panx-1. Further, our data showed a significant negative correlations between glutamate level and EAATs (Figure 7). In the present study, the upregulation of Cxs in glia cells seems to contribute to glutamate release, contrary to the downregulation of astrocyte EAATs that appears to be involved in glutamate uptake.
4 DISCUSSION
This study suggests a role of NFκB in the homeostatic regulation of glutamate, thereby potentiating neuronal cell death and EEG changes observed after the activation of astrocyte and microglia. This is explained by the following results: (1) the cortical Cx43, Cx32, and Panx-1 expressions were significantly enhanced by LPS but were inhibited by BAY-117082. The opposite was observed for EAATs; (2) the alterations in EAATs, Cx43, Cx32, and Panx-1 expressions were significantly associated with increased glutamate level and exaggerated neuronal cell death; and (3) NFκB -dependent cytokines release contributes also to the concomitant activation of the astrocytes and microglia. Our results provide another novel evidence to support that neuro-inflammation, glutamate balance, and the expression patterns of EAATs, Cxs, and Panx-1 could be modulated by opioids as well as nicotinic receptors activation. But the greatest effect was noted when galantamine or morphine was combined with BAY-117082.
For each rat, EEGs displayed normal cortical activity before LPS and any drug administration as determined by the amplitude, frequency, and shape of the waveforms. However, EEG analysis indicated significantly changes in delta and theta power of the LPS-treated groups, indicative of a disruption of normal brain function. These findings are consistent with the greater degree with acute cell death observed mainly in LPS-treated groups. No focal or epileptiform abnormalities were observed in either group. Aggravated damage of brain cells may disarranges and reduces the synchronization of excitation, and increases the slow-wave component of the EEG. (H. Chen et al., 2012) Our findings are consistent with Van denBoogaard et al. (2010), who found that LPS infusion was associated with predominant slow waves. Furthermore, predominant theta and delta activities were reported to be associated with enhanced cerebral dysfunction and mortality in septic patients. (Hosokawa et al., 2014) The enhanced neuronal cell death, induced by LPS, could be explained by cytokines and glutamate overproduction. This is further supported by in vitro studies demonstrating the effect of LPS as well as cytokines on glutamate level and subsequent neuronal apoptosis (Sharma et al., 2016; Ye et al., 2013). Previous data denoted that prefrontal cortical is the site of interaction between glutamatergic system and the dopaminergic systems, and that dyshomeostasis of glutamatergic neurotransmission within prefrontal area is involved in mental dysfunctions. In our study, photography was done in hippocampus, CA1, CA2, CA3, CA4, dentate and prefrontal cortex regions, however, neuro-inflammation and apoptosis were obviously found in the prefrontal cortical area. These findings, taken together with those published earlier (Gluck et al., 2002; Kashani et al., 2008; Kulijewicz-Nawrot et al., 2013; Kumar et al., 2017; Melendez et al., 2005; Montag et al., 2008; T. Zhang, et al., 2020), all support the hypothesis that prefrontal cortical is the most severely affected during neuroinflammatory process associated with various brain disorders.
Neuronal activity was resumed in galantamine, BAY-117082 as well as cotreated rats. The ability of galantamine and BAY-117082 to reverse the effects of LPS on the EEG waves could be attributed to the reduction of neurotoxicity and the facilitation of the synaptic transmission. This is supported by our results and previous works (Alvarez-Jimenez et al., 2018; Gascoyne et al., 2018; Santos et al., 2002; Semmler et al., 2008; Simpraga et al., 2018) No dissimilarity, however, could be noted in rats receiving single or combined treatments where the increase in EEG activity was similar in all rats. The EEG technique can monitor neurochemical release, uptake and metabolism. A strong correlation exists between EEG frequency patterns and the levels of discrete neuromodulators that reflect brain dysfunction. The dominance of a particular EEG wave patterns depends on receptor subtype and the relative levels of distinct neurotransmitters. Glutamate is the most excitatory neurotransmitter in the brain, but numerous neurotransmitter molecules work constantly to maintain the brain functioning, including cholinergic and monoaminergic (serotonin, dopamine, and noradrenaline) neurotransmitters. Neurodegeneration can result from the alterations in the balance of neurotransmitters (Arai et al., 2004; Harris et al., 2014; Kim et al., 2020; Korte-Bouws et al., 2018; Tyagi et al., 2008; Vakalopoulos, 2014). Inflammation was previously reported to alter the neurotransmitter metabolism, and thus affects frontal neuronal activity (Yamanashi et al., 2021). In our study the combination of BAY-117082 and MOR/GAL synergistically decreased glutamate, whether this synergistic effect exists between BAY-117082 and MOR/GAL on other neurotransmitters need further investigation.
In our study, exposure to LPS and/or different medications modulated microglia and astrocyte activities, remodeled Cxs, Panx-1, and EAATs, and thus affected glutamate homeostasis and neuronal survival. Microglia and astrocyte responses not only contribute to neuronal protection but also exacerbate brain injury (Ikegami et al., 2019). Exaggerated activation of microglia is crucial for the development of an activated phenotype in astrocytes leading to the production of a neurotoxic inflammatory environment (Bachtell et al., 2017; Kirkley et al., 2017; Mayhew et al., 2015). In addition to cytokines, activated microglia release a large amount of glutamate in the extrasynaptic space (Haroon et al., 2017). Astrocytes provide a mechanism of efficient glutamate recycling and form a barrier that prevents the glutamate spillover (Haroon et al., 2017). However, the factors and molecular mechanisms that could regulate glutamate balance are not fully understood.
We and others (Borges et al., 2012; Norden et al., 2016; Takaki et al., 2012) have found that the administration of LPS was associated with increases in astrocyte and microglial activities. Activated microglia colluded with astrocytes, after LPS, to cause the elevation of TNF, IL1, and glutamate and to enhance neuronal cell damage. Compared with other treatments, morphine had less immunosuppressive effects. Similarly, morphine precondition was found to prevent the deleterious effects of LPS on microglia cells (Gwak et al., 2010). Previous data could be explained by a mu (μ) opioid receptor, which could modulate NFKB via promoting induction over degradation of IkB. This effect could be attributed to increasing AP-1, decreasing cAMP levels, and calcium mediated induction of the deubiquitinating enzyme ubiquitin-specific protease 15 (Usp15). Moreover, morphine acting through mu (μ) opioid receptor reduces the amount of Toll like receptor 4 on the immune cells, therefore, affects the NFkB activation (Börner & Kraus, 2013; Y. L. Chen et al., 2006; Eisenstein, 2019; Franchi et al., 2012; Khabbazi et al., 2019; P. Zhang et al., 2020).
Studies on glial cell cultures and brain slices revealed the role of a7 nAChR in regulating their activation (Egea et al., 2015). Similar to our results, galantamine had been shown to prevent glutamate-induced neuronal cell death in the cortex, due to activation of a7 nAChR and reduction of microglia and astrocyte activities (Akaike et al., 2010; Golime et al., 2018; Shimohama & Kawamata, 2018; Takada-Takatori et al., 2006).
The obvious anti-inflammatory effects of galantamine could be attributed to activation of janus kinase2/signal transducer and activator of transcription3 (Jak2/STAT3) pathway, by a7 nAChR, which on the one hand reduces NF-κB nuclear translocation, and on the other hand, stimulates nuclear factor erythropoietin-2-related factor 2/heme oxygenase 1 (Nrf2/HO-1) (a key regulator of oxidative stress) (Egea et al., 2015; Shimohama & Kawamata, 2018). Our findings together with the previous data (Decourt et al., 2016) suggest that galantamine alone, as compared with a combined galantamine and BAY 11-7082, is less sufficient to achieve the most effective control of inflammation.
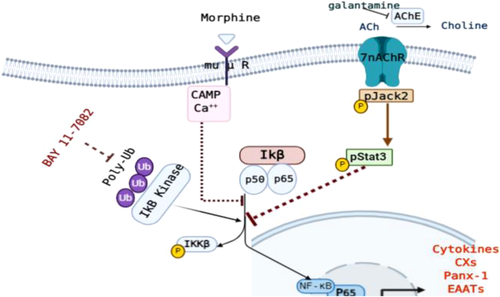
We also showed that a direct blocking phosphorylation of IKKB through targeting components of the ubiquitin system (Ubc13, UbcH7 and LUBAC), using BAY 11-7082, reduced microglial and astrocyte activations. NF-κB is a key signaling event in controlling microglia—astrocyte cross talk. The activation of NF-κB is essential for mediators (such as TNF, IL1, and glutamate) to be produced by activated microglial cells after administration of LPS. The aforementioned mediators induce neuronal damage, leading to reactive microgliosis that drives the neurodegeneration. The inhibition of NF-κB signaling blocks its effects on target inflammatory gene promoters and represses the transcription of activated astrocytes and microglia (Kaltschmidt et al., 2005). Although each single treatment can produce a reduction in NF-κB activity, the overall protective effect of the combined treatment with BAY 11-7082 and galantamine/morphine was obvious, implicating that various mechanistic pathways were involved in the concomitant activations of microglia and astrocyte, as summarized in Figure 8.
For proper neuronal function, it is essential to maintain the homeostasis of glutamate. The microglia and astrocytes act synergistically to regulate glutamate levels. Connexion, pannexin as well as EAATs expressions reflect the ability of an activated microglia and astrocyte cells to control the balance between glutamate uptake and release (Bachtell et al., 2017; Gajardo-Gómez et al., 2016).
Concomitant activation of microglia and astrocyte was found to be associated with increased Cx32 and Cx43 in astrocytes as well as microglia, which potentiate glutamate-induced neurotoxicity. Results obtained in cellular culture models suggest that these particular proteins influence the extent of neurotoxicity. Cx43 and Cx32 activations under pathological conditions facilitate glutamate release and promote neuronal cell death (Freitas-Andrade et al., 2019; Froger et al., 2010; Haroon et al., 2017; Orellana et al., 2011) However, decreased astrocytic Cx43 was found to aggravate cell death and inflammation after stroke (Kozoriz et al., 2010).
In addition to Cxs, Panx-1 seems to be involved in LPS induced neurotoxicity.
Activation of Panx-1 by glutamate is more detrimental to neurons than different mechanisms known to contribute to calcium overload (such as NMDA, AMPA, TRPM, and P2X receptors and CaV1.2 channels) (Orellana et al., 2011; Shestopalov & Slepak, 2014b). Moreover, Panx-1 is recognized as a gateway for molecules released from pathogens to enter and activate NF-κB in glial cells. Activation of Panx-1 leads to sustained cytokines release, which further stimulates Panx-1, thereby perpetuating a vicious circle of inflammation and neurodegeneration (Shestopalov & Slepak, 2014b; L.-Y. Wu et al., 2017). This is further supported by an experimental model of retinal ischemic injury where genetic ablation of Panx-1 was associated with attenuation of interleukin production and protection of retinal neurons from injury (Dvoriantchikova et al., 2012).
Consistent with studies (Haroon et al., 2017; Olmos & Lladó, 2014), releasing of TNF-α and IL1 resulted in concomitant downregulation of EAATs and increased glutamate levels. This is explained by the crucial role played by EAATs in the glutamate reuptake, which prevents glutamate spillover from the synapse (ODonovan et al., 2017).
Downregulations of Cxs and Panx-1 and upregulation of EAATs were observed after NF-κB suppression. These data, in conjunction with the pattern of astrocyte and microglia activities, strongly implicate the NF-κB pathway as an important component of glutamate homeostasis. Our results are in line with previous studies showed that NF-κB inhibitor BAY 11-7082 significantly downregulated Cx43 expression in H9c2 cardiomyocytes (X. Wu et al., 2013; Zhao et al., 2006) Moreover, NF-κB blockage alleviates TNF induced glutamate neurotoxicity by increasing EAATs expression and functionality (Haroon et al., 2017).
Our data support evidence for association of inflammation with neurotoxic astrocyte profile, which is characterized by the upregulation of Cxs and downregulation of glutamate transport (EAATs) (Gomes et al., 2019; Tolosa et al., 2011; Trias et al., 2018). Inflammation also led to an increased Panx-1 that provides a positive inflammatory feedback loop (Li et al., 2018; L.-Y. Wu et al., 2017). NFKB is the main pathway that target genes, which are involved in inflammation development and progression. We propose that NFkB is likely a central regulator of Cxs, EAATS and Panx-1 levels during inflammation. Thus, NF-κB inhibition by single or combined treatments restored the glutamate level increased by altered transporter expression levels. We suggest that each treatment could act directly or indirectly to block IkB phosphorylation and stabilize NFKB, but the combination of Bay 11-7082 and MOR/GAL may exhibit enhanced efficacy in suppression P-IkB/NFKB pathway and restoring glutamate imbalance caused by altered transporters (Figure 8).
5 CONCLUSIONS
In this study, we demonstrated that direct IKK inhibition ameliorates inflammatory induced neurotoxicity. NF-κB signaling pathway can serve as a key target for therapeutic intervention, due to its ability to control multiple genes involved in glutamate release and reuptake. It is plausible to believe that Cxs, Panx-1, and EAATs hold great potential in regulating glutamate neurotoxicity. Thus, the remodeling of the expressions of Cxs, Panx-1, and EAATs represents an unexplored approach to prevent neuronal cell death during neuroinflammation. Both morphine and galantamine partially alleviate NF-κB activation, reduce cytokines release and modulate Cxs, Panx-1, and EAATs expressions, and giving BAY 11-7082 along with them enhanced their effects. We anticipate our findings will provide novel approaches to diagnosis and therapeutic strategies for several inflammatory-related neurological disorders.
ACKNOWLEDGMENTS
The skilful technical assistance of Afaf, Aza and Tarek is appreciated. This work was supported by research grants from the Faculty of Medicine, Cairo University.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Maha Gamal, Shimaa Magdy, Nancy F. Samir, and Nivin Sharawy: contributed to the study conception and design. Shimaa Magdy, Laila Rashed, Basma Emad Aboulhoda, Haitham S. Mohammed, Nancy F. Samir, and Nivin Sharawy: collected the data. Nivin Sharawy: analyzed the data and presented the results. All the authors have participated in paper writing and revision. All authors read and approved the final manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The raw data that support the findings of this study are available from the corresponding author upon reasonable request.




