The NLRP3 inflammasome: Multiple activation pathways and its role in primary cells during ventricular remodeling
Abstract
Inflammasomes are a group of multiprotein signaling complexes located in the cytoplasm. Several inflammasomes have been identified, including NLRP1, NLRP2, NLRP3, AIM2, and NLRC4. Among them, NLRP3 was investigated in most detail, and it was reported that it can be activated by many different stimuli. Increased NLRP3 protein expression and inflammasome assembly lead to caspase-1 mediated maturation and release of IL-1β, which triggers inflammation and pyroptosis. The activation of the NLRP3 inflammasome has been widely reported in studies of tumors and neurological diseases, but relatively few studies on the cardiovascular system. Ventricular remodeling (VR) is an important factor contributing to heart failure (HF) after myocardial infarction (MI). Consequently, delaying VR is of great significance for improving heart function. Studies have shown that the NLRP3 inflammasome plays an essential role in the process of VR. Here, we reviewed the latest studies on the activation pathway of the NLRP3 inflammasome, focusing on the effects of the NLRP3 inflammasome in primary cells during VR, and finally discuss future research directions in this field.
1 INTRODUCTION
Ventricular remodeling (VR) is a progressive pathological change of the heart structure, which can develop in three different modes according to different underlying diseases, including concentric remodeling, eccentric hypertrophy, and myocardial infarction (MI; Galli & Lombardi, 2016). The process of VR is complicated, but the main underlying changes are found in cardiac microstructures, including cardiomyocytes apoptosis, cardiac fibrosis, vascular endothelial damage, and extracellular matrix deposition (Ma et al., 2017; Z. Wang et al., 2019). Chronic kidney disease and obesity are also thought to be related to the occurrence of VR in addition to MI, hypertension, and diabetes (Bajaj et al., 2020; Sarashina et al., 2017; Schlett et al., 2018; Yoshie et al., 2020). A large number of clinical and experimental studies have shown that inflammation plays an essential role in VR, and the NLRP3 inflammasome has received widespread attention as a central molecule in the inflammatory response. Perhaps unsurprisingly, studies have demonstrated that the NLRP3 inflammasome is large amount activated during VR and promotes its progression (Sokolova et al., 2019; Yang et al., 2017).
The NLRP3 inflammasome is a multiprotein complex that interacts with caspase-1 to promote the maturation and release of pro-inflammatory factors such as IL-1β and IL-18, which in turn induce pyroptosis (Haque et al., 2020; Kelley et al., 2019). The NLRP3 inflammasome is activated by a wide range of stress-related injuries and immunological inflammation, with various activation pathways. According to current research, the primary activation pathways includes microRNAs (miRNAs), long Noncoding RNAs (lncRNAs), mitochondrial reactive oxygen species (ROS), and ions (Bhatta et al., 2020; Z. Chen et al., 2019; Wu et al., 2020; Ying et al., 2019). Recent studies have found that activation of the NLRP3 inflammasomes contributes to the fundamental structural changes of VR. For example, cardiac pressure overload causes Ca2+ influx, which triggers the activation of the NLRP3 inflammasome in cardiomyocytes, leads to the recruitment of cardiac macrophages, and promotes myocardial fibrosis (Suetomi et al., 2018). The downregulation of miRNA-495 after MI promotes the u-regulation of the NLRP3/caspase-1/IL-1β pathway, aggravates the death of vascular endothelial cells (VECs), and promotes endothelial dysfunction (Zhou et al., 2018). These findings demonstrate that NLRP3 inflammasome activation plays an important role in the VR process. Therefore, it is vital to clarify the relationship between the NLRP3 inflammasome and VR for developing effective NLRP3 inflammasome inhibitors and slowing the progression of heart failure (HF).
In this review, we introduce the structure and function of the NLRP3 inflammasome in detail and describe the underlying activation pathways, including the roles of miRNAs, lncRNAs, lysosomes, mitochondria, Ca2+, and K+. Based on convincing evidence, we discuss the effects of NLRP3 inflammasome activation on the heart's microstructure and provide a comprehensive model of how the NLRP3 inflammasome promotes VR through the primary cells. Finally, we introduce possible future directions that may be worth studying.
2 STRUCTURE AND FUNCTION OF THE NLRP3 INFLAMMASOME
The NLRP3 inflammasome is a multiprotein complex that is mainly located in the cytoplasm and widely expressed in many cell types. It can be activated through pathogen-related molecular patterns (PAMP) or damage-related molecular patterns (DAMP) to mediate the maturation and release of the pro-inflammatory factors IL-1β and IL-18 (Eren & Ozoren, 2019; Swanson et al., 2019; Yang et al., 2020). The NLRP3 inflammasome is composed of three parts, a sensor, adapter, and effector. The trisomic NLRP3 protein, which belongs to the NLR protein family, acts as a sensor for the NLRP3 inflammasome. It contains a central nucleotide-biding domain (NBD), a carboxy-terminal leucine-rich repeat (LRR) domain, and a variable amino-terminal pyrin domain (PYD). The variability of the PYD makes NLRP3 different from other sensors in the NLR protein family. Apoptosis-associated speck-like protein, which contains a caspase recruitment domain (ASC), is an adapter of the NLRP3 inflammasome that consists of a caspase-1 protease recruitment domain (CARD) and a PYD. Pro-caspase-1 is the effector of the NLRP3 inflammasome. It consists of a CARD, a large central catalytic domain (P20), and a small carboxy-terminal catalytic subunit domain (P10). PYD and CARD can be stimulated to induce aggregation by PAMPs derived from bacteria and viruses, or DAMPs such as adenosine triphosphate (ATP), oxidized mitochondrial DNA (ox-mtDNA), and α-synuclein. When NLRP3 is stimulated, it forms oligomers and the inhibitory state is released. The PYD of NLRP3 combines with the PYD of ASC to aggregate it, and ASC recruits pro-caspase-1 through its CARD to form a CARD-CARD linker, eventually forming the NLRP3 inflammasome (Figure 1). This assembly is different from NLRP1 and NLRC4, which have their own CARD sensors, and is also called assembly dependent on ASC adaptors (de Alba, 2019; Sharif et al., 2019; Swanson et al., 2019).
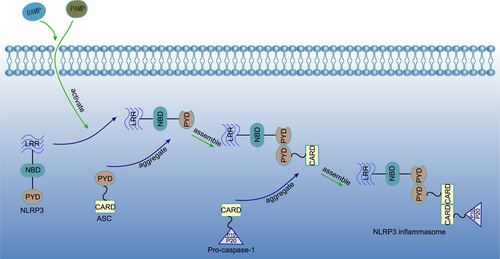
As a key sensor of immunological inflammation, NLRP3 is considered to be related to many diseases. In Alzheimer's disease, the accumulation of amyloid β can activate the NLRP3 inflammasome, leading to the production of IL-1β and inducing tau hyperphosphorylation. This in turn reduces the plasticity of synapses, causing memory impairment (Ising et al., 2019; Ren et al., 2019; Stancu et al., 2019). In the course of chronic fibrotic diseases, redox imbalance leads to the activation of the NLRP3 inflammasome, promoting collagen deposition, and amplifying the pro-inflammatory reaction cascade, which promotes fibrosis through the NLRP3/IL-1β axis (Cai et al., 2016; Meng et al., 2019). Continuous NLRP3 overexpression was found in experimental models of inflammatory bowel disease (IBD) and affected patients. There are complex interactions between the NLRP3 inflammasome, mucosal immune response, and intestinal homeostasis. At the initial stage, when the integrity of the intestinal epithelium is destroyed, the activation of the NLRP3 inflammasome can promote the repair and regeneration of intestinal mucosa. However, it appears to enhance the inflammatory response with the chronic progression of the disease, which may be related to thioredoxin interacting protein (TXNIP) dependent NLRP3 inflammasome activation. However, various research teams have come to different conclusions. Studies have shown that these different findings may be related to differences in the intestinal flora between individuals (Zhao et al., 2019; Zhen & Zhang, 2019). In addition, recent research results indicate that NIMA-related kinase 7 (NEK7) in macrophages can easily bind NLRP3 to activate the NLRP3 inflammasome. This is considered a new direction for the study of IBD (X. Chen et al., 2019). Moreover, the activation of the NLRP3 inflammasome has been deeply studied in the fields of atherosclerotic diseases, viral infectious diseases, diabetes, kidney disease, and cancer (Akosile et al., 2020; Coulon et al., 2019; M. Wang, Yang, et al., 2019; Yu et al., 2020).
3 NLRP3 INFLAMMASOME ACTIVATION PATHWAYS
3.1 Organelles
3.1.1 Lysosomes
Desorption or rupture of the lysosome membrane can activate the NLRP3 inflammasome (Baljon et al., 2019; Katsnelson et al., 2016; Zamani et al., 2020). After the lysosome is destroyed, a variety of factors are released into the cytoplasm. Among them, lysosomal cysteine cathepsins play an important role. Cysteine cathepsins are a family of proteases, among which cathepsins B, C, L, S, and Z are the most relevant for NLRP3 inflammasome activation (Figure 2). These five cathepsins complement each other and can also independently activate the NLRP3 inflammasome (Cao et al., 2019; Liao et al., 2019). Cathepsin B has a C-terminal exopeptidase activity, and it can bind to the LRR of NLRP3, and acts as a direct ligand to cause the activation of the NLRP3 inflammasome (Bruchard et al., 2013; Zamani et al., 2020). Cathepsin B−/− animals have been used to prove that cathepsin B is involved in NLRP3 inflammasome activation and that its overexpression can increase the production of caspase-1 (p10) subunits in adipocytes (Mizunoe et al., 2017). Pretreatment of cells with the cathepsin inhibitor CA-074Me significantly reduced the release of IL-1β. However, since CA-074Me is not specific for cathepsin B, this experiment cannot rule out the effects of other cathepsins on the activity of the NLRP3 inflammasome (Baljon et al., 2019; Shridas et al., 2018). Cathepsin B is a bidirectional regulator of lysosome biogenesis and autophagy, and the balance between them will determine whether the NLRP3 inflammasome is activated (Qi et al., 2016). Cathepsin C is involved in the synthesis of IL-1β in neutrophils through the processing of upstream proteases. Cathepsin C−/− mice show reduced IL-1β levels, and cathepsin C can increase the activity of caspase-1. However, the lack of cathepsin C cannot completely eliminate the production of IL-1β (Campden & Zhang, 2019). Cathepsin L may compensate for cathepsin B in some cases and play an indirect role in the activation of the NLRP3 inflammasome (Tang, 2018). Cathepsin S can compensate for the loss of other cathepsins in the activation of NLRP3 inflammasome. Cathepsin Z is nonredundant for the activation of the NLRP3 inflammasome by melanomycin, ATP, and uric acid. Still, it is not clear whether cathepsin Z has catalytic activity under these conditions. Due to the unique properties of cathepsin Z, this enzyme may independently promote NLRP3 inflammasome activation through another mechanism (Allan et al., 2017; Orlowski et al., 2017). The instability and rupture of lysosome membranes are the basis of NLRP3 activation, and the released cathepsins activate the NLRP3 inflammasome through cross-reactive and overlapping signal transduction pathways.
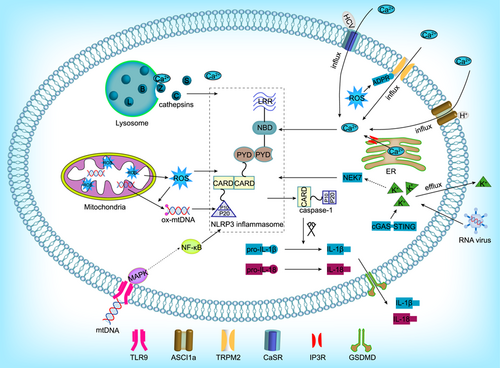
3.1.2 Mitochondria
Mitochondria are considered the main source of ROS in cells, and their dysfunction can lead to large ROS increases (Qiu, Luo, et al., 2016). The excessive production of ROS is an important upstream event and a potent activator of the immune system. ROS destroy mtDNA and interact with the NLRP3 inflammasome during inflammation, activating it to amplify the maturation and release of IL-1β (Liu et al., 2017; Qiu, Ji, et al., 2016; Xu et al., 2017). In studies on neurodegenerative diseases, it was found that mitochondrial damage is a key mechanism of neurodegeneration. Consequently, the ROS/NLRP3 inflammasome/IL-1β signaling pathway may be a potential drug target for the prevention and treatment of postoperative cognitive dysfunction (Gao et al., 2017; Liu et al., 2017; Wei et al., 2019). In a model of H9C2 cardiomyocyte injury induced by high glucose levels, the overexpression of mitochondrial aldehyde dehydrogenase 2 (ALDH2) was found to eliminate the accumulation of 4-hydroxynonenal (4-HNE) and lactate dehydrogenase (LDH), which reduces the production of ROS, thereby inhibiting the activation of the NLRP3 inflammasome. In the same study, the protein levels of ASC, Caspase-1, and IL-18 were significantly reduced, effectively supporting this conclusion (Cao et al., 2019). In microglia, mitochondrial membrane permeability transition pore (MPTP) treatment promoted the binding of NLRP3 to ASC, which led to the activation of the NLRP3 inflammasome, and ROS production was found to mediate this process. Cell-culture experiments using the MitoTEMPO ROS scavenger also confirmed this hypothesis (Lee et al., 2019). The mechanism of ROS-dependent NLRP3 inflammasome activation has been extensively studied. The overproduction of ROS is sensed by the complex of thioredoxin (TRX) and TXNIP. After the decomposition of the complex, TXNIP binds to the LRR of NLRP3, resulting in the activation of the NLRP3 inflammasome (Minutoli et al., 2016). Another potential mechanism of mitochondrial NLRP3 inflammasome activation is the release of mtDNA. Mitochondrial MPTP is destroyed upon mitochondrial dysfunction, after which mtDNA from the mitochondria enters the cytoplasm and is oxidized. Intracytoplasmic ox-mtDNA can directly activate the NLRP3 inflammasome to trigger the innate immune response (Y. Wang, Meng, et al., 2019). Unlike intracytoplasmic mtDNA, extracellular mtDNA can interact with toll-like receptor 9 (TLR9) to induce activation of mitogen-activated protein kinases (MAPK) and nuclear factor-κB (NF-κB), which promote NLRP3 inflammasome activation (Figure 2). Although the molecular mechanism by which TLR9 activates NLRP3 is not clearly known, inhibition of TLR9 can significantly reduce the activation of inflammasomes and inflammatory pancreatic injury in mice with acute pancreatitis (G. Wu et al., 2019). Although the exact pathway of mitochondrial NLRP3 inflammasome assembly and activation is still unclear, promoting mitochondrial autophagy, inhibiting the overproduction of ROS, and release of mtDNA can at least be considered the main directions for studying the inhibition of NLRP3 inflammasome activation.
3.2 Ions
3.2.1 Ca2+
Many studies have shown that Ca2+ flux is the key to promoting NLRP3 inflammasome activation. The concentration of Ca2+ is low in the cytoplasm, which allows the spontaneous flux of Ca2+ from the extracellular space or intracellular storage vesicles. Intracellular Ca2+ is mainly derived from the endoplasmic reticulum (ER). The inositol triphosphate receptor (IP3R) is a Ca2+ channel mainly located in the ER. It can activate the NLRP3 inflammasome by releasing Ca2+ into the cytoplasm under stress-related stimulation (Wang et al., 2020). Studies have confirmed that NLRP3 inflammasome activation mediated by Ca2+ can be blocked by inhibiting the IP3R channel (Gong et al., 2018). Another source of intracellular Ca2+ is the lysosomes. Membrane instability caused by particulate matter can cause Ca2+ to enter the cytoplasm from lysosomes, thereby activating the NLRP3 inflammasome. In addition to Ca2+ stored in the cell, Ca2+ influx from the extracellular space also participate in the activation of the NLRP3 inflammasome and the release of cytokines. Accordingly, removing Ca2+ from the extracellular environment or intracellular storage pool, thereby inhibiting Ca2+ influx into the cytoplasm, has been shown to inhibit ASC oligomerization and pro-caspase-1 processing (Bagur & Hajnoczky, 2017; Zhang et al., 2017). Ca2+ enters the cytoplasm through different mechanisms and becomes a key point for NLRP3 activation. Ca2+ can activate the NLRP3 inflammasome by activating channel proteins. Under stress, intracellular ROS increase and promote the production of ADP ribose (ADPR). ADPR binds to the TM region of transient receptor potential channel protein M2 (TRPM2) in the cytoplasm to activate it, causing the influx of extracellular Ca2+, which in turn promotes the assembly of the NLRP3 inflammasome. Accordingly, knockout or inhibition of TRPM2 was found to eliminate this pathological process (Wang et al., 2020; M. Wang, Zhang, et al., 2019). The calcium-sensing receptor (CaSR) is a widely distributed G protein-coupled receptor that plays a key role in maintaining Ca2+ homeostasis. Ca2+ is the main agonist of CaSR. In THP-1 macrophages, CaSR activation increases the mRNA expression of NLRP3 and ASC (D'Espessailles et al., 2020). In hepatitis, Ca2+ influx is an intermediate link in the activation of the NLRP3 inflammasome in liver macrophages. Hepatitis C virus (HCV) core protein can promote the influx of Ca2+, which can activate and transmit the phospholipase-C signal, promoting NLRP3 inflammasome assembly, and the maturation of IL-1β. This in turn activates the IL-1β release signal from macrophages, thus establishing the inflammatory tissue microenvironment typical of hepatitis (Negash et al., 2019). HCV core protein may affect the level of Ca2+ in the cytoplasm by regulating CaSR (D'Espessailles et al., 2018). Additionally, extracellular Ca2+ can be released into the cytoplasm under certain stress conditions. Acid-sensitive ion channel 1a (ASCI1a) is a cation channel-specific for extracellular acidosis, which can transport Ca2+ and increase the intracellular Ca2+ content (Bhowmick et al., 2017; Gonzalez-Inchauspe et al., 2017). The accumulation of inflammatory metabolites in the joints causes the pH of the joint fluid to decrease, which activates ASIC1a and Ca2+ influx, promoting the expression of ASC, NLRP3, and caspase-1 (p10). (Figure 2) The use of chelating agents can alleviate this phenomenon (X. Wu et al., 2019). However, some studies have found that Ca2+ plays a dual role in the activation of the NLRP3 inflammasome. Ca2+ can promote cyclic adenosine phosphate (cAMP) synthesis by activating soluble adenylate cyclase, thereby inhibiting the activation of the NLRP3 inflammasome. In this regard, the balance of Ca2+ and cAMP signaling determines the exact result of NLRP3 inflammasome activation (Xu et al., 2019).
3.2.2 K+
The activation of NLRP3 the inflammasome by K+ has an opposite concentration response to that of Ca2+, since K+ efflux triggers the activation of the NLRP3 inflammasome and pyroptosis. NEK7 is an important downstream mediator of K+ efflux. NEK7 bridges adjacent NLRP3 subunits, thus mediating the activation of the NLRP3 inflammasome (H. Liu et al., 2019; Sharif et al., 2019), and this reaction can be prevented by inhibiting the formation of the NEK7-NLRP3 complex. When primary cortical neurons were transfected with NEK7-shRNA and switched to medium with no K+ or high K+, there was no K+ efflux into the medium, and IL-1β was released. When the K+ efflux stopped, IL- 1β release decreased, and the K+ efflux directly increased the upregulation of NEK7 expression and the activation of caspase-1. It also triggered the formation of the NEK7-NLRP3 complex, but K+ efflux did not affect the activation of caspase-1 in NEK7 knockout cells. Stimulation of primary cortical neurons with lipopolysaccharide (LPS) + ATP can induce similar K+ efflux, and knockdown of the NEK7 gene leads to low levels of IL-1β release. A high concentration of KCl can inhibit K+ efflux, which was found to also inhibit the formation of the NLRP3-NEK7 complex, and eliminate the activation of caspase-1 (Y. Chen et al., 2019). These data suggest that K+ efflux triggers NEK7, which is necessary for caspase-1 activation and NLRP3 inflammasome assembly. The replication of cytopathogenic RNA viruses, such as vesicular stomatitis virus or encephalomyocarditis virus, promotes necrosis or lytic cell death and K+ efflux, which in turn activate the NLRP3 inflammasome and IL-1β maturation. The critical step in the induction of NLRP3 inflammasome activation by RNA viruses is K+ efflux caused by the cytotoxic effect of its replication. In fact, inflammasome activation is negligible when the infection does not promote cell death (da Costa et al., 2019; Figure 2). Like cytopathogenic RNA virus infection, stimulation of the cGAS-STING axis in human myeloid cells can induce a cell death program and initiate K+ efflux upstream of NLRP3 (Gaidt et al., 2017). Rupture of the plasma membrane and K+ efflux from the cells are necessary and sufficient upstream signaling events during the activation of the NLRP3 inflammasome, which is followed by the release of the highly inflammatory cytokines IL-1β and IL-18 to produce a series of effects.
3.3 Noncoding RNAs
3.3.1 miRNAs
miRNAs are an evolutionarily conserved group of noncoding RNAs that fine-tune important physiological responses by targeting the expression of key genes at the posttranscriptional level. Many miRNAs have been confirmed to act as regulators of immune and inflammatory responses. There is a close relationship between the expression of specific miRNAs and activation of the inflammatory response (Wang et al., 2016). Recent studies have shown that miRNAs are involved in the regulation of NLRP3 inflammasome activation. miRNA-155 enhances inflammatory signals by targeting the two negative regulators SHIP1 and SOCS1. Some miRNAs targeting negative regulators of the NLRP3 inflammasome are upregulated after inflammasome activation. These results suggest that miRNAs can promote NLRP3 inflammasome activation, which may be mediated by specific negative modulators targeting the inflammasome (Ojcius et al., 2019). Many miRNAs are considered to be key regulators of sepsis, and miRNA-21 is upregulated under these conditions. Further studies have found that miRNA-21 can participate in the initial stage of NLRP3 inflammasome activation by regulating tumor necrosis factor α-induced protein 3(A20), and can also control the assembly of several protein complexes, including NLRP3, ASC, and caspase-1, by regulating the formation of ASC (Xue et al., 2019). miRNA-21 also induces pyroptosis by targeting A20 to block the gasdermin-D (GSDMD) mediated release of caspase-1, which in turn cleaves GSDMD and releases the cleaved GSDMD-N domain to induce pyroptosis (Tonnus & Linkermann, 2017). In angiotensin II-induced liver fibrosis, miRNA-21 can promote the assembly of NLRP3 inflammasome complexes by targeting sprouty1 and Smad7 (Ning et al., 2017). miRNA-335 is closely related to oxidative stress (Y. Liu et al., 2019) and was found to be overexpressed in non-small cell lung cancer cells, where it can mediate tumor cell proliferation and apoptosis through the miRNA-335/SOD2/ROS signaling pathway. miRNA-335 inhibitors and sponging by lncRNA XIST can reverse this phenomenon, which is thought to be related to the activation of the NLRP3 inflammasome by miRNA-335 (J. Liu, Yao, et al., 2019; Yu et al., 2019). Therefore, miRNAs can not only directly bind to specific sites of NLRP3 to regulate inflammasome assembly, but also indirectly activate the NLRP3 inflammasome by regulating intermediate molecules, demonstrating the possibility of bidirectional NLRP3 inflammasome regulation under different pathological conditions (Tables 1 and 2).
| miRNA/lncRNA | Activation pathways | Key effects | Key references |
|---|---|---|---|
| miRNA-155 | Regulate SHIP1 and SOCS1 | Enhance NLRP3 inflammasome activation signal | (Ojcius et al., 2019) |
| miRNA-21 | Regulate A20 | Promote ASC protein synthesis and improve caspase-1 activity | (Tonnus & Linkermann, 2017) |
| miRNA-335 | SOD2/ROS axis | Direct activation of NLRP3 inflammasome | (J. Liu, Yao, et al., 2019) |
| lncRNA(NEAT1) | Sponging miRNA-3076-3P | Promote NLRP3, ASC, and caspase-1 protein expression | (M. Zhang et al., 2019) |
| Transfer of itself from the nucleus to the cytoplasm | Directly promote NLRP3 inflammasome assembly | (P. Zhang et al., 2019) | |
| lncRNA (H19) | Sponging miRNAs and regulate PDCD4 | Raise caspase-1 cutting pro-IL-1β and pro-IL-18 | (Wan et al., 2020) |
| lncRNA(LINC00339) | Sponging miRNA-22-3P | Same as above | (Z. Song et al., 2019) |
| lncRNA(LINC00969) | miRNA-335-3P/TXNIP axis | Activation of NLRP3 inflammasome | (Yu et al., 2019) |
| lncRNA (XIST) | Sponging miRNA-335 | Same as above | (J. Liu, Yao, et al., 2019) |
| lncRNA (ANRIL) | miRNA-122-5P/BRCC3 axis | Decrease the level of NLRP3 protein ubiquitination | (Hu et al., 2019) |
- Abbreviations: ASC, apoptosis-associated speck-like protein, which contains a caspase recruitment domain; BRCC3, complex subunit 3; PDCD4, programmed cell death 4; ROS, reactive oxygen species; SHIP1, SH-2 containing inositol 5ʹ-polyphosphatase 1; SOCS1, suppressor of cytokine signaling 1; A20, tumor necrosis factor α-induced protein 3; SOD2, superoxide dismutases 2; TXNIP, thioredoxin interacting protein.
| Conditions | Mechanisms | Impacts | Key references |
|---|---|---|---|
| I/R | Release ROS and activated caspase-1 | Cardiomyocyte pyroptosis | (Ren et al., 2020; X. Wang et al., 2019) |
| TXNIP activation, inhibition TRX-1, and ROS↑ | |||
| NLRP3 inflammasome and mitochondria cross-talk and NLRP3 protein↑ | Cardiomyocyte damage and necrosis | (Darwesh et al., 2019; Wang et al., 2017) | |
| TXNIP activation NLRP3 inflammasome and inhibition TRX-2 | |||
| Mitochondrial dysfunction, Mfn-2↑, and NLRP3 inflammasome associated protein↑ | |||
| MI | Cell rupture, Ca2+ outflow, CaSR activation, NLRP3 inflammasome activation, and IL-1R↑ | Myocardial cell damage, necrosis, enlarged infarct size | (Ong et al., 2018; Weil & Neelamegham, 2019) |
| Trigger macrophages AMPK/ERK1/2 axis | |||
| M0 type macrophages are transformed into M1 | |||
| T2DM | Trigger MALAT1/miRNA/NLRP3 axis | VECs pyroptosis | (Xin et al., 2020; Y. Z. Zhang et al., 2019) |
| Obesity | PA↑ | Decreased cardiomyocyte viability and apoptosis | (Mangali et al., 2019; Y. Z. Zhang et al., 2019) |
| CRP | Trigger NF-κB/ROS/NLRP3 axis, promote the adhesion of leukocytes to the vascular endothelium, and promote the transfer of LDL to the subendothelial | Exacerbate AS, damage VECs | (Bian et al., 2019; Stachon et al., 2017) |
| LPS | Combine TLR4, trigger ROS/TXNIP/NLRP3/HMGB1 axis, and inhibition ZO-1/2 | Loss of vascular endothelial tightness | (X. Wang et al., 2019; X. Zhou et al., 2019) |
| Ox-LDL | Trigger the AMPK/PP2A axis of macrophages | Exacerbate AS, damage VECs | (J. Song et al., 2019; L. Zhang, Lu, et al., 2019) |
| Trigger the MEK1/ERK1/2 axis of macrophages | |||
| Nicotine | Trigger ROS/TXNIP/NLRP3/HMGB1 axis and inhibition ZO-1/2 | Loss of vascular endothelial tightness | (Luo et al., 2006; Y. Zhang et al., 2019) |
| Lysosome ruptures, release of cathepsin B, NLRP3 inflammasome activation, and inhibition ZO-1/2 | |||
| PM2.5 | M0 type macrophages are transformed into M1 | Chronic damage and necrosis of cardiomyocytes | (Alhamdi et al., 2019; L. Du et al., 2019) |
| High-salt diet | NFAT5↑ and binding to NLRP3 mRNA promoter | VECs injury | (Berry et al., 2017; Ma et al., 2019) |
| Daily stress | α1-AR↑, NADPH oxidase↑, and NLRP3 inflammasome associated protein ↑ | Cardiomyocyte damage and necrosis | (Xin et al., 2020) |
- Notes: The table is mainly described in three levels. The first level refers to diseases, including I/R, MI, T2DM, and obesity. The second level refers to molecules, including CRP, LPS, and ox-LDL. The third level refers to external factors, including nicotine, PM2.5, high-salt diet, and daily stress.
- Abbreviations: ↑, indicates upregulation; α1-AR, α1-adrenergic receptor; AMPK, adenosine monophosphate-activated protein kinase; AS, atherosclerosis; CaSR, Calcium-sensing receptor; CRP, C-reactive protein; ERK1/2, extracellular signal-regulated kinase 1/2; HMGB1, high mobility group box 1; I/R, ischemia–reperfusion; LPS, lipopolysaccharide; MEK1, mitogen-activated protein kinase 1; Mfn-2, mitofusins-2; MI, myocardial infarction; NADPH, nicotinamide adenine nucleotide phosphate; NF-κB, nuclear factor-κB; NFAT5, nuclear factor of activated T cells 5; ox-LDL, oxidized low-density lipoprotein; PA, palmitic acid; PP2A, protein phosphatase 2A; ROS, reactive oxygen species; T2DM, type 2 diabetes mellitus; TLR4, toll-like receptors 4; TRX-1, thioredoxin-1; TRX-2, thioredoxin-2; TXNIP, thioredoxin interacting protein; VEC, vascular endothelial cell; ZO-1/2, zonula occludens-1/2.
3.3.2 LncRNAs
LncRNAs are a subclass of noncoding RNAs, ranging in size between 200 bp and 10 kb, which are involved in the regulation of man cellular activities, such as gene expression, cell growth, differentiation, pyroptosis, and tumor progression (X. Li et al., 2017; Tang et al., 2020; Y. Zhang et al., 2018). In cell-culture experiments, RNA-seq analysis showed that lncRNA NEAT1 could promote NLRP3 inflammasome activation by inhibiting the expression of miRNA-3076-3p. Treating cells with miRNA-3076-3p mimics can reverse the increased expression of NLRP3, caspase-1, and ASC promoted by NEAT1. In animal experiments, knocking down NEAT1 reduced the inflammatory response in an experimental autoimmune model of myocarditis, which was found to be related to the regulation of NLRP3 inflammasome activation. NEAT1 acts as a competitive endogenous RNA (ceRNA) that reduces miRNA-3076-3p by promoting NLRP3 expression (M. Zhang et al., 2019). In recent years, there has been increasing evidence that ceRNAs act as a bridge between lncRNAs and miRNAs (Wang et al., 2017, 2018; Xi et al., 2017). The lncRNA H19 plays an important role in the induction of inflammation following ischemia–reperfusion (I/R), where it forms a ceRNA network in the ischemic cascade. H19 acts as a ceRNA that sponges miRNAs to promote the expression of programmed cell death 4 (PDCD4), thus activating the NLRP3 inflammasome and triggering neuroinflammation. In addition, H19 can promote the phosphorylation and nuclear transfer of transcription factors, thus increasing the expression of target genes, and recruiting more caspase-1 to cleave pro-IL-1β and pro-IL-18 into their respective mature forms, leading to aseptic inflammation (Wan et al., 2020). Another study in HK-2 cells confirmed the relationship between the lncRNA LINC00339 and miRNA-22-3p. Bioinformatic analysis indicates that LINC00339 contains a possible binding site for miRNA-22-3p (Z. Song et al., 2019). In addition, the lncRNA LINC00969 was shown to promote the activation of the NLRP3 inflammasome through the miRNA-335-3p/TXNIP axis in intervertebral disk degeneration (Yu et al., 2019). The activation of the NLRP3 inflammasome can be regulated by de-ubiquitination (Humphries et al., 2018). The ubiquitinase BRCA1-BRCA2, which contains complex subunit 3 (BRCC3), can reduce the ubiquitination level of NLRP3 protein and thereby promote the activation of the NLRP3 inflammasome (Cheng et al., 2020). This process was confirmed to be related to the lncRNA ANRIL, encoded at the INK4 locus. ANRIL promotes the activation of the NLRP3 inflammasome by sponging miRNA-122-5p to upregulate the expression of BRCC3. When HK2 cells were transfected with an ANRIL overexpression vector, the protein levels of IL-1β, IL-18, and NLRP3 were significantly upregulated, while silencing ANRIL had the opposite effect (Hu et al., 2019). During direct activation by an inflammasome activation signal, the lncRNA Neat1 dissociates and translocates from the nucleus of macrophages to the cytoplasm and directly binds to the NLRP3 inflammasome to promote assembly and subsequent caspase-1 zymogen processing, which promotes IL-1β production and pyroptosis (P. Zhang et al., 2019). Finally, evidence of lncRNAs activating the NLRP3 inflammasome has been widely found in cancer studies (Tang et al., 2020; Xu & Xi, 2019).
4 REGULATORY EFFECTS OF THE NLRP3 INFLAMMASOME IN PRIMARY CELLS DURING VR
VR is an adaptive response of the heart in a pathological state, leading to secondary ventricular systolic dysfunction during the process of injury repair. Many cells are involved in this complex process, including cardiomyocytes, macrophages, and VECs (X. Zhang et al., 2019). Severe VR can lead to a poor prognosis and even death, and the NLRP3 inflammasome plays a regulatory role in primary cells during VR and accelerates its progression.
4.1 Effects of the NLRP3 inflammasome on the remodeling of cardiomyocytes
Myocardial I/R injury is characterized by noninfectious damage to cardiomyocytes. At the initial moment of myocardial I/R, the burst of ROS in the damaged mitochondria can induce the oligomerization and assembly of NLRP3, promote the activation of caspase-1, release IL-1β, trigger cardiac inflammation, and promote cardiomyocyte death by pyroptosis. Accordingly, inhibiting the expression of NLRP3 protein can protect cardiomyocytes from I/R injury. Studies have shown that NLRP3 upregulation after I/R is due to the crosstalk between the NLRP3 inflammasome and mitochondria, which is mediated by the ROS produced by dysfunctional mitochondria. Further studies have also confirmed that cytochrome P450 derived epoxy derivatives of eicosapentaenoic acid and docosahexaenoic acid can alleviate cardiomyocyte injury by maintaining mitochondrial function, which in turn inhibits the activation of the NLRP3 inflammasome. This protective effect may also be related to the reduction of ROS levels (Wang et al., 2017). AS an activator and oxidant of the NLRP3 inflammasome, TXNIP participates in the I/R injury cascade. In recent studies, TXNIP protein expression in cardiomyocytes and mitochondria was found to increase significantly after ischemia, which is consistent with the increase in NLRP3 inflammasome activation. Under normal physiological conditions, TXNIP is mainly located in the nucleus. Under I/R stress, TXNIP is transported into the cytoplasm, where it inhibits the antioxidant activity of TRX-1 and activates the NLRP3 inflammasome. This shuttles TXNIP to the mitochondria, where it inhibits TRX-2 activity, and initiates the death process of cardiomyocytes (Ren et al., 2020; X. Wang et al., 2019; Ye et al., 2017). Furthermore, the increased expression of mitochondrial mitofusins-2 (Mfn-2) in cardiomyocytes after I/R injury is also associated with an increase in the levels of markers of NLRP3 inflammasome formation. Mitochondrial Mfn-2 may also be a signal for the activation of the NLRP3 inflammasome in cardiomyocytes (Darwesh et al., 2019). As a factor related to immunological inflammation, the NLRP3 inflammasome itself is closely related to immune cells. Neutrophils are the main source of IL-1β in the infarcted area of the heart. Ca2+ outflow due to cardiomyocyte lysis after MI can activate the CaSR of infiltrating neutrophils. CaSR in turn activates the NLRP3 inflammasome, resulting in the release of large amounts of IL-1β from neutrophils, and IL-1β further upregulates IL-1R in cardiomyocytes, which eventually leads to cardiomyocyte apoptosis. Inhibition of IL-1R activation in a model of acute myocardial infarction (AMI) was found to reduce cardiomyocyte apoptosis, which is consistent with the results of clinical studies (Ren et al., 2020).
The pathogenesis of cardiovascular diseases is complex, and the increase of blood glucose and blood lipids are independent risk factors. These risk factors were also confirmed to play a role by activating the NLRP3 inflammasome. Insulin resistance is one of the main characteristics of type 2 diabetes mellitus (T2DM). The effects of insulin resistance on the cardiovascular system are manifested in the destruction of cardiac structure and systolic function, resulting in VR and cardiac dysfunction (Govindsamy et al., 2018; Louwen et al., 2018). In obese patients, the level of palmitic acid (PA) is significantly increased, and excessive PA will lead to the production of ceramide, which can activate the NLRP3 inflammasome in cardiomyocytes (Mangali et al., 2019). Data have shown that PA increases the apoptosis rate of cardiomyocytes, reduces cell activity, and increases ROS production. The rupture of mitochondrial membranes is a specific marker of apoptosis. PA reduces mitochondrial membrane potential, as evidenced by the transfer of red fluorescent Q2 to green fluorescent Q4, resulting in mitochondrial dysfunction. ROS release is a potential factor of cardiomyocyte injury caused by PA. The presence of hydroxyl radicals can be visualized using DCFH-DA as a probe. In one study, PA induced more ROS production than in the control group, but ROS production in H9C2 cells pretreated with fatty acids decreased significantly. In addition, PA treatment increased the expression and complex formation of NLRP3. These results suggest that cardiomyocyte injury caused by obesity may be related to NLRP3 inflammasome activation caused by excessive PA accumulation (Y. Z. Zhang et al., 2019).
The NLRP3 inflammasome can also be activated in cardiomyocytes under stress, which is related to the excessive secretion of catecholamines. Animal studies have shown that α1-adrenergic receptor (α1-AR) mediates this process. By comparing mice injected with the α1-AR agonist phenylephrine (PE) with wild-type mice, the expression level of NLRP3 protein, caspase-1 (p20), and IL-18 in the experimental group increased, and the difference was more evident in the early stage of stress. In NLRP3−/− mice, PE did not increase the levels of caspase-1 (p20) and IL-18, which indicated that NLRP3 deficiency blocked the activation of the NLRP3/caspase-1/IL-18 pathway. According to the ejection fraction, cardiac systolic, and diastolic dysfunction in NLRP3−/− mice was also improved. ROS play a vital role in the activation of the NLRP3 inflammasome, while nicotinamide adenine nucleotide phosphate (NADPH) oxidase is the source of intracellular superoxide anion and promotes ROS production. An α1-AR agonist can also promote ROS production in rat cardiomyocytes through NADPH. Therefore, ROS may be involved in the activation of the NLRP3 inflammasome after α1-AR over-activation (Xin et al., 2020). The chronic activation of α1-AR can also upregulate cytokines due to pressure overload, promoting the activation of the NLRP3 inflammasome, and eventually leading to VR (Koren et al., 2017; R. Li et al., 2017).
4.2 Effects of the NLRP3 inflammasome on the integrity of VECs
As an important aspect of VR, vascular remodeling is often caused by angiogenesis and endothelial damage, which involves VECs (Alexandru et al., 2020; Lother et al., 2019; Segers et al., 2018). As a barrier against inflammation and thrombosis, VECs play an important role in maintaining the homeostasis of the vascular environment (Polunina et al., 2018; X. Zhang et al., 2018). VEC dysfunction promotes VR by affecting angiogenesis, reducing capillary density, and promoting atherosclerosis (AS), thereby affecting the balance of oxygen supply and energy uptake of the heart. This pathological change was confirmed in the process of ventricular transformation from concentric to eccentric hypertrophy in patients with aortic stenosis (Yan et al., 2019; Żak et al., 2019; Zeriouh et al., 2019). It is suggested that VEC dysfunction is closely related to VR.
Vascular inflammation is the main cause of VEC dysfunction, which leads to vascular wall thickening, microthrombosis, and acute cardiovascular events. The activation of the NLRP3 inflammasome is related to endothelial dysfunction. In the past two years, forkhead-box 1 (Foxp1) has become a focus of research on vascular endothelial dysfunction. Foxp1 regulates many genes involved in cell proliferation and differentiation and is highly expressed in VECs. The loss of Foxp1 has been shown to lead to severe VR (J. Liu, Zhuang, et al., 2019). A recent study has established a Foxp1 knockout mouse model and found that the loss of endothelium-specific Foxp1 expression aggravated AS. Based on this, chromatin immunoprecipitation analysis found that the NLRP3 inflammasome is the direct target of Foxp1. The binding site is located in the promoter region of NLRP3, Caspase-1, and IL-1β genes. This may also be why the NLRP3 inflammasome is activated in VECs under blood flow shear stress (Zhuang et al., 2019). In addition, recent studies have shown that pyroptosis is also involved in the occurrence and development of AS (Xu et al., 2018). The endothelial pyroptosis model based on the human VEC line EA.hy926 stimulated by high glucose concentrations confirmed this conclusion. Further studies found that the lncRNA MALAT1/miRNA-22/NLRP3 axis may be the key pathway of pyroptosis in VECs exposed to a high-glucose environment (Y. Song et al., 2019). VECs act as a barrier against inflammation. IL-1β released after the activation of the NLRP3 inflammasome regulates adhesion molecules and promotes leukocyte adhesion to VECs, which can form pro-inflammatory lipid-loaded foam cells and cause AS. Therefore, the activation of the NLRP3 inflammasome is the main driving force of AS, which is consistent with previous research (Stachon et al., 2017; van der Heijden et al., 2017). C-reactive protein (CRP) is an inflammatory marker that is widely regarded as a major predictor of cardiovascular events and participates in the endothelial inflammatory response. Recent studies have concluded that the NLRP3 inflammasome can be used as a predictor of CRP levels, indicating that NLRP3 inflammasome activation heralds an increase in cardiovascular risk. The subcutaneous retention of low-density lipoprotein (LDL) is the initial step in AS formation. Previous studies have shown that CRP promotes AS by directly increasing LDL transport across the endothelial barrier (Bian et al., 2014). Recently, it was found that CRP can upregulate the activity of NF-κB in VECs, induce the expression of NLRP3 and pro-IL-1β, as well as promote the activation of the NLRP3 inflammasome and IL-1β maturation by increasing ROS levels. In an in vitro model of trans-cellular LDL transport, VECs and CRP were incubated for 24 h, and the expression of proteins involved in the NLRP3 inflammasome activation signaling pathway was upregulated, indicating that NLRP3 inflammasome activation is involved in CRP-mediated trans-VEC LDL transport, promoting the inflammatory response of VECs (Bian et al., 2019).
The loss of endothelial connective tissue integrity can lead to vascular remodeling. In LPS-induced vascular injury, the NLRP3 inflammasome is activated, resulting in dysfunction of the endothelial junction. By inhibiting the activation of the NLRP3 inflammasome, the function of the endothelial junction proteins zonula occludens-1/2(ZO-1/2) is restored, which indicates that NLRP3 inflammasome activation can affect the function of ZO-1/2. It causes the loss of endothelial connective tissue integrity, leading to vascular injury and remodeling (Zhou et al., 2019). This process is complex and involves the expression of many vital factors. First, LPS was confirmed to bind to TLR4, a process that increased the release of ROS from VECs. Second, the expression of TXNIP was upregulated by the increase of ROS, and the NLRP3 inflammasome was found to be activated by excessive TXNIP levels. Finally, the activated NLRP3 inflammasome induces the release of high mobility group box 1 (HMGB1) and reduces the expression of ZO-1/2, leading to endothelial dysfunction (X. Wang et al., 2019; Zhou et al., 2019). HMGB1 is a newly discovered vascular permeability factor, which mediates high vascular permeability and promotes vascular remodeling by VECs (Chen et al., 2015). It has long been recognized that nicotine is harmful to the cardiovascular system, leading to endothelial dysfunction and microvascular damage (Luo et al., 2006). Nicotine activates the NLRP3 inflammasome and affects VEC function via mechanisms similar to the LPS pathway described above. In addition, nicotine also leads to the release of cathepsin B and enhances the cathepsin B-dependent activation of the NLRP3 inflammasome by inducing lysosome damage in VECs (Y. Zhang et al., 2019). Similarly, the NLRP3 inflammasome was found to be activated in VECs exposed to a high-salt environment, resulting in endothelial injury and vascular remodeling. These phenomena were confirmed in human umbilical vein endothelial cells exposed to high salt concentrations. The results indicated that nuclear factor of activated T cells 5 (NFAT5) is necessary for VECs to activate the NLRP3 inflammasome under high-salt stimulation, and it can directly bind to the promoters of NLRP3 and IL-1β mRNA, affecting their transcription (Ma et al., 2019). NFAT5 induced under high-salt stimulation, low oxygen, and mechanical stress (Berry et al., 2017).
In summary, the activation of the NLRP3 inflammasome in VECs can be induced by high glucose, high salt, fat accumulation, and other pathological microenvironments. This may be the fundamental pathological changes through which cardiovascular risk factors exert their effects in the initial stages of heart damage. This may be especially true for the risk-increasing effects of T2DM and AS. Whether it is the influence of the discussed risk factors, the direct impact of vascular endothelial inflammation, or the pyroptosis of VECs, NLRP3 inflammasome activation reduces the integrity of the vascular endothelium, damaging the protective barrier of VECs, and thereby promoting VR. Therefore, maintaining the protective function of the endothelial barrier should be a focus of research on the prevention and treatment of VR.
4.3 Effects of the NLRP3 inflammasome on cardiac macrophages
In addition to typical stress-inducing microenvironments, immune-inflammatory injury also affects the heart microstructure and leads to VR. The NLRP3 inflammasome is expressed by various immune cells, such as macrophages, T cells, and B cells (M. Zhang et al., 2019). Due to the immune-inflammatory response of the heart, peripheral macrophages attached to the heart or macrophages originally present in the heart can participate in VR via the activation of the NLRP3 inflammasome. This results in pathological changes such as promoting AS, expanding the area of MI, and promoting myocardial fibrosis (L. Zhang, Liu, et al., 2019; L. Zhang, Lu, et al., 2019).
There is increasing evidence that AS affects the cardiac microstructure, whereby oxidized LDL (ox-LDL) crucially aggregates under the cardiac vascular endothelium and stimulates macrophages to secrete pro-inflammatory cytokines. Notably, IL-1β and IL-18 induce pro-inflammatory macrophage phenotypes, which are related to the activation of the NLRP3 inflammasome and the formation of lipid-laden macrophages, or so-called foam cells (Hoseini et al., 2018; Karasawa & Takahashi, 2017; Tabas & Bornfeldt, 2016). Recent studies have shown that challenge with ox-LDL significantly increases the protein expression of NLRP3 and pro-IL-1β in primary mononuclear macrophages, as well as increasing the release of IL-1β and caspase-1 (P10) subunits, which are regulated by the adenosine monophosphate-activated protein kinase (AMPK) and protein phosphatase 2A (PP2A) pathways. Interestingly, the diabetes drug metformin inhibits the activation of the NLRP3 inflammasome and reduces VR (L. Zhang, Lu, et al., 2019). Another study showed that MEK1/ERK1/2 is also involved in the activation of the NLRP3 inflammasome in macrophages stimulated by ox-LDL. It was found that the phosphorylation levels of MEK1, ERK1/2, and NF-κB were all increased. In addition, the expression levels of NLRP3, Caspase-1, IL-1β, and IL-18 were also significantly higher than in the control group, while miRNA-181a was found to inhibit the activation of NLRP3 inflammasome by targeting MEK1. This reduced the proliferation of macrophages induced by ox-LDL, and protected the cardiac microstructure (J. Song et al., 2019). Similar to these two pathways, the activation of macrophage NLRP3 inflammasome in the acute inflammation phase of AMI is related to the activation of the AMPK/ERK1/2 signaling pathway, which leads to the deterioration of HF and infarct size enlargement, as well as rapid progress of VR (Ong et al., 2018; van Amerongen et al., 2007; L. Zhang, Liu, et al., 2019). miRNA-155 is a critical molecule that regulates the AS signaling pathway in inflammation (Li et al., 2016). It has also been reported that its overexpression can increase the phosphorylation of ERK1/2 and NF-κB, promoting the activation of the NLRP3 inflammasome and the release of IL-1β (Yin et al., 2019).
Unlike most other immune cells, macrophages can transform into different phenotypes that play different roles according to the needs of the environment, and this change often involves the NLRP3 inflammasome. In the inflammatory stage, M0 macrophages transform into M1 macrophages through the classical activation pathway, and then turn into M2 macrophages during anti-inflammatory repair after the inflammation subsides, which is reflected in the process of heart repair after MI (X. Liu et al., 2019). In the inflammatory phase after MI, macrophages mainly exhibit the pro-inflammatory M1 phenotype, with an activated NLRP3 inflammasome, as well as the secretion of IL-1β, tumor necrosis factor α (TNF-α), and matrix metalloproteinase-9 (MMP-9) to promote phagocytosis, proteolysis, and inflammation (Weil & Neelamegham, 2019). The anti-inflammatory repair phase follows when the inflammatory response mediated by the NLRP3 inflammasome is weaker than in the acute MI stage. This long-term mild inflammatory response will also aggravate myocardial injury, and the subsequent progressive VR will still increase the risk of secondary HF (Prabhu & Frangogiannis, 2016). A recent study found that VR related to PM2.5 is mediated by macrophage polarization and activation of the NLRP3 inflammasome. Moreover, exposure to high concentrations of PM2.5 can cause cardiac dysfunction in mice. The mRNA and protein expression levels of NLRP3, ASC, Caspase-1, IL-1β, and IL-18 in the myocardial tissue were all higher in the experimental group. In mice exposed to PM2.5, the polarization of M0 macrophages into the M1 phenotype is accompanied by NLRP3 inflammasome activation, which is the main pathological mechanism leading to VR under high PM2.5 concentrations (X. Du et al., 2019). Biodegradable chitosan particles were found to selectively induce the release of IL-1β by macrophages. Further studies found that chitosan scaffolds with a higher degree of acetylation can induce typical pro-inflammatory signals mediated by M1 macrophages, leading to the release of IL-1β, which has been shown to be related to the activation of the NLRP3 inflammasome (Alhamdi et al., 2019; Smith et al., 2017; Vasconcelos et al., 2019). Moreover, the degradation products of some stents gradually promote VR to a certain extent.
The activation of the NLRP3 inflammasome in cardiac macrophage is involved in both the process of acute cardiac injury, such as AMI, and chronic inflammatory infiltration, such as the progression of AS and cardiac repair after MI. This leads to acute exacerbation of VR, or even to progressive VR. Therefore, the activation of the NLRP3 inflammasome in cardiac macrophages during the process of VR cannot be ignored. In addition, as immune cells, macrophages can respond to external pathogens, which may be a primary mechanism through which external environmental changes affect VR.
5 CONCLUSIONS AND PROSPECTS
Most of the research on VR is based on AMI or myocardial I/R models. This type of animal model induces great amounts of damage to the heart, which makes it easier to quickly draw conclusions on the effect of NLRP3 inflammasome activation on VR. Nevertheless, it is easy to overlook how the NLRP3 inflammasome acts on the heart in many real situations. Although the inflammatory storm caused by the activation of the NLRP3 inflammasome in acute heart disease is clearly very harmful, the long-term mild inflammation mediated by the activation of the NLRP3 inflammasome in chronic heart disease can also lead to progressive VR and eventually damage cardiac function to the point of no recovery. It therefore stands to reason that heart function still gradually declines under long-term active treatment of coronary heart disease. The studies done to date mainly explain the modes of NLRP3 inflammasome activation leading to VR from the aspects of cardiomyocyte injury, VECs dysfunction, and macrophage infiltration, covering almost all the known pathological changes that lead to VR. However, the mechanism of NLRP3 inflammasome activation has not been thoroughly studied. Instead, more attention was paid to the impact of NLRP3 inflammasome activation on cardiac structure, which may also be the reason why the NLRP3 inflammasome activation pathway described above is less mentioned when reviewing the effects of NLRP3 inflammasome activation on VR. In fact, the activation of the NLRP3 inflammasome is not mediated by a single pathway, whereby the classical ion pathway, the organelle pathway, and the recently studied noncoding RNA pathway all promote each other.
The activation of the NLRP3 inflammasome is a complex process that involves many upstream events. Therefore, there are many different inhibitors of the NLRP3 inflammasome. For example, mito-TEMPO can block the release of ROS (Wei et al., 2019), aspirin can block redox signals (Zhou et al., 2019), and the mushroom Coriolus versicolor can inhibit the activation of NF-κB (Y. Wang, Li, et al., 2019). Similarly, α-Bisabolol and KGA-2727 can protect the myocardial ultrastructure of rats with MI by inhibiting the activation of the NLRP3 inflammasome (Nagoor Meeran et al., 2020; Sawa et al., 2020). In view of the pathological changes caused by multiple pathways, single-target drugs often fail to achieve the desired efficacy. Therefore, to prevent the negative effects of the NLRP3 inflammasome in VR, which is caused by complex pathological mechanisms, research on multiple drug targets is obviously a strategy worth considering. In addition, the development of inhibitors that directly act on the NLRP3 inflammasome, rather than upstream events, is also a promising research direction. Related experiments have been carried out in this area (Coll et al., 2019; C. Zhang et al., 2019). Although the research on the NLRP3 inflammasome and changes to the heart structure that cause VR is relatively comprehensive, it seems that the myocardial extracellular matrix and cardiac fibroblasts have not received enough attention as far as the current research is concerned. Whether NLRP3 inflammasome activation can interfere with VR by affecting the extracellular matrix of cardiomyocytes and fibroblasts is unknown, which may be a new research direction. All these theoretical approaches are needed for the basic understanding of the NLRP3 inflammasome and its role in VR, which will hopefully enable the development of NLRP3 inhibitors to ameliorate VR by reducing the inflammatory response.
ACKNOWLEDGMENT
This study was supported by the National Natural Science Foundation of China (Grant nos. 81804046, 81774232, and 30672734).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Zhipeng Yan and Zhongwen Qi wrote the manuscript. Xiaoya Yang, Nan Ji, and Yueyao Wang collected materials. Qi Shi and Meng Li revised the manuscript, and Yaping Zhu and Junping Zhang gave instructive advices. All authors read and approved the final manuscript.




