Roles of apoptotic chondrocyte-derived CXCL12 in the enhanced chondroclast recruitment following methotrexate and/or dexamethasone treatment
Abstract
Intensive use of methotrexate (MTX) and/or dexamethasone (DEX) for treating childhood malignancies is known to cause chondrocyte apoptosis and growth plate dysfunction leading to bone growth impairments. However, mechanisms remain vague and it is unclear whether MTX and DEX combination treatment could have additive effects in the growth plate defects. In this study, significant cell apoptosis was induced in mature ATDC5 chondrocytes after treatment for 48 h with 10−5 M MTX and/or 10−6 M DEX treatment. PCR array assays with treated cells plus messenger RNA and protein expression confirmation analyses identified chemokine CXCL12 having the most prominent induction in each treatment group. Conditioned medium from treated chondrocytes stimulated migration of RAW264.7 osteoclast precursor cells and formation of osteoclasts, and these stimulating effects were inhibited by the neutralizing antibody for CXCL12. Additionally, while MTX and DEX combination treatment showed some additive effects on apoptosis induction, it did not have additive or counteractive effects on CXCL12 expression and its functions in enhancing osteoclastic recruitment and formation. In young rats treated acutely with MTX, there was increased expression of CXCL12 in the tibial growth plate, and more resorbing chondroclasts were found present at the border between the hypertrophic growth plate and metaphysis bone. Thus, the present study showed an association between induced chondrocyte apoptosis and stimulated osteoclastic migration and formation following MTX and/or DEX treatment, which could be potentially or at least partially linked molecularly by CXCL12 induction. This finding may contribute to an enhanced mechanistic understanding of bone growth impairments following MTX and/or DEX therapy.
1 INTRODUCTION
Although intensifying use of chemotherapeutic agents such as methotrexate (MTX) and glucocorticoids including dexamethasone (DEX) has achieved great success in the management of pediatric malignancies, high prevalence of chemotherapy-associated bone growth impairments has been found in cancer patients and/or survivors which could persist into adulthood and is with significant clinical burden (Crofton et al., 2000; Fan et al., 2011; Haddy et al., 2001; Su et al., 2017). For example, patients of acute lymphoblastic leukemia (ALL), the most common pediatric cancer, have an over 80% of survival rate following multiagent chemotherapy involving the combination treatment of MTX and DEX (Mitchell et al., 2009). However, they have been observed to develop a lower bone mass and achieve a considerable decrease in height velocity during and after chemotherapy treatment (Viana & Vilela, 2008). As there is still a lack of a clear understanding of its pathogenesis and there are no preventative treatments, there remains an urgent requirement to increase mechanistic understanding and to develop preventive strategies and effective treatments for cancer chemotherapy-associated bone growth impairments (Su et al., 2017).
The growth plate cartilage towards both ends of a long bone is well-established to act as a template for bone matrix deposition and crucial for endochondral ossification longitudinal bone growth in childhood and adolescence (Xian, 2007). During endochondral ossification bone growth, resorption of the calcified cartilage and/or excavation of cartilage canals for vascular invasion are critically for infiltration of highly specialized skeletal cells such as resorbing chondroclasts/osteoclasts and bone-forming osteoblasts (Mackie et al., 2011), which are important in remodeling or converting hypertrophic growth plate cartilage into woven bone (primary spongiosa). Cartilage resorption is generally characterized by a sequence of events including osteoclast precursor cell migration, invasion or homing, followed by the differentiation into activated chondroclasts at the resorption site. Chondroclasts are mineralized cartilage-resorptive osteoclasts at the growth plate-metaphysis junction, that are derived from osteoclast precursor cells or the macrophage/monocyte lineage cells of bone marrow origin (Fan et al., 2011; Vu et al., 1998; Xian et al., 2007). While osteoclast precursor cell recruitment and osteoclast differentiation involved in bone resorption are better understood (Kikuta & Ishii, 2013), recruitment of precursor cells and their differentiation into chondroclasts involved in cartilage resorption remain relatively more elusive. Nonetheless, dysregulation and alterations in chondroclast recruitment and activity are known to contribute to growth plate abnormalities and bone growth impairments (Gerber et al., 1999; Odgren et al., 2016). Despite the unclear mechanisms, it has been suggested that bone growth impairments induced by cancer chemotherapy are, at least in part, attributable to the disturbances of growth plate structure due to the induction of chondrocyte apoptosis and increased recruitment/formation of chondroclasts (Fan et al., 2011; Vu et al., 1998; Xian et al., 2007). Recently, the tight coupling between apoptotic bone cells and resorptive cells in response to chemotherapeutic agent treatment has been revealed (Shandala et al., 2012), indicating an existence of underlying intercellular communication within growth plate (between apoptotic chondrocytes and osteoclast precursor cells), which could be associated with the aggravated cartilage loss and bone growth impairments.
Since skeletal and immune systems interact with each other and have several regulators in common (e.g., cytokines and chemokines; Caetano-Lopes et al., 2009) as well as some cytokines and/or chemokines play critical roles in multiple skeletal cell regulation pathways including chondrocyte apoptosis and osteoclastic differentiation (Gronthos & Zannettino, 2007; King et al., 2012; Yu et al., 2003), we proposed that a specific subset of cytokine(s)/chemokine(s) derived from apoptotic chondrocytes have the potential to coordinate osteoclastic or chondroclastic recruitment/formation and, therefore, could be a promising treatment target for chemotherapy-induced bone growth impairments. As the two most frequently and intensively used drugs in pediatric cancer, individual MTX or DEX treatment is evident to cause growth plate dysfunction, leading to endochondral ossification disruption, the thinning of the growth plate and bone growth impairments (Fan et al., 2012; Olney, 2009; Xian et al., 2007; Zaman et al., 2012), which have been suggested to be related to the upregulated cytokine/chemokine expression (King et al., 2012). However, in clinical medication management, most chemotherapeutic regimens are composed of different agents from multiple classes, such as the combination or sequential usage of MTX and DEX being the key component for ALL chemotherapy in children (Veerman et al., 2009). It is still not clear whether and/or how combination treatment of DEX with MTX would have synergistic or antagonistic effects on skeleton impairments, revelation of which is important to the underlying mechanisms and management strategies for skeletal impairments.
ATDC5 is a well-characterized chondrogenic cell line which exhibits a sequential differentiation process analogy to the in vivo chondrocyte chondrogenesis and hypertrophy within the growth plate during endochondral ossification bone growth (Shukunami et al., 1997). This cell line has been widely used as an optimized in vitro model for exploring the underlying molecular mechanisms related to, or for investigating therapeutic interventions for, endochondral ossification bone growth (Yao & Wang, 2013). Relevant to the current work, this cell line has been used to study secretion of signalling molecules that regulate cell–cell communication between chondrocytes and osteoclast precursor cells (Wang et al., 2014; Zaman et al., 2019). Using MTX ± DEX treatment models in mature ATDC5 chondrocytes, the present project aimed to compare the effects of MTX and/or DEX treatment on chondrocyte apoptosis, cytokine/chemokine expression and their potential in osteoclast precursor cell migration and formation. In addition, as a step for in vivo validation of these potential relationships, MTX treatment effects on chemokine expression and chondroclast recruitment were analysed in the proximal tibial growth plate in young rats treated acutely with MTX.
2 MATERIALS AND METHODS
2.1 Cell culture
Mouse ATDC5 chondrogenic cells were seeded into the basal medium (alpha minimum essential medium; Sigma) which contains 5% fetal bovine serum (FBS; Invitrogen), l-glutamine (2 mM; Sigma), Hepes (15 nM; Sigma) and penicillin/streptomycin (100 U/ml; Invitrogen) at a density of 5 × 104 cells/well. Following reaching confluency (Day 0), cells were then cultivated in chondrogenic differentiation medium which was the basal medium additionally supplemented with sodium selenite (5 ng/ml; Sigma), transferrin (10 μg/ml; Invitrogen) and l-ascorbate-2-phosphate (300 μM; Sigma). The medium was changed every 2 days and the cells were incubated at 37°C and in 5% CO2 and harvested at multiple time points (Days 0, 4, 10, and 20) for identifying the stage of chondrocyte hypertrophy for the subsequent experiments with MTX and/or DEX treatment.
2.2 Alcian blue staining
To visualize the process of chondrogenic differentiation, Alcian blue staining was performed at various time points (Days 0, 4, 10, and 20). In brief, following the fixation in 10% formalin for 30 min, ATDC5 cells were stained with 0.1% Alcian blue (Waldeck GmbH & Co. KG.) in 0.1 M hydrochloric acid (HCl; Sigma) overnight. Aside from image capture, Guanidine-HCl solution (6 M; Sigma) was used to extract the dye and the intensity of staining was determined by using spectrophotometry at 600 nm as described (Su et al., 2016; 1420 Victor3; Perkin Elmer).
2.3 MTX and/or DEX treatment and conditioned medium generation
To clarify the effects of MTX ± DEX treatment on hypertrophic chondrocytes, ATDC5 cells were either treated with MTX at 10−5 M and/or DEX at 10−6 M on Day 18 (time point for chondrocyte hypertrophy determined from the above time course study) for 48 h, or with normal chondrogenic differentiation medium as an untreated control. Parts of treated or untreated cells were trypsinised and collected on Day 20 for further gene expression analyses. Additionally, the conditioned medium was collected from ATDC5 cells with/without MTX and/or DEX treatment on Days 18 and 20, respectively. The clinical chemotherapy-relevant concentrations (MTX at 10−5 M and/or DEX at 10−6 M) were chosen in this study as they were found within the ranges of their plasma levels in patients receiving these chemotherapeutic agents (Li et al., 2004). In addition, exposure for 48 h to these agents individually at these concentrations has been previously found to cause significant apoptosis in bone cells (osteoblasts and osteocytes; Shandala et al., 2012; Weinstein et al., 1998) as well as in mature ATDC5 chondrocytes as shown in our pilot experiment (data not shown).
2.4 Cleaved caspase-3 immunofluorescence analyses
Cleaved caspase-3 immunofluorescence assay was carried out, in triplicate and repeated three times, to assess the effects of MTX and/or DEX treatment on chondrocyte apoptosis. Briefly, treated cells were incubated for 30 min with 2 μM caspase-3/7 Green Detection Reagent (C10423; Invitrogen) in phosphate-buffered saline (Invitrogen) with 5% FBS (Invitrogen). After being fixed with 10% formalin (Sigma), the cells were counter-stained with 1 µg/ml 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI; Sigma). The densities (cell number/mm2) of cleaved caspase-3 positive (cleaved caspase-3+) cells were then calculated.
2.5 Chemokine/cytokine PCR array
To systematically investigate and compare the effects of MTX and/or DEX treatment on the expression of cytokines/chemokines, a mouse cytokines & chemokines RT2 Profiler™ PCR Array (PAMM-150ZD; QIAGEN Science) was conducted according to the manufacturer's instructions. Glyceraldehyde-3-phosphate dehydrogenase was utilized as internal reference gene and the gene expression levels were calculated by using comparative CT (2−ΔCT) method.
2.6 Real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Aside from further verifying expression changes in the selected cytokine(s)/chemokine(s) (e.g., CXCL12, identified to be prominently induced by the above PCR array studies), qRT-PCR analyses were also conducted in triplicate and repeated three times for the treatment effects on the expression of some cartilage matrix protein genes (e.g., Type II collagen or Col-2 and Type X collagen or Col-X) and apoptosis regulatory molecules (e.g., B-cell lymphoma 2 or Bcl-2, Bcl-2-associated X protein or Bax, First apoptosis signal or Fas and Fas ligand or Fas-L). Primers of interest genes (Table 1) were designed using NCBI Primer-Blast software and cyclophilin-A was used as internal reference gene.
| Gene | Forward primer (5’–3’) | Reverse primer (5’–3’) |
|---|---|---|
| Col-2 | GGGCTCCCAGAACATCAGCTACCA | TCGGCCCTCATCTCCACATGATTG |
| Col-X | GGCAGCAGCACTATGACCCAA | ACAGGCCTACCCAAACGTGAGTCC |
| Bcl-2 | CAGCATGCGACCTCTGTTTG | TCTGCTGACCTCACTTGTGG |
| Bax | CGAGAGGTCTTCTTCCGTGT | GAGCACCAGTTTGCTAGCAA |
| Fas | AAGATCGATGAGATCGAGCACA | AAGCTTGACACGCACCAGTCT |
| Fas-L | GAGCTGTGGCTACCGGTGATAT | ACTCACGGAGTTCTGCCAGTTC |
| CXCL12 | ATCAGTGACGGTAAGCCAGTCA | TGCACACTTGTCTGTTGTTGCT |
| CycA | CGTTGGATGGCAAGCATGTG | TGCTGGTCTTGCCATTCCTG |
- Abbreviations: CycA, cyclophilin-A; RT-PCR, real-time reverse transcription polymerase chain reaction.
2.7 Enzyme-linked immunosorbent assay (ELISA)
The protein concentrations of the selected cytokine(s)/chemokine(s) (e.g., CXCL12) in conditioned medium were assessed by using using ELISA kits (R&D Systems) as previously described (Georgiou et al., 2012).
2.8 Osteoclast precursor cell migration assay
To determine the potential of the selected cytokine(s)/chemokine(s) (e.g., CXCL12, with the prominent induction following MTX and/or DEX treatment) in the conditioned medium in promoting osteoclast precursor cell recruitment, mouse RAW264.7 osteoclast precursor cells were subjected to an in vitro chemotaxis assay by using Boyden chambers with 8.0 mm pore size PET membranes (3422; Corning Costar Corp.). Briefly, RAW264.7 cells were cultured in the upper chambers at a density of 5 × 104 cells/well and the bottom chambers were filled with the collected conditioned medium from ATDC5 cells with/without MTX ± DEX treatment. Basal medium supplemented with/without specific recombinant chemokine protein such as recombinant mouse CXCL12 protein (80 ng/ml; MAB350; R&D Systems) was employed as control groups. Following 6-h incubation, all chamber inserts were placed into new plates (3513; Corning Costar Corp) and the migrated cells on the lower side of the membrane were stained with 0.5% toluidine blue (Sigma) for density quantification. The above migration assay in combination with specific cytokine/chemokine functional blockade was conducted to further determine the roles of cytokine(s)/chemokine(s) in osteoclast precursor cell migration. The selected neutralizing antibodies such as anti-CXCL12 antibody (10 µg/ml; MAB310; R&D Systems) were incubated with the conditioned medium for 2 h and osteoclast precursor migration assay was subsequently carried out in a similar fashion. All assays were conducted in triplicate and repeated three times.
2.9 Osteoclast formation assay
To investigate the proosteoclastogenic potential of the selected cytokine(s)/chemokine(s) (e.g., CXCL12) expressed by MTX ± DEX-treated chondrocytes, RAW264.7 cells were also employed for an in vitro osteoclast formation assay. In brief, RAW264.7 cells were cultivated in basal medium supplemented with 30 ng/ml receptor activator of nuclear factor-κB ligand (RANKL; Peprotech) at a density of 5 × 103 cells/well for 2 days to offer a baseline stimulus to trigger osteoclast formation. On Day 3, different conditioned medium including those from MTX ± DEX-treated and -untreated ATDC5 cells were added to the wells in a 1:1 ratio with basal medium, after which the concentration of RANKL was adjusted to maintain at 30 ng/ml. In addition, basal medium supplemented with/without 30 ng/ml RANKL (Peprotech) and/or specific recombinant chemokine protein such as recombinant mouse CXCL12 protein (80 ng/ml; MAB350; R&D Systems) was used in control groups. On Day 9, tartrate-resistant acid phosphatase (TRAP; a cytochemical marker for osteoclasts/chondroclasts) staining was performed and the densities (number/mm2) of multinucleated osteoclasts, which were in ruby red in color and with three or more nuclei, were calculated as described (King et al., 2012). Similar to the migration assay, the selected neutralizing antibodies such as anti-CXCL12 antibody (10 µg/ml; MAB310; R&D Systems) were added into the conditioned medium 2 h before the commencement of the osteoclast formation assay. All assays were conducted in triplicate and repeated three times.
2.10 MTX acute treatment in young rats and chondroclast analyses in tibial growth plate
Five-week old male rats were given five consecutive once daily subcutaneous injections of either saline or MTX at 0.75 mg/kg (n = 6 per group). At Day 9 after the first injection, a time point previously shown to have the most significant growth plate and bone damage (Xian et al., 2007), proximal tibial bones were collected. The left tibial growth plate cartilage was dissected, stored at −80°C, and was used for RT-PCR analyses of CXCL12 messenger RNA (mRNA) expression (as described above). The right proximal tibias were fixed in 10% formalin, decalcified and processed for paraffin-embedded section (4 μm) for hematoxylin and eosin-TRAP histological staining as described (King et al., 2012; Shandala et al., 2012). The densities of TRAP-stained chondroclasts (cells/mm perimeter) were analyzed at the growth plate-metaphysis transitional border on four serial sections of each rat.
2.11 Statistical analyses
Data were analysed by one-way analysis of variance and Tukey's post hoc tests by using Prims 8 software (GraphPad). The p value of less than .05 was considered as statistically significant. In the figures, the symbols *, **, and *** represent p < .05, 0.01, and 0.001, respectively.
3 RESULTS
3.1 Identification of the hypertrophic differentiation stage of ATDC5 chondrogenic cells
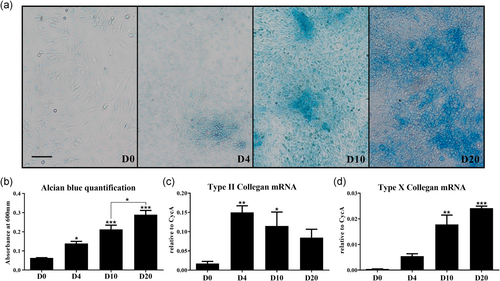
To identify suitable culture time period of hypertrophic (mature) chondrocytes for MTX and/or DEX treatment, Alcian blue staining for cartilage matrix proteoglycan and qRT-PCR analyses for typical chondrogenesis markers were conducted in an ATDC5 chondrogenic cell differentiation/maturation time-course study. While Alcian blue positive cells (colonies) were not detected until Day 4, the typical nodular structures were observed from Day 10, which increased in size and number with time in culture (Figure 1a). During the phase between Days 10 and 20, Alcian blue-positive cells appeared to expand by the accumulation of cartilage matrix resulting from the ongoing differentiation. Consistently, there was a progressive and significant increase in Alcian blue absorbance from Days 0 to 20 (p < .05, p < .001, and p < .001 on Days 4, 10, and 20, respectively, compared to Day 0; Figure 1b). In parallel, mRNA expression levels of Col-2 were elevated on Day 4 (peak level, p < .01 compared to Day 0) and Day 10 (p < .05) and then declined by Day 20 (Figurr 1c). Additionally, the expression levels of Col-X, a marker of chondrocyte hypertrophy, were gradually increased and reached a peak on Day 20 (p < .001 compared to Day 0; Figure 1d), suggesting reaching mature hypertrophic stage of the differentiation by Day 20.
3.2 Induced apoptosis of ATDC5 chondrocytes following MTX and/or DEX treatment
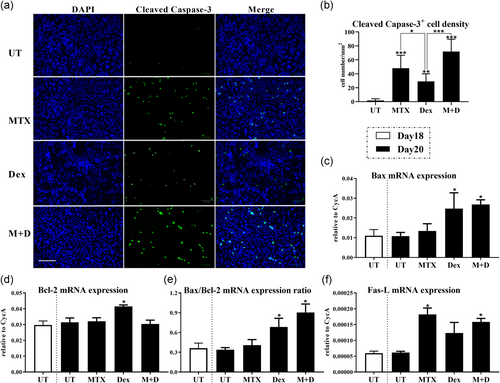
With the above results demonstrating ATDC5 cells reaching hypertrophic stage towards Day 20, treatment of ATDC5 cells with/without 48 h MTX (10−5 M) and/or DEX (10−6 M) was carried out from Days 18 to 20 in culture. Immunofluorescent analysis for cleaved capase-3, a chief marker for cell apoptosis, was then performed to evaluate the treatment effects on ATDC5 cell apoptosis. Quantification of the cleaved caspase-3+ cells showed a considerable increase in chondrocytes among all MTX and/or DEX treatment groups in comparison to -untreated cells on Day 20 (p < .001, p < .05, and p < .001, respectively; Figure 2a,b). However, despite the nonstatistical differences between MTX alone and combination with DEX treatment groups, a relatively lower density of cleaved caspase-3+ cells was detected after individual DEX treatment when compared to individual MTX treatment (p < .05); and MTX and DEX combination treatment induced an even higher number of apoptotic cells in comparison to individual DEX treatment (p < .001; Figure 2b). qRT-PCR analysis was also conducted to investigate the alterations in expression of apoptosis regulatory genes. Although there were no differences observed in each regulatory gene expression during normal chondrogenesis between Days 18 and 20 in culture (Figure 2c–f), DEX treatment had considerable effects on the expression of proapoptotic protein Bax and antiapoptotic protein Bcl-2 (p < .05; Figure 2c,d). The Bax/Bcl-2 ratio was thus strikingly increased after individual DEX or DEX and MTX combination treatment (p < .05; Figure 2e). On the contrary, no statistical differences in Bax/Bcl-2 expression were found following MTX treatment (Figure 2c–e). Furthermore, the expression of Fas maintained in low and unchanged levels following MTX ± DEX treatment in the current study (data not shown). A pronounced trend of Fas-L upregulation was observed in response to individual MTX or MTX and DEX combination treatment (p < .05), whereas there were statistically insignificant differences in Fas-L expression between untreated control and DEX treatment groups (Figure 2f).
3.3 Prominent induction of CXCL12 in ATDC5 chondrocytes following MTX and/or DEX treatment
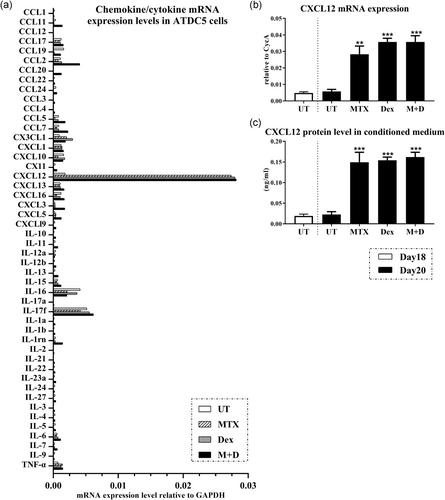
Diverse responses of cytokine/chemokine expression in chondrocyte were found upon MTX and/or DEX treatment (Figure 3a). For example, compared to that in untreated group, expression was downregulated upon MTX treatment for interleukin (IL)-17f, CCL2, and CCL7, but they were upregulated after individual DEX treatment or the combination treatment of MTX with DEX; while the expression levels of IL-5, IL-9, and CCL12 were all inhibited following MTX ± DEX treatment. Interestingly, in the current in vitro model, CXCL12 displayed the most prominent induction in each treatment group and thus selected to be examined further (Figure 3a). While there was a lack of CXCL12 upregulation during normal chondrogenesis from Days 18 to 20 in culture, we found a notable induction in CXCL12 mRNA expression (p < .01, p < .001, and p < .001, respectively) in response to MTX and/or DEX treatment (Figure 3b). Consistently, the protein concentrations of CXCL12 in the conditioned media within all treatment groups were also significantly increased when compared with those of the conditioned media in untreated group (p < .001; Figure 3c). There were no statistical differences in CXCL12 mRNA expression levels and protein concentrations amongst these three (MTX, DEX, and MTX + DEX combination) treatment groups.
3.4 The capability of CXCL12 in conditioned medium from MTX and/or DEX-treated ATDC5 chondrocytes in stimulating osteoclast precursor cell migration
To determine the potential in osteoclast/chondroclast precursor cell recruitment of CXCL12 induced by chondrocytes following MTX and/or DEX treatment, osteoclast precursor RAW264.7 cell migration assay was performed with different media (Figure 4). Interestingly, in basal medium, exogenous recombinant CXCL12 protein was found to be associated with the significantly augmented migration (p < .05). In addition, the conditioned medium from each MTX ± DEX treatment group appeared to have the capability to enhance cell migration (p < .05, p < .01, and p < .05 in MTX, DEX, MTX + DEX treatment groups, respectively, compared to untreated control), which could be evidently repressed after CXCL12 blockade by using the neutralizing antibody for CXCL12 (p < .05).

3.5 The capability of CXCL12 in conditioned medium from MTX and/or DEX-treated ATDC5 chondrocytes in stimulating osteoclastic formation
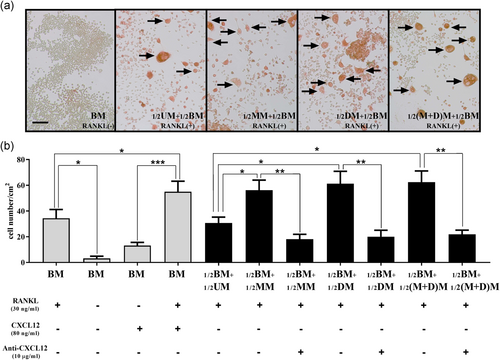
As shown in Figure 5, in basal medium, osteoclastic differentiation could not be triggered without the presence of RANKL in current model. In addition, although the CXCL12 supplementation showed a trend of promoted osteoclastogenesis in basal medium, there were no statistical differences between basal medium groups supplemented with/without exogenous recombinant CXCL12 protein in the absence of RANKL while CXCL12 in combination with RANKL supplementation could significantly enhance osteoclast differentiation when compared to CXCL12 alone (p < .05), suggesting exogenous recombinant CXCL12 protein exerts no or only weak effects on osteoclastic differentiation. Interestingly, in the presence of RANKL, conditioned medium from each of MTX ± DEX treatment groups could significantly stimulate osteoclast formation when compared to the conditioned medium from the untreated control group (Figure 5a), as observed by the increased densities of TRAP+ multinuclear cells in the culture (p < .05; Figure 5b). Similar to the findings in the migration assay, these promoting effects of the conditioned medium among three treatment groups were markedly inhibited by the neutralizing anti-CXCL12 antibody (p < .01 in MTX, DEX, and MTX + DEX treatment groups when compared to the -untreated group; Figure 5b). There were statistically insignificant differences in TRAP+ multinuclear cell densities among the three treatment groups (conditioned media from MTX, DEX, and MTX + DEX treatment groups).
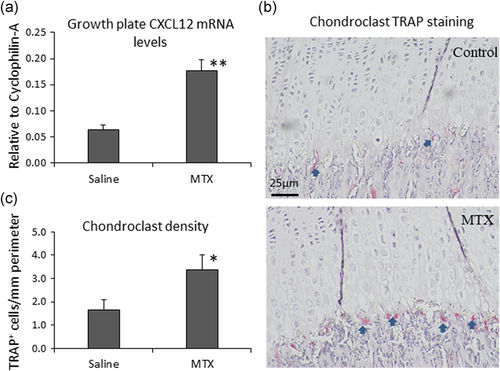
3.6 Increased chondrocyte apoptosis and CXCL12 expression in the growth plate and a higher chondroclast density at the growth plate-metaphysis transition zone in MTX-treated rats
As a step to validate the possibility of CXCL12 acting as a potential molecular linker between MTX treatment-induced chondrocyte apoptosis and chondroclast recruitment in vivo, a rat model of chemotherapy was set up with acute MTX treatment (mimicking the MTX treatment protocol in childhood oncology), and CXCL12 expression and chondroclast recruitment were analysed in the proximal tibial growth plate (Figure 6). Consistent with our previous observations (Xian et al., 2007), MTX treatment caused a higher level of chondrocyte apoptosis particularly at the hypertrophic zone at Day 9 after the first of the five-consecutive daily MTX injections; data not shown). By RT-PCR analyses, MTX treatment was found to significantly increase CXCL12 mRNA expression in the growth plate (p < .01 compared to saline control; Figure 6). In addition, by TRAP staining, more TRAP-positively stained cartilage-resorbing chondroclasts were found present at the transition border zone between hypertrophic growth plate cartilage and metaphysis bone (p < .05 compared to saline control; Figure 6b,c). These in vivo data support the above in vitro observations and suggest that MTX treatment increases chondrocyte apoptosis and induces CXCL12 expression at the growth plate, which consequently causes increased recruitment of chondroclasts that resorb the mineralized hypertrophic growth plate cartilage.
4 DISCUSSION
Intensive use of MTX or DEX treatment has been shown to considerably interrupt the steady-state skeletal cells within bone growth unit which results in bone growth impairments (Fan et al., 2012; Olney, 2009; Xian et al., 2007; Zaman et al., 2012). Although the underlying mechanisms remain unclear, based on previous studies with individual chemotherapy drug treatments, it is suggested that specific cytokine(s)/chemokine(s) (e.g., CXCL12) derived from apoptotic growth plate chondrocytes following MTX or DEX treatment have the potential to promote osteoclastic recruitment and differentiation at the chondro-osseous junction between the growth plate and metaphysis bone and cause uncontrolled cartilage and/or bone loss. However, as chemotherapy drugs are often used in combination, it is unclear whether the combination drug treatment would result in additive or counteractive effect in the induction of the pro-osteoclastogenic cytokines/chemokines. MTX and DEX are commonly used sequentially or in combination to treat childhood cancers (particularly ALL) which are known to cause significant bone growth impairments. To delineate any potential additive or counteractive effects of MTX and DEX combination treatment on induction of the pro-osteoclastogenic cytokines/chemokines, the current study utilized MTX ± DEX treatment models in cultured ATDC5 chondrocytes to compare whether MTX and/or DEX treatment-induced chondrocyte apoptosis could potentially up-regulate expression of some cytokines/chemokines which are responsible for the enhanced osteoclastic migration and formation. Herein, following establishing an ideal time period of in vitro chondrocyte hypertrophy (the maturing/mature stage of differentiation) for drug treatment, significantly induced chondrocyte apoptosis and upregulated cytokine/chemokine (particularly chemokine CXCL12) expression levels were observed after mature ATDC5 chondrocytes had been treated with 10−5 M MTX and/or 10−6 M DEX for 48 h. In addition, conditioned medium from each MTX and/or DEX treatment group (shown to have a higher content of CXCL12 protein compared to untreated control) could noticeably stimulate osteoclast precursor cell migration and osteoclast formation, which was inhibited by the neutralizing anti-CXCL12 antibody. Interestingly, in comparison to individual MTX/DEX treatment in culture, apart from some additive effects on apoptosis induction, the combination treatment of MTX and DEX appeared to have no additive or counteractive effects on CXCL12 induction and the potentials of apoptotic chondrocyte-derived CXCL12 in osteoclast precursor migration and formation.
4.1 A feasible in vitro model for mature chondrocyte MTX and/or DEX treatment studies
In normal endochondral ossification process of bone formation and bone lengthening in the growth plate, chondrocyte hypertrophy is a mandatory step allowing subsequent calcification, apoptosis and vascularization during endochondral bone growth (Mackie et al., 2011). As previous in vivo studies have demonstrated that MTX or DEX treatment could significantly induce chondrocyte apoptosis (principally among the terminally differentiated hypertrophic chondrocytes; Chrysis et al., 2003; Xian et al., 2007; Zaman et al., 2012), the current in vitro study also used mature chondrocytes in culture. Thus, the time course of chondrogenic differentiation was firstly evaluated to find a suitable time point/period for the MTX and/or DEX treatment in mature chondrocytes. The ATDC5 chondrogenic cell line was used in the current study as it could exhibit the entire spectrum of chondrocyte differentiation during endochondral ossification (Shukunami et al., 1997). To assess chondrogenesis, histochemical analyses of cartilage glycosaminoglycans by using Alcian blue staining (Terry et al., 2000) were conducted, and gene expression analyses of Col-2 were carried out which showed induction commencing at the early stage of chondrogenesis as also previously reported (Zuscik et al., 2008). Our gene expression analyses indicated that chondrocyte hypertrophy is accompanied by the reduction of Col-2 (a marker of chondrogenesis) and the increased expression of Col-X (a marker of hypertrophy; Ballard & Ballard, 1995). Therefore, aside from the observed ongoing increase in Alcian blue staining and absorbance reading starting from Day 4 upon differentiation culture, RT-PCR analyses for Col-2 expression suggested that ATDC5 chondrogenic cells undergo active phrase of differentiation (chondrogenesis) during Days 4 and 10, and the late phrase of differentiation (hypertrophy) towards and/or from Day 20. Thus, for the subsequent in vitro experiments of this study, MTX and/or DEX treatment was given during the late stage of chondrogenic differentiation of ATDC5 cells (between Days 18 and 20).
4.2 Different pathways for the chondrocyte apoptosis induced by MTX or DEX treatment and the additive effect of MTX + DEX combination in apoptosis induction
The adverse effects of MTX and/or DEX therapy on the skeleton are evident to be associated with undesired chondrocyte apoptosis, which can lead to the thinning of the growth plate and bone growth impairment (Xian et al., 2007; Zaman et al., 2012). Two major signaling pathways have been well-characterized to be associated with induced apoptosis, namely the intrinsic pathway where the release of apoptogenic protein cytochrome c from mitochondria is regulated by members of the Bcl-2 family proteins, and the extrinsic pathway where by the interaction between death receptor Fas and its ligand Fas-L induces apoptosis (Pfeffer & Singh, 2018). As an activated form of cysteine protease, cleaved caspase-3 is considered to act as the major executioner of apoptosis in both intrinsic and extrinsic signaling cascades (Boland et al., 2013). In the current study, significantly increased numbers of cleaved caspase-3+ cells were observed in three MTX and/or DEX treatment groups when compared to the untreated control, suggesting apoptosis is indeed induced in mature ATDC5 chondrocytes following MTX and/or DEX treatment. In addition, consistent with previous studies which showed that chondrocyte apoptosis in growth plate induced by MTX or DEX treatment is related to the Fas/Fas-L and Bcl-2/Bax expression, respectively (Fan et al., 2012; Xian et al., 2007; Zaman et al., 2012), the current study demonstrated that MTX treatment caused a remarkable Fas-L induction but no significant differences in Bax and Bcl-2 expression in chondrocytes. On the other hand, despite the elevated expression in proapoptotic protein Bax and antiapoptotic protein Bcl-2, the relative concentration of Bax and Bcl-2 (Bax/Bcl-2 ratio), which acts as the determiner for cell susceptibility to apoptosis, was sharply increased by the DEX individual treatment. Interestingly, MTX and DEX combination treatment caused a higher density of cleaved caspase-3+ cells when compared to DEX induvial treatment, and it enhanced expression of both Fas-L and Bax/Bcl-2 ratio. These findings indicated chondrocyte apoptosis induced by MTX or DEX treatment is regulated by the two different pathways in culture and the combination treatment of MTX and DEX appears to have synergistic or additive effects on chondrocyte apoptosis (in comparison to DEX induvial treatment) in this in vitro model.
4.3 Apoptotic chondrocyte-derived CXCL12 is one determinant for osteoclastic recruitment and differentiation following MTX and/or DEX treatment
MTX or DEX treatment has been previously shown to cause bone cell apoptosis which subsequently leads to a local increase in recruitment of osteoclasts resulting in osteoclastic bone loss (Shandala et al., 2012; Weinstein et al., 1998). However, although MTX or DEX treatment is known to cause thinning of the growth plate with increased chondrocyte apoptosis (Fan et al., 2012; Xian et al., 2007; Xian et al., 2008; Zaman et al., 2012; Zaman et al., 2019), it is less clear how treatment with these drugs can increase chondroclast recruitment following chondrocyte apoptosis and thus contribute to the growth plate thinning. The current study has sought to identify a potential molecular connection between the induced chondrocyte apoptosis and the enhanced osteoclastic migration/differentiation following MTX and/or DEX treatment. Through PCR array assays, candidate gene mRNA and protein expression confirmation analyses, our in vitro model studies demonstrated that chemokine CXCL12 exhibited the most dominant induction in apoptotic chondrocytes in each of MTX and/or DEX treatment groups; and that when compared to MTX or DEX individual treatment, MTX and DEX combination treatment appeared to have no synergistic or antagonistic effects in CXCL12 induction.
Previous studies have shown that CXCL12 participated in multiple homeostatic, developmental, and pathological pathways within the skeletal microenvironment. It is demonstrated that the death of articular chondrocytes is accompanied by a higher concentration of CXCL12 at the chondro-osseous transitional zone (Wei et al., 2006), and that, under a pathological circumstance, CXCL12 is capable of eliciting deleterious effects in the articular cartilage via upregulation of Col-X and matrix metalloprotease (MMP)-13, leading to enhanced chondrocyte hypertrophy and articular cartilage degradation (Wei et al., 2010). CXCL12 is also evident to significantly stimulate the chemotactic recruitment and migration of circulating osteoclast precursor cells partly via upregulation of MMP-9 to penetrate the underlying basal lamina barrier in the process of their migration (Yu et al., 2003), and to exert its direct effect on osteoclast differentiation via promotion of multinucleated cell fusion, size, and TRAP activity (Wright et al., 2005). In the present work, it was found that both exogenous recombinant CXCL12 protein and the CXCL12 contained in the conditioned medium from MTX, DEX, or their combination-treated chondrocytes had the capacity to act as a chemoattractant for osteoclast precursor cells in vitro. On the other hand, although CXCL12 alone (in the absence of RANKL) had no or only a weak capacity to trigger osteoclast differentiation in culture, with the supplementation of RANKL, conditioned medium from MTX, DEX, or their combination -treated chondrocytes, which has a higher content of CXCL12, could considerably promote osteoclast formation, to the extents of 166.7%, 181.5%, 185.2%, respectively, higher than the conditioned medium of untreated cells. Importantly, these promoting effects of the conditioned media could be inhibited by prior treatment with the neutralizing antibody for CXCL12 in each treatment group, further suggesting CXCL12 derived from apoptotic chondrocytes is an important osteoclastic recruitment and stimulating factor following MTX and/or DEX treatment. Furthermore, MTX and DEX combination treatment had no synergistic/additive or antagonistic effects on the pro-osteoclastic potential when compared to individual MTX or DEX treatment.
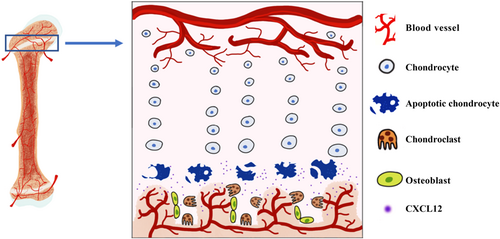
Apart from our above in vitro data (that has identified CXCL12 being a major player in chondroclast/osteoclast recruitment following MTX ± DEX treatment), the current study has also provided in vivo evidence that MTX treatment increases chondrocyte apoptosis and CXCL12 expression in the growth plate and induces a higher recruitment of cartilage-resorbing chondroclasts at the growth plate-metaphysis transition zone. These findings together have indicated the critical roles of apoptotic chondrocytes in coordinating osteoclastic recruitment and differentiation, which occurs potentially via CXCL12 induction in the apoptotic growth plate chondrocytes following MTX and DEX individual or combination treatment (Figure 7). Our findings are consistent with previous observations that support an essential role for hypertrophic chondrocytes in recruiting osteoclasts/chondroclasts by supplying critical signalling molecules including vascular endothelial growth factor, RANKL, and tumor necrosis factor alpha (TNF-α) that are important in regulating endochondral ossification bone growth and/or endochondral bone fracture healing (Alblowi et al., 2013; Gerber et al., 1999; Odgren et al., 2016; Zaman et al., 2019).
4.4 Future directions
However, future work is required to investigate mechanistic profiles of MTX and/or DEX treatment in causing the skeletal impairments and to clarify the roles of other molecules (e.g. IL-17f and TNF-α) induced in apoptotic chondrocytes (to lower extents compared to CXCL12 induction) after MTX and/or DEX chemotherapy. In addition, as CXCL12 exerts biological effects through binding with receptor CXCR4, future studies are required to further investigate the potential roles of CXCL12-CXCR4 axis in MTX and/or DEX treatment-associated growth plate cartilage resorption and thinning and the potential therapeutic effect with administration of a CXCR4 inhibitor as used in a rodent bone cancer-induced bone lysis model (Diamond et al., 2009). Furthermore, while previous studies have demonstrated that cancer chemotherapy including MTX chemotherapy can cause significant damage to the bone marrow microvascular system accompanying the aggravated bone loss (Hassanshahi et al., 2017; Hassanshahi et al., 2019a; 2019b; 2019c), further studies are required to clarify any possible effects of MTX and DEX treatments on vasculogenesis and/or angiogenesis at the growth plate/metaphysis junction. This is important as vasculogenesis and/or angiogenesis are crucial for remodelling calcified hypertrophic growth plate cartilage into woven bone (primary spongiosa) and thus for the coupling of cartilage scaffold production and new endochondral bone formation during bone growth (Gerber et al., 1999). Finally, as there have been no comparative studies which have directly compared the bone growth impairment outcomes resulting from MTX, DEX, or MTX + DEX combination treatment, future in vivo studies will be required to clarify/compare the effects and potential mechanisms of MTX, DEX, or MTX + DEX combination treatments in causing bone growth impairments.
5 CONCLUSION
The findings from the present study showed a molecular connection between induced chondrocyte apoptosis and enhanced osteoclastic migration and formation following MTX and/or DEX treatment, which could be potentially or at least partially linked by CXCL12 induction. It is likely that such effects could result in aggravated calcified growth plate cartilage resorption, leading to the bone growth impairments. In addition, in comparison to MTX or DEX individual treatment, apart from its enhancement of chondrocyte apoptosis, MTX and DEX combination treatment had no synergistic/additive or antagonistic effects in CXCL12 induction and function in osteoclastic migration and formation in vitro. In the present work, data obtained from the cultured ATDC5 chondrocytes have been found to be consistent with our in vivo data obtained from tibial growth plate of young rats treated with MTX. Together, our in vitro and in vivo data suggest that, by enhancing CXCL12 expression, chemotherapy drug (MTX and/or DEX) treatment-induced chondrocyte apoptosis may lead to more recruitment of chondroclasts that can resorb the hypertrophic growth plate cartilage causing bone growth impairments. Data from the present work and findings from future studies will help us to achieve a better understanding how cancer chemotherapeutic treatment with MTX ± DEX can impair bone growth and to develop further studies to evaluate whether CXCL12 could serve as a novel candidate for developing therapeutic strategies for managing bone growth impairments in children receiving chemotherapy with these drugs.
ACKNOWLEDGEMENTS
This work was supported in part by grants from National Health and Medical Research Council of Australia (Grant No.: 1127396), Channel-7 Children's Research Foundation (Grant No.: 171494), and Natural Science Foundation of China (Grant No.: 81671928).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Yu-Wen Su: Investigation (lead); formal analysis (equal); writing—original draft (supporting); writing—review and editing (supporting); funding acquisition (supporting). Jian Fan: Investigation (supporting); formal analysis (equal); writing—original draft (supporting); writing—review and editing (supporting); funding acquisition (supporting). Chia-Ming Fan: Methodology (supporting); investigation (supporting); formal analysis (supporting). Yaser Peymanfar: Methodology (supporting). Cory Xian: Conceptualization; funding acquisition (lead); formal analysis (equal); writing—original draft (lead); writing—review and editing (lead); project administration; supervision.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




