Retracted: Paeoniflorin promotes angiogenesis and tissue regeneration in a full-thickness cutaneous wound model through the PI3K/AKT pathway
Abstract
The treatment of wounds remains a clinical challenge because of poor angiogenesis under the wound bed, and increasingly, the patients' need for functional and aesthetically pleasing scars. For the wound healing process, new blood vessels which can deliver nutrients and oxygen to the wound area are necessary. In this study, we investigated the pro-angiogenesis ability and mechanism in wound healing of paeoniflorin (PF), which is a traditional Chinese medicine. In our in vitro results, the ability for proliferation, migration and in vitro angiogenesis in human umbilical vein endothelial cells was promoted by coculturing with PF (1.25–5 μM). Meanwhile, molecular docking studies revealed that PF has excellent binding abilities to phosphatidylinositol-3-kinase (PI3K) and protein kinase B (AKT), and consistent with our western blot results, that PF suppressed PI3K and AKT phosphorylation. Furthermore, to investigate the healing effect of PF in vivo, we constructed a full-thickness cutaneous wound model in rats. PF stimulated the cellular proliferation status, collagen matrix deposition and remodeling processes in vitro and new blood vessel formation at the wound bed resulting in efficient wound healing after intragastric administration of 10 mg·kg−1·day−1 in vivo. Overall, PF performed the pro-angiogenetic effect in vitro and accelerating wound healing in vivo. In summary, the capacity for angiogenesis in endothelial cells could be enhanced by PF treatment via the PI3K/AKT pathway in vitro and could accelerate the wound healing process in vivo through collagen deposition and angiogenesis in regenerated tissue. This study provides evidence that application of PF represents a novel therapeutic approach for the treatment of cutaneous wounds.
1 INTRODUCTION
Due to the increasingly trauma, burns, and chronic diseases, skin wounds remains a major public health problem all over the world, and can cause pain, infections, and even amputation in millions of patients (Eberhardt & Raffetto, 2014; Valencia, Falabella, Kirsner, & Eaglstein, 2001). The structure and function of injured skin can be repaired and regenerated by the wound healing process (S.-H. Hwang et al., 2016; Muralidhar, Babu, Sankar, Reddanna, & Latha, 2011). In general, the wound healing process is a complicated procedure, which can be classically divided into clot formation, inflammation, proliferation, and remodeling stages (Hosemann, Wigand, Göde, Linger, & Dunker, 1991; B.-S. Kim, Pallua, Bernhagen, & Bucala, 2015; Watelet, Bachert, Gevaert, & Van Cauwenberge, 2002), and these processes involves various growth factors, parenchymal cells and extracellular matrix (ECM). Recently, bioactivity biomaterials have been widely used for enhancing angiogenesis on wound healing (C. Wang, Wang, Gao, et al., 2018; C. Wang, Wang, Xu, et al., 2019; M. Wang, Wang, Chen, et al., 2019); however, it is difficult to generalize the results derived from these studies to clinical practice. For fast vascularization, improving regeneration and accelerating the healing process, finding a new pharmacological approach is necessary and this can be a potential therapeutic strategy for wound healing (Branski, Gauglitz, Herndon, & Jeschke, 2009; Duscher et al., 2016; Harding, Morris, & Patel, 2002).
It is well-known that endothelial cells are important cells in angiogenesis, and are responsible for many biological activities from pre-existing blood vessels, such as proliferation, adhesion, and transmigration (Folkman, 1995). Due to the capacity for conveying nutrition and oxygen to the wound site, new blood vessel formation is imperative for tissue repair and acts as a scaffold for neodermis and increased cell survival. Several growth factors have shown their bioactivities, such as promoting cell proliferation, migration, differentiation and angiogenic activities of endothelial cells (Bir et al., 2014; Scharpfenecker et al., 2007), and vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) have been investigated in treating ischemic disease. However, there are some disadvantages in these growth factors, such as high expense, short protein half-life and undesired side effects, which limits their clinical use potential (S. E. Epstein, Kornowski, Fuchs, & Dvorak, 2001). Therefore, seeking a therapeutic and affordable medicine, which could accelerate angiogenesis in the wound bed for skin wound patients, is a potential alternative treatment (Y.-S. Kim et al., 2011).
Paeoniflorin (PF) is a major pharmacologically active ingredient derived from Paeonia lactiflora Pall or Paeonia veitchii Lynch, which is a traditional Chinese medicine and has been used to treat various diseases (Arentz et al., 2017; Parker et al., 2016). Recently, PF has been investigated in that it is responsible for a lot of therapeutic activity, such as antibacterial, anti-inflammatory, antioxidant antiviral, immunoregulatory, and antinociceptive (Chu et al., 2017; Xu et al., 2017). Moreover, an increasing amount of evidence confirms that PF can improve cardiac function and angiogenesis (Chen, Dong, He, Li, & Wang, 2018; Xin et al., 2016). Previous research has reported that growth factors such as bFGF, PDGF, and VEGF could regulate angiogenesis, and several signal proteins including phosphatidylinositol-3-kinase (PI3K), protein kinase B (Akt) (Zhang et al., 2018) and mTOR (Gupta & Qin, 2003; Munoz-Chapuli, Quesada, & Angel Medina, 2004) participate in the wound healing process. Interestingly, a research study confirmed that suppression of PI3K and AKT can promote the cells proliferation, migration, differentiation (Zhang et al., 2018). However, the potential angio-modulatory and wound healing roles of PF remain poorly understood.
Consequently, in this study, our hypothesis is that PF could promote angiogenesis and accelerate wound healing through suppressing the PI3K/AKT signaling pathway. The proangiogenic effect and the underlying mechanism of PF were investigated in vitro. Rats comprising the full-thickness cutaneous wound model were used to evaluate the therapeutic potential of PF in vivo.
2 EXPERIMENT AND METHODS
2.1 Reagents and antibodies
PF (purity ≥ 98%) was obtained from Herbpurify (Chengdu, China). Calcium fluorescein-AM were provided by Yeason (Shanghai, China). Dimethylsulfoxide (DMSO) was purchased from Sigma Aldrich (St. Louis, MO). PF was dissolved in DMSO at concentrations of 20 mM, and diluted appropriately with cell culture medium (final DMSO concentration<1%). Monoclonal antibodies specific for p-PI3K, PI3K, p-AKT, AKT were purchased from Cell Signaling Technologies (Beverly, MA). Polyclonal antibody cell nuclear antigen (PCNA), α-smooth muscle actin (α-SMA), and cytokeratin were purchased from Millipore (Darmstadt, Germany). Monoclonal antibodies specific for collagen I, collagen III, and fluorescein isothiocyanate-labeled and horseradish peroxidase-labeled secondary antibodies were purchased from Abcam (Cambridge, UK). Hoechst 33258, crystal violet and 4',6-diamidino-2-phenylindole (DAPI) were obtained from Beyotime (Shanghai, China).
2.2 Human umbilical vein endothelial cells (HUVECs) culture
HUVECs were obtained from iCell Bioscience (Shanghai, China). The cells were expanded and maintained in RMPI 1640 containing heat-inactivated 10% fetal bovine serum (FBS), 5% horse serum, penicillin (final concentration, 100 U/ml), and streptomycin (final concentration, 0.1 mg/ml) in a humidified atmosphere of 5% CO2 and 95% air at 37°C in the dark.
2.3 Assessment of cellular viability and proliferation
The cytotoxicity of PF toward HUVECs was measured by a cell counting kit-8 (CCK-8; Dojindo Co., Kumamoto, Japan) according to the manufacturer's protocol. In brief, HUVECs were cultured in 96-well plates (5,000 cells per cm2) at 37°C for 24 hr and then incubated with different concentrations of PF (1.25, 2.5, 5, 10, and 20 μM) for 48 hr. After treatment, the cells were washed with phosphate-buffered saline (PBS), 100 µl of RMPI 1640 containing 10 µl of tetrazolium substrate was added, and the plates were cultured in the dark for another 1 hr. The absorbance was read at 450 nm using a spectrophotometer (Thermo Fisher Scientific).
Cell proliferation was detected by 5-ethynyl-20-deoxyuridine (EdU) assay using a Cell-Light EdU DNA Cell Proliferation Kit (RiboBio, Shanghai, China). The nucleic acids in all cells were stained with Hoechst 33342 Dye. EdU pulse-chase incorporation and cell proliferation rate were applied according to the manufacturer's instructions. Images were taken using a fluorescence microscope (Nikon).
2.4 Cell adhesion assay
2.4.1 Cell-matrix adhesion assay
The cell-matrix adhesion assay was performed, as previously described (Tang et al., 2011). Briefly, HUVECs (1 × 104 cells per well) were incubated with different doses of PF (0, 1.25, 2.5, and 5 μM), then collected and seeded into six-well plates, which were pre-coated with human fibronectin (1 μg/ml, F0895, Sigma). Finally, the cells were incubated at 37°C for 30 min followed by washing with PBS three times. After the floating cells washed out, the adherent cells were then fixed with 4% paraformaldehyde for 30 min followed by Hoechst 33258 (Beyotime, Shanghai, China) staining for another 30 min. The nuclei were visualized and counted under a fluorescence microscope (Nikon) on five random fields.
2.4.2 Cell–cell adhesion assay
The effect of intercellular adhesion with PF was assayed by a cell–cell adhesion assay (Tang et al., 2011). Briefly, treated HUVECs were stained with a cyto-membrane tracker, calcein-AM, for 30 min at 37°C, and then seeded onto another group HUVECs, which were completely confluent and stained with Hoechst 33258. After 30 min, followed by three gentle washes with PBS, the attachment and spread of seeded cells were monitored and recorded with a fluorescence microscope (Nikon).
2.5 Cell migration assay
For evaluating the capacity of migration in HUVECs, a transwell (Corning, 8 μm) assay was used. Briefly, in the transwell system, the upper and lower chambers contained 1 × 105 cells and RMPI 1640 with 1% FBS with different doses of PF (0, 1.25, 2.5 and 5 μM). After a coculture of 12 hr, the upper chamber was fixed in 4% paraformaldehyde for 30 min at room temperature followed by washing with PBS three times and then stained with crystal violet. Finally, the cells on the membrane of the upper chamber were carefully removed by cotton swab. The migrated cells were examined using an inverted microscope (Nikon, ECLIPSE Ti, Japan). All experiments were performed three times, and migrated HUVECs were counted for statistical analysis on five random fields.
2.6 In vitro tube formation assay
To evaluate the HUVEC morphogenesis and tube formation capacity after PF treatment, a tube formation assay on growth factor reduced Matrigel (BD Biosciences) was performed. Briefly, growth factor reduced Matrigel was thawed at 4°C overnight and then placed into a μ-Slide (10 μl per well, IBIDI, Germany) followed by incubation at 37°C for 1 hr to solidify. A total of 5000 cells per well, which were pretreated with different concentrations of PF (0, 1.25, 2.5 and 5 μM) were seeded in the Matrigel precoated μ-Slide. Finally, the μ-Slide was incubated at a cell incubator for 2 hr. Tube formation was observed and quantified under an inverted light microscope (Nikon) on five independent fields.
2.7 Establishment of a wound model
All the Sprague-Dawley (SD) rats were obtained from the Animal Center of the Academy of Medical Science of Zhejiang Province, and all procedures were approved by the Institutional Animal Care and Use Committee of Zhejiang University. Twenty-four SD rats were anesthetized with 10% chloral hydrate (3 ml/kg, i.p.). After shaving and sterilization, two full-thickness wounds were made on each side of the rat's back and the diameter of the wound was about 20 mm. After surgery, PF was dissolved in saline and intragastrically delivered at a dose of 10 mg/kg immediately. In the next few days, rats of the PF treatment group were delivered a dose of 10 mg·kg−1·day−1 until the rats were killed, while rats of the vehicle control group were delivered equivalent normal saline. All the treated rats were housed in individual cages, and at 7, 10, 14 and 21 days, the wounds were photographed. The wound area was calculated using Image-Pro Plus 6.0 software.
2.8 Histology, immunohistochemistry and immunofluorescence
The rats (n = 12 per group) were killed with 10% chloral hydrate (4 ml/kg, i.p.) at specific time points postoperation. First, 0.5 cm sections of skins were fixed with 4% paraformaldehyde for 6 hr and then embedded in paraffin. Skin samples were dissected out in about 5-μm-thick longitudinal sections for hematoxylin and eosin (H&E) staining, Masson staining, immunohistochemistry and immunofluorescence analyses. Collagen and vascularization assays were performed by Masson and H&E staining (Day 7), according to the manufacturers’ instructions. Images were acquired using light microscopy (Olympus, Tokyo, Japan). For immunohistochemistry analyses, the dewaxed and hydrated sections were placed in boiling buffer (citrate buffer; pH 6.0) for 10 min. Each section was then incubated in one drop of 3% H2O2 for 10 min and in one drop of primary monoclonal antibodies of anti-collagen I (1:300) or anti-collagen III (1:300) at 4°C overnight. The sections were washed with PBS, incubated with HRP goat anti-rabbit (1:400; Abcam) for 1 hr, washed again with PBS and incubated with a drop of a DAB chromogen kit (ZSGB-BIO, Beijing, China), then counterstained with hematoxylin (Beyotime Institute of Biotechnology, China) for 5 min. Sections were then counterstained, differentiated and blued. Finally, the sections were dehydrated, dried, sealed, aired and photographed using a Nikon ECLPSE 80i (Nikon, Japan), and analyzed by Image-Pro Plus 6.0 software. For immunofluorescence analysis, before culture with primary antibodies, the tissue sections (Day 7) were managed similar to immunohistochemistry analyses. Then, the sections were incubated with the rabbit monoclonal anti-α-SMA (1:200), mouse monoclonal anti-PCNA (1:200) and rabbit monoclonal anti-cytokeratin (1:200) antibody followed by washing four times with PBS and incubated with AlexaFluor 568 and AlexaFluor 488 donkey anti-rabbit/mouse secondary antibodies for 1 hr at 37°C. Next, the sections were washed with PBS three times, incubated with DAPI for 1 min, washed with PBS and sealed with a coverslip. All images were captured by a fluorescence microscope (Nikon, Japan). Fluorescence intensity was calculated by Image-Pro Plus 6.0 software.
2.9 Molecular modeling
PI3K (PDB ID: 5ITD) and AKT (PDB ID: 3QKK) were chosen for docking studies (Hoegenauer et al., 2016; Kallan et al., 2011). Each protein was prepared for docking after being downloaded from PDB (https://www.rcsb.org/). After being minimized using PyMoL (version 1.7.6), the lowest energy conformations for docking were determined via default parameters. Protein–ligand docking analysis was conducted using AutoDockTools (version 1.5.6), which can provide ligand binding flexibility with the binding pocket residues. The images were finally generated using UCSF PyMoL.
2.10 Western blot analysis
Western blot analysis was used to examine the amount of intact p-PI3K and p-AKT. Sodium dodecyl sulfate polyacrylamide gel electrophoresis was utilized for separation followed by protein blotting on a polyvinylidene fluoride membrane (Bio-Rad Laboratories, Hercules, CA). The membrane was blocked with 5% bovine serum albumin in Tris-buffered saline (TBS) with 0.05% Tween 20 for 1 hr, then incubated with the appropriate primary antibodies: anti-PI3K (1:1,000), anti-p-PI3K (1:1,000), anti-AKT (1:1,000) and anti-p-AKT (1:1,000) overnight. The membranes were washed with TBS three3 times and incubated with horseradish peroxidase-conjugated secondary antibodies for 2 hr at room temperature. Signals were visualized using the ChemiDicTM XRS + Imaging System (Bio-Rad Laboratories, Hercules, CA), and band densities were quantified using Image-Pro Plus 6.0 software.
2.11 Statistical analysis
The results are presented as mean ± SD. One-way analysis of variance (ANOVA) plus Tukey's test or Kruskal–Wallis analysis (non-parametric ANOVA) plus Dunn's multiple comparisons (when the data failed the assumptions of one-way ANOVA) were used to test differences between groups. Statistically significance was set at *p < .05, **p < .01 versus the indicated group.
3 RESULTS
3.1 PF enhanced proliferation capacity in HUVECs
The chemical structure of PF is shown in Figure S1. The cytotoxicity assay of PF on HUVECs was performed using a CCK-8 kit. Cells were seeded in a 96-well plate and cocultured with different doses of PF (0, 1.25, 2.5, 5, 10 and 20 μM) for 48 hr, followed by CCK-8 analysis. As shown in Figure 1a, the cell viability was significantly increased in a dose-dependent manner 0–5 μM) after PF treatment. Thus, in our following experiments, the concentration of PF used was between 1.25 and 5 μM. A proliferation assay of PF on HUVECs was performed using a Cell Proliferation Kit. Results showed that the proliferation of HUVECs can be dose-dependently promoted by the PF treatment (Figure 1b,c), which was also consistent with the CCK-8 data.
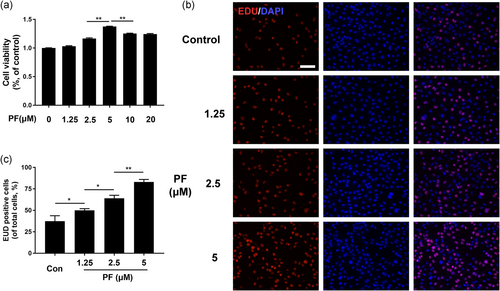
3.2 PF regulated adhesion ability in HUVECs
To evaluate the cell-matrix adhesion ability in HUVECs after PF treatment, human fibronectin was used as an ECM substitute. PF-treated cells could attach to the ECM gel more easily than control cells and were enhanced in a dose-dependent manner (Figure 2a,b). For cell–cell adhesion, inversely, this significantly reduced the adhesive ability of PF-treated cells to the HUVECs monolayer and decreased this in a dose-dependent manner (Figure 2c,d). Thus, PF could regulate adhesion ability in HUVECs.
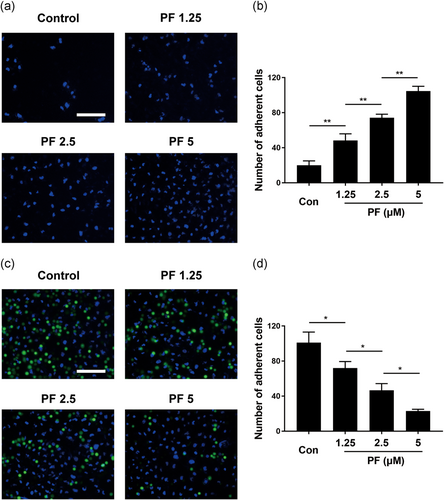
3.3 PF promoted migration and tube formation of HUVECs
In the initial stage of angiogenesis, the targeted migration of endothelial cells plays a vital role. To evaluate the motility of HUVECs after PF treatment, a transwell assay was used. Interestingly, PF could remarkably drive HUVEC migration to the lower chamber (Figure 3a,b), which also showed it accelerated cell migration in a concentration-dependent manner 1.25–5 μM). Moreover, the capillary tube formation of HUVECs was investigated using an in vitro angiogenesis assay. Compared to the control group, the PF-treated group (Figure 3c,d) remarkably increased the number of sprouting tubules in HUVECs, which suggested that the ability of HUVECs to form tube-like structures could be strongly promoted by PF.
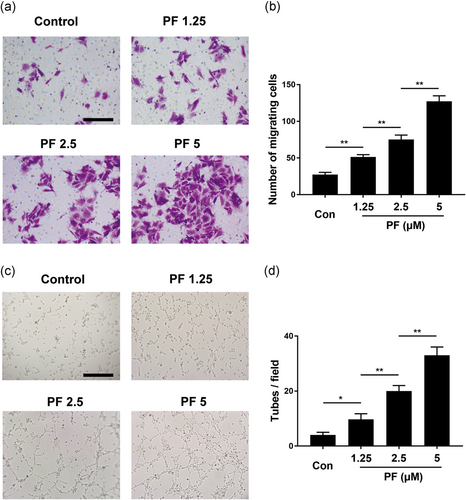
3.4 Molecular docking and the PI3K/AKT pathway
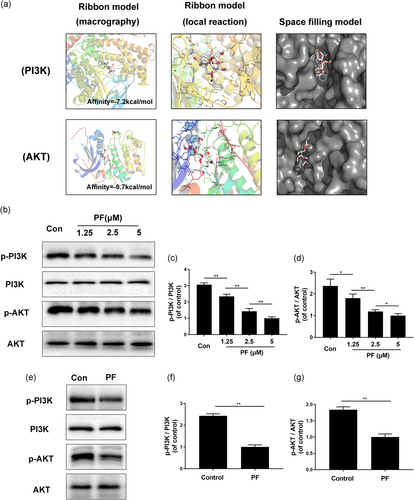
To examine whether PF has a direct affinity to related proteins of the PI3K and AKT, we performed a molecular docking analysis of PF with the respective proteins involved in these two pathways, according to the diverse binding pockets of the antagonist (Hoegenauer et al., 2016; Kallan et al., 2011). The chemical structure of PF, [(1R,2S,3R,5R,6R,8S)-3-(β-d-glucopyranosyloxy)-6-hydroxy-8-methyl-9,10-dioxatetracyclo[4.3.1.02,5.03,8]dec-2-yl]methyl benzoate, is shown in Figure S1. By studying all the models returned, we found that PF formed some favorable connections and docked nicely within the PI3K and AKT binding sites (Figure 4a). In Figure 4a, the macro views and the views of the local interactions of protein residues are shown in a ribbon model. Meanwhile, space filling models are used to directly illustrate the coverage of PF in the related protein structures. According to the docking with the PI3K structure (Figure 4a), some important hydrogen bonds were formed between PF and PI3K concerning ASP805, THR856 and ASP933, with a high affinity of −7.2 kcal/mol. Moreover, a docking study with AKT was performed. We found that some important hydrogen bonds were formed between PF and LEU166 of AKT with a high affinity of −9.7 kcal/mol. All images were generated using PyMOL. Furthermore, to confirm the change of protein level in vitro and in vivo, western blot analysis was performed. The results showed that after PF treatment, p-PI3K and p-AKT expression were significantly decreased and exhibited in a concentration-dependent manner in vitro (Figure 4b–d). Moreover, in vivo western blot was consistent with the in vitro results (Figure 4e–g). Taking these results together, regulating the PI3K/AKT pathway may play a vital role in angiogenic ability of PF in vitro and in vivo.
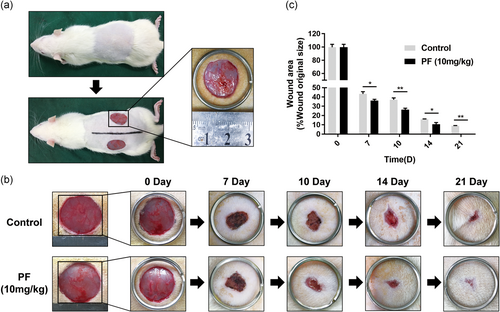
3.5 PF accelerated cutaneous wound healing in rats
Based on our in vitro results, PF showed its angiogenic effects and we supposed that it could also enhance angiogenesis in vivo and facilitate cutaneous wound healing. Therefore, a full-thickness cutaneous wound model of rats was used to evaluate the ability of promoting wound repair after PF treatment. The cutaneous wound model is shown in Figure 5a. The groups treated with PF (10 mg·kg−1·day−1) showed wound healing more quickly than the control groups (Figure 5b). As shown in Figure 5b, the wound areas shrank and closed most rapidly over the first 7 days. In PF-treated groups and control groups, the wounds achieved nearly 50% and 35% healing, respectively. On Day 10 and 14, the healing rate of the PF group accelerated and healing still occurred at a significantly higher rate than control, especially at Day 14. By Day 21, wounds in the PF-treated group were almost closed, while some of the wounds remained unhealed in control groups (Figure 5c), demonstrating that PF could accelerate wound healing in vivo.
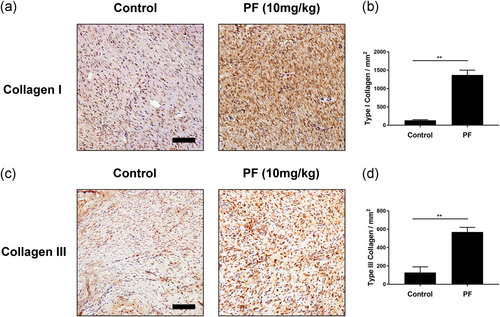
3.6 PF accelerated collagen deposition and remodeling
During the wound healing process, such as formation of granulation tissue and scars, could be directed by collagen deposition and arrangement (Clark, 1993; Merkel, DiPaolo, Hallock, & Rice, 1988). To evaluate the collagen deposition and remodeling, we used Masson Trichrome Staining and immunohistochemical staining of collagen I and III. As shown in Figure S2, strong and regular blue staining could be observed in PF-treated groups by Masson staining compared with control, demonstrating abundant and arranged regenerated collagen deposition. Furthermore, we performed immunohistochemical staining of collagen I and III, which are the newly formed ECM (Figure 6a,c). By quantifying the immunohistochemical results, as expected, both collagen I and III expression were enhanced by PF treatment (Figure 6b,d) at Day 7.
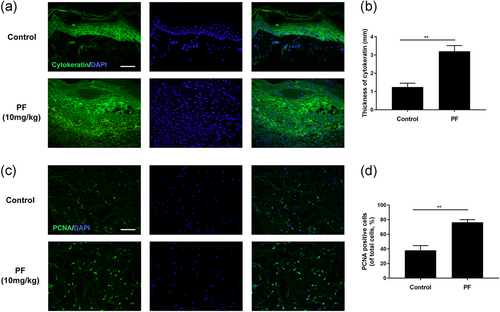
3.7 PF stimulated cytokeratin expression and cell proliferative activities
For evaluating the re-epithelialization status in the wound area, we performed immunofluorescence to detect cytokeratin. On Day 7, a thicker neo-epidermis was observed in PF-treated groups than that in control group (Figure 7a,b), suggesting the re-epithelization rate of wounds in PF-treated groups were faster than the untreated control. Furthermore, a cell proliferation biomarker, PCNA, was detected by immunofluorescence. As shown in Figure 7c,d, PCNA-positive cells were promoted in PF-treated wounds than the control on Day 7, demonstrating PF could stimulate cell proliferation in the wound area.
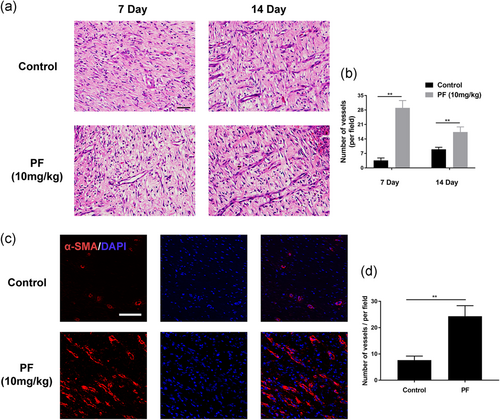
3.8 PF promotes angiogenesis in vivo
As shown in Figure 8a,b, the number of vessels on the wound bed were observed remarkedly higher in the PF-treated groups than the control groups by H&E staining at Day 7 and 14, demonstrating PF treatment can significantly promote the capillary vessel formation in the wound bed in vivo. During the wound healing process, neovascularization is required and we immunostained the vessel marker, α-SMA, to detect the angiogenesis status (Figure 8c,d). The number of newly formed vessels which were stained by α-SMA was significantly enhanced by PF treatment. Taking the H&E and immunofluorescence results together, PF boosts angiogenesis in full-thickness wounds and further leads to fast healing of wounds.
4 DISCUSSION
Newly-formed capillary vessels are pivotal for tissue regeneration due to the capacity for transporting nutrition and oxygen to the cells in the wound site (Zeng & Zhu, 2014). Therefore, our group investigated the effects of a traditional Chinese medicine called PF on boosting angiogenesis and wound healing. In our in vitro experiment, HUVECs could enhance their capacities of proliferation, migration and angiogenesis after PF dose-dependent treatment, which concerned the downregulation of PI3K/ATK signaling pathway. In our in vivo experiment, intragastric delivery of PF (10 mg·kg−1·day−1) significantly sped up the wound healing process, characterized by quicker wound closure, faster deposition and remodeling of the collagen matrix, more obvious keratinization and more newly formed capillary vessels at the wound sites. As PF could enhance angiogenesis by downregulating the PI3K/AKT signaling in vitro, and remarkedly promote wound healing in vivo, it can be considered as a promising medicine for ischemic wound therapy.
In the wound healing process, the cells involved in the wound area rely on newly formed capillary vessels, which could provide sufficient oxygen and nutrients. Additionally, acting as a scaffold to support granulation tissue and neodermis formation is another function (Gurtner, Werner, Barrandon, & Longaker, 2008; Martin, 1997). In our study, the ability for HUVECs proliferation, adhesion, migration and tube formation was regulated by PF treatment, demonstrating that PF had pro-angiogenic effects. In general, the angiogenic process could be divided into a few stages, including endothelial cell proliferation, separation and migration, adherence to the ECM and differentiation (Ingber, 2002; Lamalice, Le Boeuf, & Huot, 2007). In our present study, PF could evidently boost HUVECs proliferation, which can be regarded as a signal of the beginning of angiogenesis. Moreover, PF-treated HUVECs remarkedly enhanced its ability for migration, which could benefit and speed up the angiogenic process and is critical in the reconstruction of the blood vessel network (Cheng & Eriksson, 2017; Eelen et al., 2018; Lamalice et al., 2007). In addition, PF could regulate the adhesion of HUVECs, with decreased cell–cell adhesion and increased cell-matrix adhesion. Finally, endothelial cells differentiate into new capillary vessels, which are confirmed by the faster tube formation rate and tube numbers (Figure 3c,d). Taken together, PF exerted effective in pro-angiogenesis in vitro, however the underlying mechanism involved in this pro-angiogensis effect remains unknown. Thus, we will investigate this in a further study.
PI3Ks are lipid kinases that phosphorylate inositol phospholipids (PtdIns), controlling spatially and temporally membrane lipid composition. Changes in the phosphorylation status of PtdIns regulate essential cell physiological functions, such as membrane and actin dynamics and cell signaling, proliferation, metabolism, and angiogenesis (Balla, 2013; Yoshioka et al., 2012). AKT, a downstream signaling molecule of PI3K and activation of PI3K/ATK pathway in cancer cells could inhibit angiogenesis (Zhang et al., 2018). In our study, PF showed a direct affinity to PI3K and AKT proteins, as confirmed by western blot results, in which the p-PI3K and p-AKT were decreased after PF treatment, demonstrating that PF could promote angiogenesis via the PI3K/AKT signaling pathway.
Furthermore, a full-thickness cutaneous wound model of rats was used to evaluate the healing effect of PF in this study. Masson and immunohistochemical staining showed that PF could promote collagen deposition and remodeling in the wound bed, especially collagen I and III, characterized by abundant arrayed collagen fibers under the wound area, indicating that wound healing rate was partly dependent on collagen synthesis. In the early stage of the wound healing process, appropriate deposition of both types of collagen fibers would be of benefit to a better outcome in the wound healing area. However, synthesis of both type I and type III collagen could decisively determine the strength and appearance of the scar, which will affect the aesthetics of the wound after healing (Brem et al., 2009; Leivonen, Häkkinen, Liu, & Kähäri, 2005; Werner, Krieg, & Smola, 2007). Furthermore, cytokeratin expression, which means the level of keratinization, could be enhanced after PF treatment, suggesting PF could improve re-epithelialization of wound regeneration. After wound injury, many types of cells will proliferate and migrate to the wound area, such as fibroblasts, macrophages and endothelial cells, to assist wound healing. As expected, a biomarker of cellular proliferative capacity, PCNA, was remarkedly promoted by the PF treatment, demonstrating that the faster proliferation rate was partly facilitating wound healing. Taken together, it has been shown that ECM deposition and remodeling, re-epithelialization, cell proliferation, neovascularization, are common indicators that reflect the healing rates of cutaneous wounds (Gurtner et al., 2008).
Angiogenesis, which partly relies on collagen synthesis and cell proliferation is essential to the wound healing process (Xing, Culbertson, Wen, Robson, & Franz, 2011). Newly-formed vessels, which could convey food and act as a scaffold for cells to stay or migrate (F. H. Epstein, Singer, & Clark, 1999), were promoted by PF treatment, as evidenced by H&E staining and α-SMA immunofluorescence staining. These results also indicated that in vitro angiogenesis enhanced by PF can be functional in wound sites in vivo, and cells such as endothelial cells and fibroblasts would proliferate faster and synthesize more collagen fibers in the wound sites during angiogenesis (Martin, 1997). Thus, the high level of angiogenesis stimulated by the PF could regulate the abundantly arrayed collagen in the wounds. Moreover, new capillary vessel formation and appropriate collagen deposition are vital in providing a satisfactory wound bed for regeneration and determining the outcome of wound healing (Bates & Jones, 2003; J. Hwang et al., 2004). The downregulated levels of p-PI3K and p-AKT were also confirmed in vivo, indicating that the PI3K/AKT signaling pathway may participate in wound healing after PF treatment and could be related to faster angiogenesis and collagen deposition. Taken together, the speed of the angiogenic ability regulated by PF may contribute to faster collagen deposition and remodeling rates, finally leading to faster and better healing in wounds.
5 CONCLUSIONS
In summary, our study demonstrated that the capacity of HUVECs in proliferation, migration and angiogenic differentiation could be promoted via the PI3K/AKT signaling pathway by PF treatment. Moreover, PF speeds up vascularization in regenerated tissue and accelerates wound healing in full-thickness cutaneous wounds due to collagen deposition and remodeling, cell proliferation, re-epithelialization, and vessel formation. These findings suggest that PF has the potential to become a promising therapeutic medicine in wound healing or vascular disease.
ACKNOWLEDGMENTS
This study was supported by the Basic Public Welfare Research Project of Zhejiang Province (No. LY19H060003 and LGF18H150008), the Medical Health Science and Technology Project of Zhejiang Province (Nos. 2020KY183 and 2019KY464) and the National Natural Science Foundation of China (grant nos. 51802227).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




