Epoxyeicosatrienoic acids inhibit the activation of NLRP3 inflammasome in murine macrophages
Abstract
Epoxyeicosatrienoic acids (EETs) derived from arachidonic acid exert anti-inflammation effects. We have reported that blocking the degradation of EETs with a soluble epoxide hydrolase (sEH) inhibitor protects mice from lipopolysaccharide (LPS)-induced acute lung injury (ALI). The underlying mechanisms remain essential questions. In this study, we investigated the effects of EETs on the activation of nucleotide-binding domain leucine-rich repeat-containing receptor, pyrin domain-containing-3 (NLRP3) inflammasome in murine macrophages. In an LPS-induced ALI murine model, we found that sEH inhibitor 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl), TPPU, profoundly attenuated the pathological injury and inhibited the activation of the NLRP3 inflammasome, characterized by the reduction of the protein expression of NLRP3, ASC, pro-caspase-1, interleukin precursor (pro-IL-1β), and IL-1β p17 in the lungs of LPS-treated mice. In vitro, primary peritoneal macrophages from C57BL/6 were primed with LPS and activated with exogenous adenosine triphosphate (ATP). TPPU treatment remarkably reduced the expression of NLRP3 inflammasome-related molecules and blocked the activation of NLRP3 inflammasome. Importantly, four EETs (5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET) inhibited the activation of NLRP3 inflammasome induced by LPS + ATP or LPS + nigericin in macrophages in various degree. While the inhibitory effect of 5,6-EET was the weakest. Mechanismly, EETs profoundly decreased the content of reactive oxygen species (ROS) and restored the calcium overload in macrophages receiving LPS + ATP stimulation. In conclusion, this study suggests that EETs inhibit the activation of the NLRP3 inflammasome by suppressing calcium overload and ROS production in macrophages, contributing to the therapeutic potency to ALI.
Abbreviations
-
- ALI
-
- acute lung injury
-
- ARA
-
- arachidonic acid
-
- ARDS
-
- acute respiratory distress syndrome
-
- ASC
-
- apoptosis-associated speck-forming complex containing CARD
-
- ATP
-
- adenosine triphosphate
-
- BALF
-
- bronchoalveolar lavage fluid
-
- COX
-
- cyclooxygenase
-
- CYP
-
- cytochromes P450
-
- EETs
-
- epoxyeicosatrienoic acids
-
- ELISA
-
- enzyme-linked immunosorbent assay
-
- GPR40
-
- G protein-coupled receptor 40
-
- HMGB1
-
- high-mobility group box 1
-
- HO-1
-
- heme oxygenase 1
-
- HIF-1α
-
- hypoxia-inducible factor-1α
-
- JNK
-
- c-Jun N-terminal kinases
-
- LOX
-
- lipoxygenase
-
- LPS
-
- lipopolysaccharide
-
- NLRP3
-
- nucleotide binding domain leucine-rich repeat-containing receptor, pyrin domain-containing-3
-
- NF-κB
-
- nuclear factor-κB
-
- PPARs
-
- peroxisome proliferator-activated receptors
-
- ROS
-
- reactive oxygen species
-
- sEH
-
- soluble epoxide hydrolase
-
- STAT3
-
- signal transducer and activator of transcription-3
-
- TPPU
-
- 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl)
-
- TREM-1
-
- triggering receptors expressed on myeloid cell-1
-
- TRPV4
-
- transient receptor potential vanilloid 4
1 INTRODUCTION
Acute lung injury (ALI) and its severe form acute respiratory distress syndrome (ARDS) are life-threatening diseases resulting from various situations, such as bacterial infection, hyperventilation, and trauma (Ferguson et al., 2013). ALI is characterized by the disruption of the alveolar-capillary interface, refractory hypoxemia, and progressive dyspnea (Bellani et al., 2016). In the lung, alveolar macrophages are the first barrier defending against pathogens by sensing injury stimulus, recruiting neutrophils, and producing various cytokines (Allard, Panariti, & Martin, 2018). Currently, no pharmacologic treatment is available for ARDS, just supportive management with lung-protective mechanical ventilation (Fan, Brodie, & Slutsky, 2018), resulting in mortality as high as 46% (Goligher et al., 2018). Therefore, it is of great significance to find an effective treatment for ALI.
Arachidonic acid (ARA) is widely involved in physiological and pathological processes (Back, Yurdagul, Tabas, Oorni, & Kovanen, 2019; Jang, Kim, & Hwang, 2020). ARA can be metabolized into prostaglandins and leukotrienes by cyclooxygenase (COX) and lipoxygenase (LOX), respectively (Y. Liu et al., 2019). Recently a third metabolic pathway of ARA has attracted wide attention, by which epoxyeicosatrienoic acids (EETs) are synthesized by cytochromes P450 epoxygenases (CYP). There are four isomers, including 5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET (Spector, Fang, Snyder, & Weintraub, 2004). Collective evidence points out that EETs exert anti-inflammatory, antioxidant, antiapoptotic, and antifibrotic effects (Node et al., 1999; Y. Zhou et al., 2016; C. Zhou et al., 2018). CYPs are highly expressed in the lung (Yang et al., 2020), and EETs are the main ARA metabolite in the lung during bacterial infection (Kiss et al., 2010), suggesting that EETs play a key role in maintaining pulmonary homeostasis. While EETs can be hydrolyzed rapidly by soluble epoxide hydrolase (sEH). Therefore, the inhibition of sEH activity is the key to stabilize EETs and amplify its protective effect (Luo et al., 2019). 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl), TPPU, is a specific sEH inhibitor (Luo et al., 2019). We have demonstrated that TPPU can increase EETs content, improve the survival rate, and reduce macrophages infiltration in bronchoalveolar lavage fluid (BALF) of ALI mice (Y. Zhou et al., 2017). Subsequently, other studies report that blocking or knockout of sEH alleviated ALI in mice (Li, Tao, Yang, & Shu, 2018; L. P. Liu, Li, Shuai, Zhu, & Li, 2018). All these findings suggest that EETs may be an endogenous protective mediator against ALI. However, the underlying mechanisms are still unclear completely.
Excessive activation of nucleotide-binding domain leucine-rich repeat-containing receptor, pyrin domain-containing-3 (NLRP3) inflammasome plays a vital role in the development of ALI (Grailer et al., 2014; Jones et al., 2014). NLRP3 deficiency is protective against hyperoxia-induced ALI (Fukumoto et al., 2013). We have reported that vasoactive intestinal peptide attenuates LPS-induced ALI in mice by suppressing the activation of NLRP3 inflammasome (Sun et al., 2018; Y. Zhou et al., 2020). The NLRP3 inflammasome activation requires two steps: priming and activation. Toll-like receptors or tumor necrosis factor receptor (signal 1) mediates the priming process via nuclear factor-κB (NF-κB) pathways (Mangan et al., 2018), leading to the upregulation of the components, including sensor molecule NLRP3, apoptosis-associated speck-forming complex containing CARD (ASC), pro-caspase-1, and interleukin precursor (pro-IL-1β, pro-IL-18; Yu & Lee, 2016). Then, under the stimulation of the pathogen-associated molecular patterns and damage-associated molecular patterns (signal 2, such as ATP and nigericin) (Mangan et al., 2018; Swanson, Deng, & Ting, 2019), reactive oxygen species (ROS), ion flow (Ca2+ flux or K+ efflux), and lysosomal homeostasis can provoke the activation of the NLRP3 inflammasome, mediating the maturation and secretion of IL-1β and IL-18 (Di et al., 2018).
In our previous study, we found that TPPU reduces the secretion of IL-1β (Y. Zhou et al., 2017). However, it is unclear whether EETs inhibit the activation of NLRP3 inflammasome in macrophages. It has been reported that EETs inhibit Ca2+ influx (You et al., 2016) and reduce mitochondrial damage (Inceoglu, Bettaieb, Haj, Gomes, & Hammock, 2017; Sarkar et al., 2014). Therefore, in this study, we investigated the effects of EETs on the activation of NLRP3 inflammasome in murine macrophages in vitro. We found that EETs inhibited the activation of the NLRP3 inflammasome by suppressing calcium overload and ROS generation in macrophages, contributing to its therapeutic potency to ALI.
2 MATERIALS AND METHODS
2.1 Animals
Male C57BL/6 mice (18-20 g) were provided by Hunan SJA Laboratory Animal Co., Ltd (Hunan, China). Mice were housed in a pathogen-free room with free access to food and water and a 12-hr light/dark cycle. All animal studies were performed according to the guidelines of the National Institutes of Health and approved by the Ethics Committee of the School of Basic Medical Science at Central South University (Changsha, China).
2.2 In vivo treatments
Mice were randomly divided into three groups: (a) the control group: intratracheal injection of saline; (b) the ALI group: intratracheal injection of LPS (5 mg/kg, from Escherichia coli O111: B4, Sigma-Aldrich, St. Louis, MO); (c) the ALI + TPPU group: intraperitoneal injection of TPPU (1 mg/kg, in 0.5% EtOH) 3 hr before the LPS injection, as previously described (Y. Zhou et al., 2017). Six hours after the LPS injection, mice were killed under anesthesia.
2.3 Pulmonary histology and injury score analysis
Right upper lungs were fixed in 10% formalin overnight, embedded in paraffin, and cut into 4-μm thick slices. The slices were stained with hematoxylin-eosin (H&E). Pulmonary injury score was measured blindly by three pathologists according to five independent variables: neutrophils in the alveolar space, neutrophils in the interstitial space, hyaline membranes, proteinaceous debris filling the airspaces, and alveolar septal thickening (Y. F. Zhang et al., 2020).
2.4 Bronchoalveolar lavage fluid collection
BALF was obtained by lavaging the lung with 0.8 ml cooled sterile saline three times, as previously described (Zhong et al., 2019). The cell-free BALF was obtained after centrifuged at 1,500 rpm for 5 min and stored at −80°C for cytokine detection.
2.5 Isolation of primary murine peritoneal macrophages
Peritoneal macrophages were recruited from C57BL/6 mice 4 days after intraperitoneal injection of 3 ml 3% thioglycolate (Sigma-Aldrich). Peritoneal macrophages were collected by lavage with cooled Roswell Park Memorial Institute (RPMI) 1640 (Gibco, Life Technologies, Carlsbad, CA), centrifuged at 800 rpm for 10 min, then washed with cooled phosphate-buffered saline. Cells were plated into 12-well plates (1 × 106 cells/well) for gene expression and 6-well plates (2 × 106 cells/well) for protein detection. The medium was replaced 2 hr later to remove suspension cells.
2.6 Treatment of macrophages in vitro
Macrophages obtained from peritoneal lavage were cultured in RPMI 1640 containing 10% fetal bovine serum and 5% penicillin/streptomycin and incubated (37°C, 5% CO2). To investigate the role of TPPU in LPS-treated macrophages, cells were grouped into: (a) the control group; (b) the TPPU group: cells were treated with TPPU (10 μM); (c) the LPS group: cells were treated with LPS (100 ng/ml); and (d) the LPS + TPPU group: cells were pretreated with TPPU (10 μM) for 1 hr, followed by LPS (100 ng/ml). Macrophages were harvested 6 hr and 12 hr after LPS treatment for gene and protein expression detection, respectively. To investigate the effects of EETs on the activation of NLRP3 inflammasome, cells were grouped into six groups: (a) the control group; (b) the LPS + ATP/LPS + nigericin group: macrophages were treated with LPS (100 ng/ml) for 135 min and ATP (2.5 mM) or nigericin (20 μM) for 30 min according to previous report (Armstrong, Bording-Jorgensen, Chan, & Wine, 2019); (c–f) the EETs group: cells were pretreated with EETs (5 μM) 10 min before activated by LPS + ATP/LPS + nigericin. After that, macrophages and supernatant were collected for the following detection.
2.7 Western blotting
Western blotting was performed as previously described (C. Y. Zhang et al., 2020). Briefly, protein samples extracted from lung tissues, cells, BALF, and supernatant were quantified with the BCA protein kit (Thermo Fisher Scientific, Waltham, MA) and subjected to electrophoresis on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The protein was transferred to polyvinylidene fluoride membranes and blocked with 5% skim milk for 1 hr at room temperature. Then, the membranes were incubated with primary antibody overnight at 4°C: rabbit anti-NLRP3 antibody (1:2,000; CST, Danvers, MA); goat anti-pro-IL-1β antibody (1:2,000; R&D, Minneapolis, MN); rabbit anti-high-mobility group box 1 (HMGB1) antibody (1:500; proteintech, Rosemont, IL); rabbit anti-pro-caspase-1 antibody (1:1,000; Abcam, Eugene, OR); rabbit anti-ASC (1:1,000, CST); rabbit anti-β-tubulin (1:2,000, Servicebio, China). The membranes were incubated with a suitable horseradish peroxidase-linked secondary antibody. The blots were visualized with chemiluminescence (Millipore, Burlington, MA) in a Chemi Doc XRS (Bio-Rad, Hercules, CA) image system and analyzed with the Image Lab Analyzer software (Bio-Rad).
2.8 RNA isolation and real-time polymerase chain reaction
Total RNA from tissues and peritoneal macrophages was isolated with RNAiso Plus kit (Takara, Kusatsu, Japan). The RNA concentration and quality were assessed by spectrophotometry (Thermo Fisher Scientific). Reverse Transcription kit (Takara) was used to convert total RNA into complementary DNA. The messenger RNA (mRNA) level of Nlrp3, pro-il-1β, pro-il-18, pro-caspase-1, and Asc was detected with SYBR (Takara) on a Real-Time PCR Detection System (CFX96 TouchTM, Bio-Rad, Hercules, CA). The relative expression of target genes was calculated using the method by normalized to β-actin (T. Liu et al., 2016). Sequences of the primers used in this study are listed in Table 1.
| Gene | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|
| Nlrp3 | TACGGCCGTCTACGTCTTCT | CGCAGATCACACTCCTCAAA |
| pro-caspase-1 | CACAGCTCTGGAGATGGTGA | CTTTCAAGCTTGGGCACTTC |
| pro-il-1β | CAGGCAGGCAGTATCACTCA | AGCTCATATGGGTCCGACAG |
| pro-il-18 | ACGTGTTCCAGGACACAACA | CAAACCCTCCCCACCTAACT |
| Asc | GACAGTACCAGGCAGTTCGT | AGTCCTTGCAGGTCAGGTTC |
| β-actin | TTCCAGCCTTCCTTCTTG | GGAGCCAGAGCAGTAATC |
2.9 Enzyme-linked immunosorbent assay
The content of IL-1β in serums and cell supernatants was evaluated by an enzyme-linked immunosorbent assay (ELISA) kit (Invitrogen, Thermo Fisher Scientific), following the application instructions. The content of IL-1β was quantified by comparison of the optical density (450 and 570 nm) with the standard curve.
2.10 Detection of ROS
An ROS assay (Jiancheng Bioengineering Institute, Nanjing, China) was used to detect intracellular ROS content. After the treatment mentioned above, a 10-μM fluorescent indicator of cytosolic ROS, dichloro-dihydro-fluorescein diacetate, was added in cell culture medium and incubated for 30 min in the dark. Immunofluorescence photograph was obtained by a fluorescence microscope, while the DCF fluorescence was detected by spectrophotometry (Thermo Fisher Scientific).
2.11 Confocal microscope fluorescent images for intracellular [Ca2+]
A confocal microscope (UltraVIEW VoX; Perkin Elmer) was used to detect fluorescence intensity of intracellular Ca2+. Briefly, macrophages were seeded onto glass-bottom cell culture dishes. After the treatment mentioned above, cells were washed with Hanks balanced salt solution and incubated with 5 μM fluo-3 acetoxymethyl (Sigma-Aldrich) at 37°C for 40 min.
2.12 Statistical analysis
All data were expressed as means ± standard deviation. Values were derived from three independent experiments. Data from multiple treatments were analyzed by analysis of variance, followed by Tukey's multiple comparison test. SPSS 22.0 (IBM, Chicago, IL) was performed for statistical analysis. Differences were considered significant at p < .05.
3 RESULTS
3.1 TPPU inhibits the activation of NLRP3 inflammasome in the lungs of ALI mice
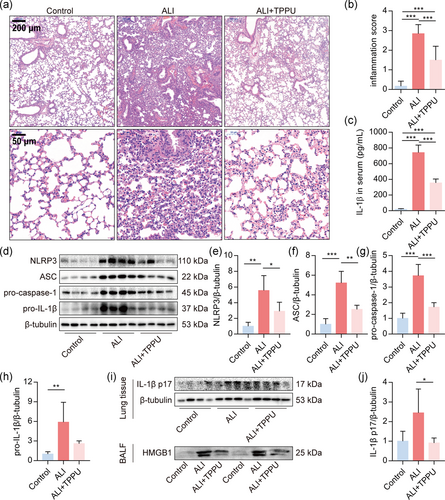
We first investigated whether blockade of sEH with TPPU repressed the activation of NLRP3 inflammasome in vivo. In accordance with our previous study (Y. Zhou et al., 2017), TPPU reduced lung tissue pathological injury (Figure 1a,b) and IL-1β content in serum (Figure 1c) in LPS-induced ALI mice. Furthermore, TPPU profoundly reduced the expression of NLRP3, ASC, pro-caspase1, and pro-IL-1β in the lungs (Figure 1d–h). In addition, TPPU decreased the expression of IL-1β p17 in lung tissue (Figure 1i,j). NLRP3 inflammasome also mediates the release of HMGB1 (Lu et al., 2012). We also found that TPPU dramatically reduced the expression of HMGB1 in BALF of ALI mice (Figure 1i). These results suggested that blocking the degradation of endogenous EETs inhibits the activation of NLRP3 inflammasome in LPS-treated mice.
3.2 TPPU inhibits the activation of NLRP3 inflammasome in macrophages
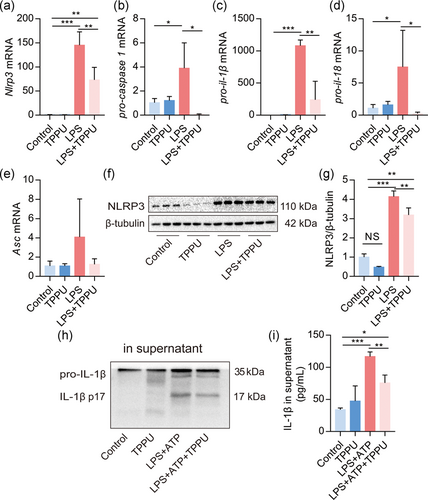
Next, we investigated the effects of TPPU on the NLRP3 inflammasome activation in primary macrophages. Results demonstrated that TPPU significantly reduced the mRNA level of Nlrp3, pro-caspase-1, pro-il-1β, and pro-il-18, but not Asc in LPS-primed macrophages (Figure 2a–e). TPPU treatment alone did affect the protein expression of NLRP3, but significantly reduced LPS-induced NLRP3 expression in macrophages (Figure 2f,g). In addition, TPPU also reduced the IL-1β p17 protein expression in the supernatant of macrophages activated by LPS + ATP (Figure 2h), which was confirmed by the result of ELISA (Figure 2i). Altogether, these data indicated that TPPU inhibits the activation of NLRP3 inflammasome in macrophages in vitro.
3.3 EETs inhibit the activation of NLRP3 inflammasome in macrophages induced by LPS + ATP
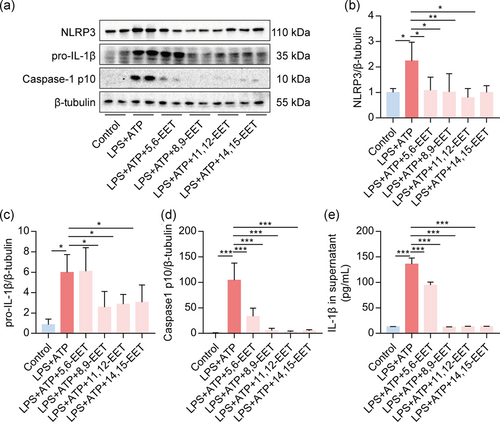
To evaluate the direct effect of EETs on activation of the NLRP3 inflammasome, we treated macrophages with LPS + ATP present or without four EETs (5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET) pretreatment for 10 min. We found that all EETs profoundly reduced the expression of NLRP3, pro-IL-1β, and caspase-1 p10 in macrophages (Figure 3a–d). All EETs decreased the secretion of IL-1β in the supernatant of activated macrophages (Figure 3e). These results suggested that EETs exert direct inhibitory effects on the NLRP3 inflammasome activation induced by LPS + ATP in macrophages.
3.4 EETs inhibit the activation of NLRP3 inflammasome in macrophages induced by LPS + nigericin
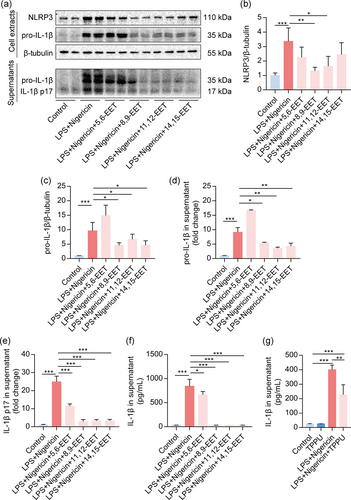
To further confirm the effects of EETs on the activation of the NLRP3 inflammasome, we treated macrophages with LPS + nigericin, which also could activate the NLRP3 inflammasome (Mangan et al., 2018). Results showed that NLRP3 protein expression was dramatically depressed by 8,9-EET and 11,12-EET but not 5,6-EET and 14,15-EET (Figure 4a,b). And 8,9-EET, 11,12-EET, and 14,15-EET dramatically decreased the expression of pro-IL-1β in cells and the supernatant (Figure 4c,d). Interestingly, all EETs reduced the IL-1β p17 in the supernatant detected by Western blotting and ELISA (Figure 4e,f). Besides, TPPU also decreased IL-1β secretion induced by LPS + nigericin (Figure 4g). Collectively, these results implied that EETs also inhibit activation of the NLRP3 inflammasome induced by LPS + nigericin in macrophages.
3.5 EETs decrease cytoplasmic ROS generation in macrophages
ROS is a critical factor of the NLRP3 inflammasome activation (Ty et al., 2019). Thus, we detected the level of intracellular ROS in macrophages. The data showed that LPS + ATP significantly increased the intensity of DCF fluorescence, which indicates increased levels of ROS (Figure 5). The intracellular ROS was reduced by pretreatment of four EETs (Figure 5). These results suggest that EETs treatment attenuates oxidative stress induced by LPS + ATP.
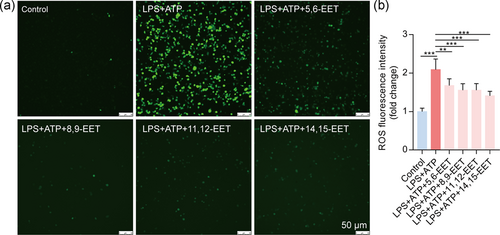
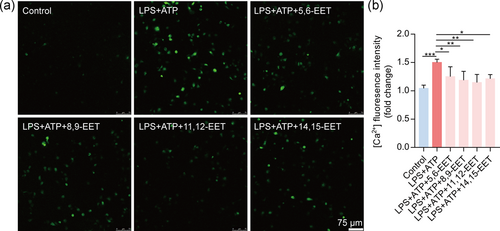
3.6 EETs attenuate Ca2+ overload in activated macrophages
NLRP3 inflammasome activation is linked with calcium mobilization (Negash, Olson, Griffin, & Gale, 2019). Calcium influx plays an essential role in the activation of the NLRP3 inflammasome (Ratajczak et al., 2019). Herein, we used a confocal microscope to determine whether EETs reduce intracellular [Ca2+]. We found that four EETs decreased the abnormal increase of [Ca2+] in the macrophage induced by LPS + ATP (Figure 6). Altogether, we speculate that pretreatment of EETs reduces NLRP3 inflammasome activation by decreasing intracellular calcium ions overload.
4 DISCUSSION
We have proved that the inhibition of sEH improves the survival rate of ALI mice and reduces pulmonary inflammation (Y. Zhou et al., 2017). In this study, we found that the sEH inhibitor TPPU effectively blocked the activation of the NLRP3 inflammasome in the lungs of ALI mice in vivo and macrophages in vitro. In addition, EETs inhibited the activation of the NLRP3 inflammasome in macrophages. Our results indicate that EETs reduce LPS-induced ALI in mice by inhibiting the activation of NLRP3 inflammasome least partially.
Accumulating evidence points out that EETs exert an anti-inflammatory effect (Dai et al., 2015; Node et al., 1999). Its mechanisms are related to the inhibition of the NF-κB pathway and activation of peroxisome proliferator-activated receptors (PPARs) and heme oxygenase 1 (Dai et al., 2015). Enhancement of EETs by sEH inhibitor reduces extracellular signal-regulated kinase phosphorylation, c-Jun N-terminal kinases, and signal transducer and activator of transcription-3, contributing to the attenuation of airway hyperresponsiveness (Jiang et al., 2020). In our previous studies, we found that inhibition of sEH suppresses LPS-induced expression of triggering receptors expressed on myeloid cell-1, an inflammatory amplifier, and inhibits the NF-κB activation in murine macrophage (Dong et al., 2017). Here, we provide a novel mechanism, for the first time, that EETs inhibit the activation of NLRP3 inflammasome in macrophages, contributing to the protective effect of EETs against ALI. In a hyperoxic ALI murine model, sEH gene knockout mice showed less NLRP3 inflammasome (Li et al., 2018). Our study also makes their conclusion more credible.
Our study systematically demonstrated the mechanism by which EETs inhibits the activation of NLRP3 inflammasome. The activation of NLRP3 inflammasome includes two steps: prime and activation (Swanson et al., 2019). The priming of NLRP3 is mainly mediated by NF-κB on transcriptional upregulation of NLRP3 inflammasome components (Swanson et al., 2019). And a line of research has proved EETs inhibit the NF-κB through different strategies, such as CYP2J2 overexpression (Chen et al., 2015), exogenous EETs (Dai et al., 2015), and sEH inhibitor (Hung et al., 2017). We also found that TPPU inhibits the NF-κB in LPS-stimulated macrophages (Dong et al., 2017). So, we believe that EETs suppress the priming of the NLRP3 inflammasome by inhibiting the NF-κB. On the other hand, ROS, ion flow (Ca2+ flux or K+ efflux), and lysosomal homeostasis are involving in the NLRP3 inflammasome activation, leading to the caspase-1-dependent secretion of proinflammatory cytokines and pyroptosis (Di et al., 2018; Q. Liu, Zhang, Hu, Zhou, & Zhou, 2018). Here, we found that EETs reduced the ROS production and Ca2+ overload, contributing to the suppression of NLRP3 inflammasome activation. It has been reported that EETs inhibit Ca2+ influx in rat cardiomyocytes (You et al., 2016). EETs could reduce the ROS production deriving from mitochondria (L. Liu et al., 2011; Waldman et al., 2016). Interestingly, mitochondrial damage causes Ca2+ overload (Sudevan et al., 2019) and produces ROS (L. Liu et al., 2011; Waldman et al., 2016). EETs can limit damage to mitochondria (Katragadda et al., 2009). So, we speculate that EETs attenuate the mitochondrial damage, then reduce the ROS production and Ca2+ overload, contributing to the suppression of the NLRP3 inflammasome activation.
Interestingly, the inhibitory effect of 5,6-EET on the NLRP3 inflammasome activation was weaker than that of the other three EETs. Studies have shown that 5,6-EET is an endogenous ligand of transient receptor potential vanilloid 4 (TRPV4; Berna-Erro et al., 2017; Hinata et al., 2018). TRPV4 induces the activation of microglia and astrocytes, and increases the protein expression of NLRP3, ASC, caspase-1, and IL-1β in the hippocampal tissues of mice, suggesting that the activation of TRPV4 can trigger the NLRP3 inflammasome (Wang et al., 2019). TRPV4 mediates macrophage phagocytosis, promotes cytokine secretion, and upregulates the production of ROS (Dutta et al., 2020). We also found a weaker inhibitory effect of 5,6-EET on intracellular ROS in activated macrophages in this study. Therefore, we believe that the weaker inhibitory effect of 5,6-EET on the NLRP3 inflammasome activation may attribute to the activation of TRPV4, which partially offsets the inhibition of the NLRP3 inflammasome.
There are still limits in this study. First, the possible receptor for EETs has been investigated in the present study. Studies have shown that PPAR-γ is required for mediating the protective effects of EETs (Dai et al., 2015; Samokhvalov et al., 2014). PPAR-γ plays a critical role in the modulation of pulmonary inflammation (Huang et al., 2019). Through the activation of PPARγ, EETs exert anti-inflammatory and antioxidative effects (W. Liu, Wang, Ding, Wang, & Zeng, 2014). Besides, G protein-coupled receptor 40 is a low-affinity EET receptor in vascular cells and arteries (Park et al., 2018). Second, the mechanism underlying the inhibitory effects of EETs on the NLRP3 inflammasome activation needs further research. The NLRP3 inflammasome activation is mediated by multiple signals, such as efflux of K+ or chloride ions (Cl−), the influx of Ca2+, lysosomal disruption, and metabolic changes (Swanson et al., 2019). It is of great necessity to explore how EETs affect Ca2+ influx and ROS production in macrophages. The recent research shows that mitotic kinase NEK7 licenses the assembly and activation of the NLRP3 inflammasome (Sharif et al., 2019). The stress granule component DDX3X interacts with NLRP3 and is required for the NLRP3 inflammasome activation (Samir et al., 2019). Src-family kinases negatively regulate the NLRP3 inflammasome by maintaining mitochondria homeostasis, reducing mtROS (Chung et al., 2018). These studies provide an important basis for further studies on the molecular mechanism of the NLRP3 inflammasome activation.
In conclusion, our results for the first time suggest that EETs inhibit the activation of the NLRP3 inflammasome, at least partly, by suppressing ROS generation and Ca2+ overload in macrophages, contributing to the therapeutic potency to ALI.
ACKNOWLEDGMENTS
We are grateful to Prof Bruce D. Hammock and Kin Sing Stephen Lee from the University of California Davis for providing the sEH inhibitor TPPU. This study was supported by the National Natural Science Foundation of China (81500065, 81670014, and 91949110), the Hunan Provincial Natural Science Foundation of China (2018JJ3701, 2019JJ70008, and 2019JJ50975), and the Traditional Chinese medicine research project of Hunan province (201926).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
C.-X. G. and Y. Z. conceived and designed the experiments. X.-Q. L., H.-H. Y., C.-Y. Z., C.-C. S., J.-H. T., J.-B. X., and X.-X. G. performed the experiments. X.-Q. L., J.-X. D., C. Z., and J.-H. T. analyzed the data. Y. Z. and C.-X. G. contributed reagents/materials/analysis tools. X.-Q. L. and Y. Z. wrote the paper. C.-X. G., Y. Z., and H. H. Y. critically reviewed the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
We declare that all the data presented in this article supporting the conclusions of the study. And we have not used other data that has already been published.




