Impacts of epidural electrical stimulation on Wnt signaling, FAAH, and BDNF following thoracic spinal cord injury in rat
Abstract
Electrical stimulation (ES) has been shown to improve some of impairments after spinal cord injury (SCI), but the underlying mechanisms remain unclear. The Wnt signaling pathways and the endocannabinoid system appear to be modulated in response to SCI. This study aimed to investigate the effect of ES therapy on the activity of canonical/noncanonical Wnt signaling pathways, brain-derived neurotrophic factor (BDNF), and fatty-acid amide hydrolase (FAAH), which regulate endocannabinoids levels. Forty male Wistar rats were randomly divided into four groups: (a) Sham, (b) laminectomy + epidural subthreshold ES, (c) SCI, and (d) SCI + epidural subthreshold ES. A moderate contusion SCI was performed at the thoracic level (T10). Epidural subthreshold ES was delivered to upper the level of T10 segment every day (1 hr/rat) for 2 weeks. Then, animals were killed and immunoblotting was used to assess spinal cord parameters. Results revealed that ES intervention for 14 days could significantly increase wingless-type3 (Wnt3), Wnt7, β-catenin, Nestin, and cyclin D1 levels, as well as phosphorylation of glycogen synthase kinase 3β and Jun N-terminal kinase. Additionally, SCI reduced BDNF and FAAH levels, and ES increased BDNF and FAAH levels in the injury site. We propose that ES therapy may improve some of impairments after SCI through Wnt signaling pathways. Outcomes also suggest that BDNF and FAAH are important players in the beneficial impacts of ES therapy. However, the precise mechanism of BDNF, FAAH, and Wnt signaling pathways on SCI requires further investigation.
1 INTRODUCTION
Traumatic spinal cord injury (SCI) has devastating effects on the physical, social, and professional well-being of patients. SCI results in permanent loss of different cells including neurons, oligodendrocytes, astrocytes, and precursors resulting in sensory and motor dysfunctions, urinary infections, and mental disorders (Bradbury & McMahon, 2006). In spite of constant development in regenerative research, there is no effective treatment that can significantly restore injured neurons after SCI. It also remains a tremendous challenge to induce remarkable neuroplasticity and dendritic growth, forming new neuronal connections and generating the possibility of neuroregeneration after SCI situation (Su, Niu, Liu, Zou, & Zhang, 2014).
Two critical treatment paradigms for SCI consist of neuroprotection and neuroregeneration approaches to restrict the injury, and trigger endogenous repair process (Tsai & Tator, 2005). Both of the above mentioned processes have been shown to be modulated by multiple signaling mechanisms, such as Wnt pathways, neurotrophic factors, and the endocannabinoid system (Boyce & Mendell, 2014; Varela-Nallar & Inestrosa, 2013). The endocannabinoid system, comprising two lipid mediators formed from plasma membrane precursors including anandamide (N- arachidonoylethanolamide [AEA]) and 2-arachidonoylglycerol (2-AG), the cannabinoid G-protein coupled receptors (CB1 and CB2), the endocannabinoid synthesizing, and degrading enzymes, such as fatty-acid amide hydrolase (FAAH; Ahmadalipour et al., 2020). Endocannabinoid system is involved in several physiological functions (Wu, 2019). Synaptic levels of the endocannabinoids are in part controlled by FAAH, which terminates the activity of anandamide (AEA; Ahmadalipour et al., 2020), and related fatty-acid amides (palmitoylethanolamide and oleoylethanolamine; Habib et al., 2019). Extensive evidence indicates that the 2-AG and anandamide (AEA) are produced “on demand” after cerebral ischemia (Amantea et al., 2007), excitotoxic damage (Hansen et al., 2001), and traumatic brain injury (Shohami, Cohen-Yeshurun, Magid, Algali, & Mechoulam, 2011) and have been suggested to act as neuroprotective and immunomodulatory mediators after lesions of the nervous system (Valvezan & Klein, 2012).
On the other hand, evidence showed that neurotrophic factors, such as nerve growth factor and brain-derived neurotrophic factor (BDNF) play a key role in the growth, survival, and differentiation of neurons (Vilar & Mira, 2016) and is involved in many beneficial intervention influences such as exercise and enriched environment (Ahmadalipour, Ghodrati-Jaldbakhan, Samaei, & Rashidy-Pour, 2018). Neurotrophic factors play a critical role in the neuronal regeneration, axonal growth, synaptic plasticity, and functional recovery after SCI (Boyce & Mendell, 2014). Furthermore, BDNF and Wnt signaling cooperates in the regulation of neuroregeneration. Also, Wnt/β-catenin signaling pathway regulates BDNF expression and dendritic spine formation (Hiester, Galati, Salinas, & Jones, 2013). In addition, enhancement of endocannabinoid signaling by FAAH inhibition has been proposed as a neuroprotective therapeutic modality (Bacil et al., 2015), however, some evidence suggests the opposite, for example, anandamide has been shown to induce cell death in primary neuronal cultures (Movsesyan et al., 2004).
The Wnt signaling transduction cascades are classified into two pathways, including canonical (Wnt/β-catenin) and noncanonical (β-catenin-independent) pathways (Sun, 2011). It is shown that the anandamide activates the noncanonical Wnt signaling pathway (DeMorrow et al., 2008). Wnt3 and Wnt7 are two of the main regulators of neural proliferation and differentiation which resulted in neurogenesis (Lie et al., 2005).
Activation of Wnt canonical signaling could lead to the phosphorylation (inhibited model) of the glycogen synthase kinase 3β (GSK3β) and accumulation of β-catenin in the cytoplasm (Clevers & Nusse, 2012). Subsequently, β-catenin translocates into the nucleus, where it acts as a transcriptional coactivator and activates the transcription of Wnt target genes such as cyclin D1 (Wisniewska, 2013). Administration of Wnt3a-secreting fibroblasts in SCI-subjected rats has been shown to enhance axonal regeneration and functional recovery (Suh et al., 2011). In addition, noncanonical Wnt signaling is facilitated by alternative signaling pathways, including intracellular calcium ion and Jun N-terminal kinase (JNK). JNK belongs to the mitogen-activated protein kinases and is involved in noncanonical Wnt signaling and planar cell polarity (Saadeddin, Babaei-Jadidi, Spencer-Dene, & Nateri, 2009). Cyclin D1 is a Wnt target gene and its expression is regulated by both JNK and Wnt signaling (Wisdom, Johnson, & Moore, 1999).
Electrical stimulation (ES) has been used as a modern therapeutic method to promote neurogenesis and functional recovery after central nervous system (CNS) injury (Huang, Li, Chen, Zhou, & Tan, 2015). Furthermore, ES has a potential regenerative role to increase the formation of newborn cells after SCI in animals expressing neural progenitor cell-associated markers (Becker, Gary, Rosenzweig, Grill, & McDonald, 2010).
The exact mechanism of ES on Wnt signaling pathways remains unknown. According to evidence, Wnt signaling plays a key role in the regulation of regeneration. On the other hand, ES therapy may promote neuronal regeneration. Therefore, in the present study, for the first time, we investigated the effect of epidural subthreshold ES on Wnt signaling pathways and its downstream targets as well as BDNF and FAAH levels in the spinal injury site using a rat SCI model.
2 MATERIALS AND METHODS
2.1 Antibodies and reagents
All antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA): Wnt3 (sc-74537), Wnt7 (sc-365459), β-catenin (sc-7963), Nestin (sc-23927), cyclin D1 (sc-8396), GSK3β (sc-7291), phosphorylated GSK3β (p-GSK3β; sc-373800), JNK (sc-7345), phosphorylated JNK (p-JNK; sc-6254), FAAH (sc-100739), BDNF (sc-546), and β-actin (sc-32233). Other chemicals and reagents were obtained from Sigma-Aldrich Co. (MO).
2.2 Animals
Forty male Wistar rats, weighing 250–280 g were obtained from Animal Centre of Tabriz University of Medical Sciences, Tabriz, Iran and were housed in clear plastic cages, four rats per cage, under a controlled temperature (25 ± 1°C) and humidity (55–60%) in a 12/12 hr light/dark cycle. Animals were given free access to standard food and water. All experimental procedures and protocols were performed according to the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health (NIH Publications No. 8023, revised 1978) and approved by the veterinary ethics committee of Tabriz University of Medical Sciences (Approval number: 1394.1116).
2.3 Study design
One week after adaptation, animals were randomly divided into four groups (10 rats per group) as follows: (a) Sham group: laminectomy surgery without contusion injury, (b) ES group: laminectomy surgery and implantation of stimulating bipolar electrode, (c) SCI group: moderate contusion injury at 10th thoracic (T10) segment of spinal cord, and (d) SCI+ES group: received ES after the SCI.
In the sham group, nine animals were maintained in the study. In other groups where animals received contusion injury and ES protocol, we missed two to three rats per group (ES group: two; SCI group: two; SCI+ES group: three). Overall, we missed eight animals in this study. Thereby, in statistical analysis, seven to nine rats were included in each group.
2.4 Spinal cord injury model
To induce SCI model, animals were anesthetized with isoflurane (4% for induction and 2.5% for maintenance), and laminectomy procedure was performed at 10th thoracic (T10) segment. Briefly, an incision was made over the lower thoracic vertebrae. After removing paravertebral muscles on both sides of the T9-T11 vertebrates, transverse processes of the vertebra column were stabilized by device clamps. Moderate contusion injury was induced using a 2.5-mm diameter of the tip by a 150-Kdyn force (Ghorbani, Shahabi, Ebrahimi-kalan, & Soltani-zangbar, 2018). After injury, incised muscles, subcutaneous tissues, and skin were sutured in layers and animals were kept in a separate cage under a warm lamp for 1–2 hr. For postoperative pain relief, buprenorphine (0.1 mg/kg) was injected subcutaneously every 6–12 hr for 48 hr. In addition, rats have received a 5-day period of antibiotics (enrofloxicin, 5 mg/kg) to avoid the possibility of infection after SCI. After surgery, the bladder of SCI animals was manually expressed twice a day to drain urine from the bladder until detecting normal function of the urinary system. On average, it took about 3–8 days after injury to return normal function of the animals' bladders.
2.5 Epidural subthreshold ES protocol
For electrode implantation, two unipolar electrodes were implanted in the upper injured site of the spinal cord under anesthesia and the wire electrodes were sutured into the muscles adjacent to the paravertebrate muscles and wires-socket remained out of the skin. The threshold of neuronal excitability in the spinal cord was recorded from each rat by muscle reflex in lower limbs. A day after ES implantation, epidural subthreshold ES (0.3–0.6 mA, 0.1 ms, 100 Hz) was applied at 10:00 a.m. for 1 hr each day for 14 consecutive days by a two-channel battery-powered electrical stimulator (WPI; A320; Badri et al., 2017).
2.6 Immunoblotting assay
One hour after finishing the last session of epidural subthreshold ES protocol (2 weeks' period), collecting the samples started. Animals were euthanized by an overdose of ketamine (100 mg/kg) and xylazine (5 mg/kg) and thoracic spinal cords at the lesion site approximately in the middle were rapidly removed and frozen in liquid nitrogen. Frozen spinal cord samples were homogenized in radioimmunoprecipitation assay lysis buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM Na2 ethylenediaminetetraacetic acid, 1 mM ethylene glycol tetraacetic acid, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, and 1 µg/ml leupeptin containing protease inhibitor cocktail; Sigma). Then, homogenates were centrifuged at 12,000g at 4°C for 10 min and the supernatant was collected. Bradford method (Bio-Rad, Hercules, CA) was used to quantify protein concentration of the supernatants. Then, equal amounts of total protein (30 mg) were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and proteins were transferred to polyvinylidene difluoride membranes. For blocking of nonspecific bindings, the membrane was incubated with blocking solution in phosphate-buffered saline (PBS) containing 0.1% Tween 20 for 2 hr at room temperature (RT). Next, the membrane was incubated with primary antibodies of Wnt3, Wnt7, β-catenin, cyclin D1, GSK3β, p-GSK3β, JNK, p-JNK, Nestin, or β-actin as loading control overnight at 4°C. Next, after washing the membrane three times with PBS, blots were incubated with a horseradish peroxidase-conjugated secondary appropriate antibody (Santa Cruz Biotechnology Inc.) diluted at 1:7,000 for 1 hr at RT. Enhanced chemiluminescence detection kit (Bio-Rad) was used to visualize the target protein bands development. The density of bands was quantified using ImageJ (NIH, Bethesda, MD). Western blot analysis was accomplished in triplicate for each sample (Mohammad Alizadeh et al., 2018).
2.7 Immunohistochemistry
Immunohistochemistry was performed on sections of the spinal cord using a FAAH Antibody (27-Y; Santa Cruz Biotechnology-sc-100739). Briefly, spinal cord sections of 40 μm were cleaned two times for 5 min each in washing solution (0.05 M Tris-buffered saline [TBS] buffer [pH 7.5], 0.025% Triton X-100). The tissue was then protected from nonspecific binding in a blocking solution (10% normal serum or with 1% bovine serum albumin in TBS) for 2 hr at RT. The tissue was then placed in primary antibody and left to incubate overnight at 4°C. After washing the sections three times for 5 min in washing solution, the slides were incubated in secondary antibody (Goat Biotinylated Polyvalent) for 10 min at RT. The tissue was then washed three times for 1 min and incubated for 10 min in an sterptoavidin-conjugated horseradish peroxidase. Another three washes of 1 min were performed and the sections were treated with a DAB substrate, until the tissue was colored (1–10 min). The tissue was then washed again three times for 1 min and the sections were counterstained with hematoxylin for 2 min in RT. They then underwent dehydration in graded ethanol, were cleared in xylene, and cover slipped with Permount mounting media.
2.8 Statistical analysis
All data were expressed as means ± SEM. Data were analyzed by SPSS (version 23.0) software using one-way analysis of variance followed by Tukey's posthoc test. In all cases, p < .05 was considered statistically significant.
3 RESULTS
3.1 The effect of ES on the activation of canonical Wnt signaling in the injured site after SCI
As shown in Figure 1, SCI significantly increased the expression of Wnt3 protein and increased p-GSK3β levels (p < .001), while the expression level of β-catenin was unaffected in the SCI group compared with the sham group. In addition, the levels of Wnt3, β-catenin, and p-GSK3β were significantly increased in the ES group compared with the sham group. Furthermore, levels of Wnt3 (p < .01), and β-catenin (p < .001), and phosphorylation of GSK3β (p < .001) were higher in SCI rats treated with ES (SCI+ES group) compared to the SCI group.
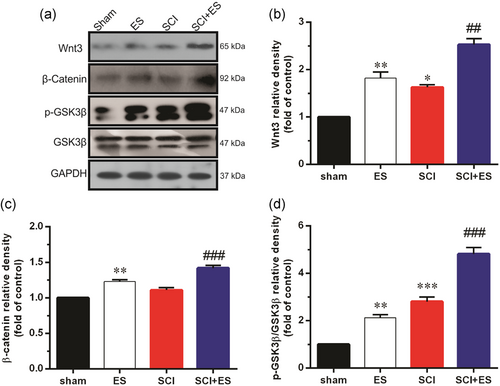
3.2 The effect of ES on the activation of noncanonical Wnt signaling in the injured site after SCI
Our results also demonstrated that SCI significantly increased Wnt7 protein and p-JNK levels, while ES in the healthy animals only increased p-JNK in comparison with the sham group. Moreover, ES significantly (p < .001) increased Wnt7 protein levels in the SCI+ES group as compared to the SCI group. However, ES had no significant effect on p-JNK in the SCI+ES group compared to the SCI group (Figure 2).
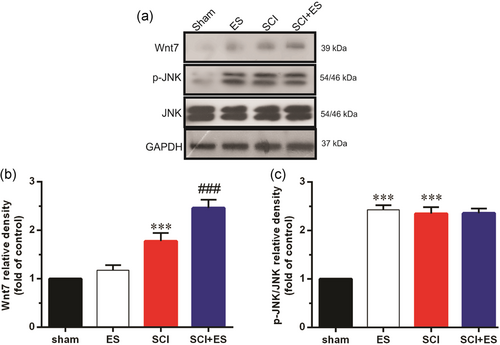
3.3 The effect of ES on the targets of Wnt signaling in the injured site after SCI
As shown in Figure 3, induction of ES in the healthy animals markedly (p < .001) increased Nestin, a neural progenitor marker, and cyclin D1 (p < .01) protein levels as compared to the sham animals. Moreover, in the SCI+ES group, protein levels of Nestin and cyclin D1 in the injured site of spinal tissue were (for both p < .001) increased after ES for 2 weeks as compared to the SCI group. Also, protein level of Nestin markedly increased in SCI group compared with sham group.
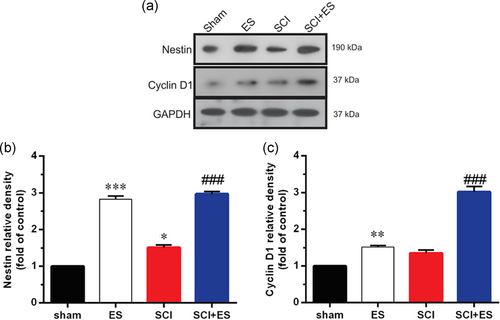
3.4 The effect of ES on the expression of BDNF protein after SC
The results of western blot analysis also showed that SCI significantly decreased the expression of BDNF as compared to the sham group (Figure 4). However, applying ES markedly increased (p < .001) the BDNF protein levels in the healthy animals in comparison with the sham group. In addition, BDNF protein levels significantly increased in the ES+SCI group as compared to the SCI group.
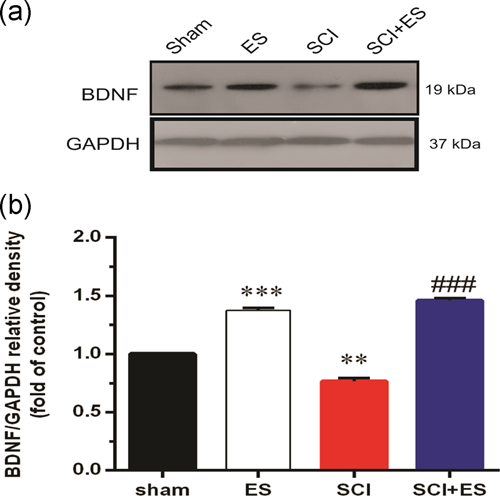
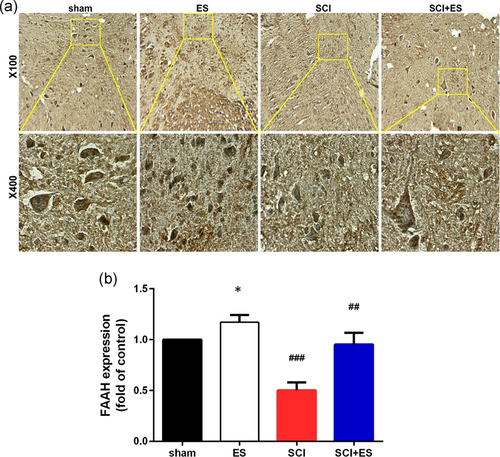
3.5 The effect of ES on the expression of FAAH after SCI
The results of immunohistochemistry showed that SCI significantly decreased the expression of FAAH enzyme as compared to the Sham group (Figure 5; p < .001). However, applying ES increased the FAAH enzyme levels in the healthy animals in comparison with Sham group, and FAAH enzyme levels significantly increased in the ES+SCI group as compared to the SCI group (p < .01).
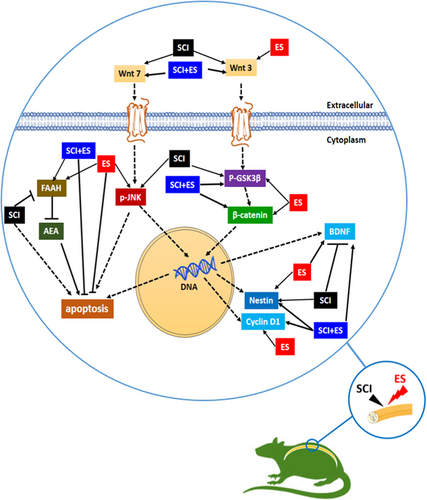
4 DISCUSSION
In this study, we examined the effects of ES on the Wnt signaling pathways, BDNF and FAAH expression after SCI in rats. We demonstrated that induction of ES at T10 segment of SCI activates canonical (Wnt3, β-catenin, and p-GSK3β), and the noncanonical (Wnt7 and p-JNK) pathways after 2 weeks. In addition, ES increased protein levels of BDNF and FAAH levels in the SCI-subjected animals (Figure 6). Recently, signaling pathways implicated in regeneration and neuronal recovery after SCI have received more attention. Several lines of evidence have shown that Wnt/β-catenin signaling has a crucial role in the regulation of axonal regeneration and is essential for neurogenesis (Hollis & Zou, 2012). GSK3β and β-catenin are two important components of the canonical Wnt pathway. Phosphorylation of GSK3β increases cellular stabilized β-catenin, which activates the canonical Wnt pathway in the nucleus by activation of the transcription of Wnt target genes involved in cell survival, proliferation, and differentiation. J. Liu et al. (2015) in an in vitro study demonstrated that Wnt/GSK3β pathway contributes to the ES-induced cell migration and inhibition of the canonical Wnt pathway eliminated this effect. Moreover, Lie et al. (2005) have shown that Wnt3 promotes neuronal differentiation in adult hippocampal neural stem cells.
Our results showed that Wnt3 was significantly induced after SCI. Zhang, Li, and Wu (2017) also indicated that electroacupuncture therapy (frequency of 2 Hz, current of 1 mA for 20 min/day for 2 weeks) promotes recovery of SCI in rats through augmentation of Wnt/β-catenin signaling. Moreover, we found that ES after SCI strongly increased the protein expression of Wnt3 and β-catenin, and increased p-GSK3β levels at the site of injury. In line with our results, H. H. Liu et al. (2013) reported that functional ES after 14 days significantly increases the expression of Wnt3 protein that plays a pivotal role in neurogenesis in the ipsilateral subventricular zone in rats after focal cerebral ischemia. Studies have shown that Wnt3 regulates the differentiation of progenitor cells toward neurons and increases the survival of newly produced neurons (Lie et al., 2005; Shruster, Ben-Zur, Melamed, & Offen, 2012). Therefore, it is likely that ES through augmentation of Wnt signaling could promote regeneration at the site of injury. We also examined the role of the noncanonical Wnt7/JNK pathway in the ES-subjected animals and found that ES markedly increased the p-JNK at the site of injury as compared to the sham group. Noncanonical Wnt pathway is induced following various pathologies, such as cancer and inflammatory diseases (Kikuchi, Yamamoto, Sato, & Matsumoto, 2011). JNK, a downstream effector of noncanonical Wnt pathway, regulates cell proliferation, migration, cytoskeleton integrity, survival, and apoptosis (Nomachi et al., 2008). In addition, studies showed that there are cross-regulatory interactions between JNK and both canonical and noncanonical Wnt signaling, which this effect progressively provokes neuroregeneration and sustained neurite elongation (Barnat et al., 2010; Saadeddin et al., 2009).
In this study, we also studied the effect of ES after SCI on the cyclin D1 level, which is a major regulator of neural development and studies have shown that cyclin D1 has a proneurogenic function and its expression is necessary for neurogenesis in the developing spinal cord of chick (Lukaszewicza & Anderson, 2011). We found that ES after SCI upregulated cyclin D1 protein expression as compared to the SCI group. Also, there was a significant increase in cyclin D1 protein expression in the ES group. It has also been demonstrated that exposure of rat Schwann cells to low-frequency ES (20 Hz) increased cyclin D1 protein expression (Gu et al., 2015). These findings suggest that the ES may affect the expression of neurogenesis-related proteins in both normal and injured spinal cord tissues.
The present study also examined Nestin protein levels in the spinal tissue in response to ES. Nestin is an intermediate filament protein, and plays a significant role in self-renewing and proliferation of neural stem cells, which is expressed under pathological conditions, including glial scar formation after CNS injury (Park et al., 2010). Evidence shows that Nestin-expressing cells are found in the site of regeneration, which act as a pool of stem/progenitor cells (Park et al., 2010). Li et al. (2013) introduced a new scaffold with the capability of delivering electrical current (20–30 mA) to provide a suitable cell growth microenvironment for neuronal stem cells (NSCs) in vitro. Their results revealed that the ES can effectively enhance the Nestin expression, a marker for NSCs. In the sham and SCI groups, the expression of Nestin remained at low levels. In our study, the expression of Nestin was upregulated in the ES-treated rats after SCI compared with SCI group, suggesting that activation of Wnt7/β-catenin signaling in canonical pathway markedly amplified spinal cord improvement in SCI rats.
BDNF has a regulatory role in the survival of neurons, neurogenesis, and differentiation of neural cells during brain development and after injury (Dougherty, Dreyfus, & Black, 2000). Substantial evidence has also proven the role of BDNF in improving axonal regeneration in the injured spinal cord and promoting functional recovery after SCI (Martínez-Gálvez et al., 2016). Furthermore, BDNF is considered as a potential target of Wnt signaling pathways and several studies have reported a functional crosstalk between the Wnt signaling cascades and BDNF. Yi, Hu, Qian, and Hackam (2012) have reported that the Wnt signaling pathways upregulate the expression of BDNF. Moreover, BDNF has been shown to initiate intracellular Wnt/β-catenin signaling cascades. In this study, BDNF significantly decreased after SCI. Contrary to our result, Gao et al. (2015) showed that 7 days after SCI, the intensity of BDNF fluorescence in the spinal cord was increased. In addition, we found that epidural subthreshold ES also increased the expression of BDNF in SCI-subjected rats accompanied by the activation of Wnt/β-catenin signaling pathway. We suggest that upregulation of BDNF by subthreshold ES activates cellular regeneration pathway as a compensatory response to protect remaining neurons from further damage induced by SCI and to provide a beneficial environment for functional recovery.
Previous studies have shown that endocannabinoids can contribute in normal spinal cord function and it is demonstrated that the endocannabinoid system is modulated after SCI (Garcia-Ovejero et al., 2009). FAAH is a key enzyme of this system, and degrades mostly anandamide, which is an important endogenous ligand of the endocannabinoid system and findings described in the literature are inconsistent about anandamide. In a controversial issue, anandamide have been proposed a neuroprotective mediator in some situation, but also neurotoxic and apoptotic factor in other studies. For example, in situations of hypoxia and glucose deprivation (Sinor, Irvin, & Greenberg, 2000), metabolic challenge (Van Der Stelt et al., 2001), and excitotoxicity (Karanian, Brown, Makriyannis, Kosten, & Bahr, 2005), anandamide has been proposed as a neuroprotective mediator which modulates inflammatory responses and reduces neuronal damage (Baker et al., 2001; Eljaschewitsch et al., 2006; Mestre et al., 2005; Ortega-Gutiérrez et al., 2005), however, anandamide also induces cell death (Sarker & Maruyama, 2003) and produces neurotoxic effects both in vitro and in vivo through multiple mechanisms independent of the CB1 (Cernak et al., 2004) receptor.
A large body of evidence shows that after many types of neural damage, the activation of endocannabinoid system is a common feature (Hansen et al., 2001; Panikashvili et al., 2001). Accordingly, our results showed a reduced level of FAAH enzyme after SCI, which can result in an increased level of endocannabinoids (mostly anandamide) and upregulated expression of FAAH in ES-treated rats after SCI which can result in a reduced level of endocannabinoids. In a previous study, Garcia-Ovejero et al. (2009) showed an increased level of anandamide and a decrease in its degradation (downregulation of FAAH) 1 and 3 days after SCI when compared to sham-operated animals. They did not find the same changes 14 days after lesion. In contrast, we showed a reduced level of FAAH enzyme on 14 days and this different results can be explained partly by different methods of lesion induction and FAAH measurements (messenger RNA vs. western blot).
The exact reason for the changes of FAAH level after SCI and ES is not clear. A number of studies have shown that activation of endocannabinoid system (particularly CB1 receptor) can induce cytotoxic effects in several cultured cell systems (Downer, Fogarty, & Campbell, 2003), including cultured hippocampal (Chan, Hinds, Impey, & Storm, 1998) and cortical neurons (Downer, Boland, Fogarty, & Campbell, 2001). Some of proposed mechanisms by which endocannabinoid system activation induces neurotoxicity, including activation of caspase-3-dependent apoptosis (Campbell, 2001), generation of reactive oxygen species (Chan et al., 1998), sustained ceramide accumulation (Galve-Roperh, Rueda, Del Pulgar, Velasco, & Guzmán, 2002), activation of the JNK cascade (Sarker & Maruyama, 2003), and sphingomyelin hydrolysis (Sánchez, Galve-Roperh, Canova, Brachet, & Guzmán, 1998). However, it should be noted that in contrast to the data supporting cannabinoid-induced neurotoxicity (Cernak et al., 2004), several studies have shown that activation of this system can induce neuroprotective effects (Mechoulam & Lichtman, 2003). Therefore, it is possible that activation of the endocannabinoid system may result in both neuroprotection and neurotoxicity, depending on a range of influences such as intensity and nature of the toxic insult, as well as the cell type under study (Guzmán, 2003). With the decrease in FAAH enzyme, the concentration of endocannabinoids is expected to increase, and this increase is accompanied by a decrease in the concentration of BDNF. These findings are more consistent with the neurotoxicity effects of endocannabinoid, and may be interpreted as a putative neurodegenerative response of this system. Previously, anandamide-CB1 receptor signaling have been shown to contribute to postnatal ethanol-induced neonatal neurodegeneration, adult synaptic, and memory deficits (Subbanna, Shivakumar, Psychoyos, Xie, & Basavarajappa, 2013). In this regard, ES may reduce neurodegeneration and improve neuroprotection after SCI by increasing FAAH expression levels that is related with decreased activity of endocannabinoid system.
In summary, our results fortify the body of evidence indicating that ES can be effective in the molecular mechanisms that have been shown to be involved in improving spinal cord injuries. We showed that epidural subthreshold ES has a potential effect on signaling pathways related to Wnt signaling, EC system, and BDNF. ES therapy increased the expression of Wnt3, Wnt7, β-catenin, cyclin D1, Nestin, FAAH, and BDNF in SCI-injured rats suggesting that ES treatment enhances Wnt/β-catenin signal transduction pathways with contribution of the endocannabinoid system and neurotrophins (BDNF). The interaction between these three important players including Wnt, ECs, and BDNF demonstrates the need for further research on the mechanisms involved in improving multiple injuries of the nervous system, especially the spinal cord, and may provide an experimental basis for the future of the clinical application of ES for the treatment of SCI. However, the precise mechanism of its action requires further investigation.
ACKNOWLEDGMENTS
The authors give special thanks to Neurosciences Research Center (NSRC), Drug Applied Research Center, and Laboratory Animal Center of Tabriz University of Medical Sciences. Tabriz, Iran. In addition, Mr. Meysam Ghorbani carried out this study in partial project fulfillment of the requirements to obtain a Master (MSc) degree in physiology. This study was supported by a grant from Tabriz University of Medical Sciences (grant number: 94/1-10/9), Tabriz, Iran.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
Data are available on request from the authors.




