MiR-216a-3p suppresses the proliferation and invasion of cervical cancer through downregulation of ACTL6A-mediated YAP signaling
Abstract
The tumor-suppressive role of microRNA-216a-3p (miR-216a-3p) has been evidenced in multiple tumors. Yet, the relevance of miR-216a-3p in cervical cancer remains undermined. The current study was designed to determine the expression and potential function of miR-216a-3p in cervical cancer. Expression of miR-216a-3p was markedly decreased in cervical cancer and functional assays revealed an inhibitory effect of miR-216a-3p on the proliferation, colony formation, and invasion of cervical cancer. Actin-like 6A (ACTL6A) was identified as a target gene of miR-216a-3p. Elevated ACTL6A expression was detected in cervical cancer, and ACTL6A inhibition exhibited a tumor-suppressive effect. ACTL6A inhibition increased yes-associated protein (YAP) phosphorylation and downregulated YAP-mediated transcriptional activity. ACTL6A restoration or YAP reactivation partially abrogated the miR-216a-3p-mediated antitumor effect in cervical cancer cells. Taken together, these data demonstrate that miR-216a-3p acts as a potential tumor-suppressive miRNA in cervical cancer, which exerts its function through inhibition of YAP signaling via targeting ACTL6A.
1 INTRODUCTION
Cervical cancer is the fourth most frequently occurring invasive neoplasm in women; it has a high incidence and mortality rate (Small et al., 2017). In China, cervical cancer continues to be a heavy social burden, since 12% of global new cases of this disease occur there (Di, Rutherford, & Chu, 2015). In spite of the advances in cancer diagnosis and therapy, the survival rate and prognosis of cervical cancer patients are still poor, especially in those at advanced stages (Hwang et al., 2001). Infection of human papillomavirus (HPV) is considered as an important cause for cervical cancer initiation (Malik, Khan, & Ahsan, 2014), along with other factors, such as genetic and epigenetic changes, early sexual activity, and poor hygiene, that also play a pivotal role in cervical cancer occurrence and progression (Uysal & Birsel, 2009). Yet, the precise molecular mechanisms underlying the malignant progression of cervical cancer cells remain largely unknown. Therefore, to identify new and key genes that exert key roles in the molecular pathogenesis of cervical cancer is imperative, since these findings will help to develop novel and promising anticancer approaches for cervical cancer.
MicroRNAs (miRNAs) are a well-known type of noncoding RNAs that are composed of ∼21 nucleotides, acting as a group of key posttranscriptional regulators (Krol, Loedige, & Filipowicz, 2010). In general, miRNAs are capable of recognizing the 3′-untranslated regions (3′-UTR) of the target gene messenger RNA (mRNA) through complementary sequences, by which miRNAs can guide an RNA-inducing silencing complex to the mRNA to degrade it and restrict transcription (Bartel, 2009). Therefore, by affecting target gene expression, miRNAs are likely to participate in regulating a wide range of biological processes, such as cell growth, differentiation, cell cycle, and apoptosis. The expression of various miRNAs are altered in tumor tissues, and these dysregulated miRNAs commonly function as oncogenes or tumor suppressors (Di Leva & Croce, 2010). Substantive studies demonstrated that miRNAs are related to cervical cancer progression, including proliferation, metastasis, and chemo-/radiotherapy resistance (Y. He et al., 2016; Pedroza-Torres et al., 2014; Srivastava et al., 2017; Torres, Torres, Maciejewski, & Harvey, 2011). Accumulating evidence shows that targeting specific miRNAs effectively restrains the growth and metastasis of cervical cancer, findings that suggest miRNAs are potential therapeutic targets for cervical cancer (Cheng, Shi, Huo, Zhao, & Zhang, 2019; H. Li et al., 2019; Y. J. Li, Wang, & Wang, 2019; Nahand et al., 2019).
Actin-like 6A (ACTL6A), a member of the SWItch-sucrose non-fermentable complex, plays a crucial role in regulating nuclear transition, chromatin remodeling, and transcription modulation (Krasteva et al., 2012). ACTL6A expression is elevated in progenitor and stem cells, and ACTL6A plays an important role in promoting cell self-renewal and repressing differentiation (Bao et al., 2013; Ho & Crabtree, 2010). ACTL6A is a potential oncogene in various cancers. High ACTL6A expression is frequently detected in various cancer tissues, including rhabdomyosarcoma, hepatocellular carcinoma, glioma, and colon cancer (Meng et al., 2017; Saladi et al., 2017; Taulli et al., 2014; S. Xiao et al., 2016; Zeng, Yang, & Xiao, 2018). Moreover, ACTL6A accelerates tumor progression by affecting various signaling pathways, and this fact suggests that ACTL6A is a potential oncogene (Ji et al., 2018; Park, Wood, & Cole, 2002).
Yes-associated protein (YAP), a crucial component of Hippo cascades, exerts an essential role in regulating development, tissue homeostasis, and pathological processes (Yu, Zhao, & Guan, 2015). In response to various stimuli, YAP is dephosphorylated and translocated into the nucleus where it promotes gene expression in association with transcriptional coactivator with PDZ binding motif (TAZ; Zhao et al., 2008). Activation of YAP/TAZ-mediated transcription contributes to the carcinogenesis of numerous cancer types, including cervical cancer (Buglioni et al., 2016; C. He et al., 2015).
miR-216a-3p has been recently proposed as a cancer-associated miRNA in several cancer types (Song et al., 2019; Wang, Li, Zhang, Li, & Yu, 2018; Wu et al., 2018). To date, whether miR-216a-3p is dysregulated in cervical cancer and contributes to the progression of cervical cancer remains unexplored. Here, we found that miR-216a-3p expression was markedly decreased in cervical cancer. A series of functional assays elucidated that miR-216a-3p exerted an inhibitory effect on the proliferation and invasion of cervical cancer. The oncogene ACTL6A was identified as a target gene of miR-216a-3p. ACTL6A knockdown resulted in an antitumor effect in cervical cancer. ACTL6A inhibition increased YAP phosphorylation (pYAP) and downregulated YAP/TAZ-mediated transcriptional activity. However, ACTL6A restoration or YAP reactivation markedly abrogated the miR-216a-3p-mediated antitumor effect in cervical cancer cells. Collectively, our findings indicate that miR-216a-3p exerts an antitumor effect in cervical cancer through the downregulation of ACTL6A-mediated YAP signaling, emphasizing a critical role of miR-216a-3p/ACTL6A/YAP signaling in cervical cancer progression.
2 MATERIALS AND METHODS
2.1 Collection of clinical tissue specimens
Human clinical samples that comprised cervical cancer tissues and adjacent noncancerous tissues were obtained from 45 cervical cancer patients at The First Affiliated Hospital of Xi'an Jiaotong University. Written informed consent about tissue donation for study purposes was obtained from all the participants before the surgery. The study design was reviewed and approved by the Clinical Research Ethics Committees of The First Affiliated Hospital of Xi'an Jiaotong University, and all experimental methods were carried out in accordance with the guidelines of the Declaration of Helsinki.
2.2 Cell lines
Human cervical carcinoma cell lines, including Ca_Ski, C-33A, HeLa, and SiHa, were obtained from the American Type Culture Collection (Manassas, VA). The human normal cervical epithelial immortalized H8 cells and 293T cells were provided by BeNa Culture Collection (BNCC, Kunshan City, China). All cells were cultured in the medium recommended by the manufacturers and grown at 37°C with an atmosphere of 5% CO2 and 95% humidity in a cell incubator.
2.3 Oligonucleotide synthesis, vector construction, and cell transfection
The oligonucleotides for miR-216a-3p mimics and inhibitor and ACTL6A small interfering RNA (siRNA) were synthesized by RiboBio (Guangzhou, China). The coding sequence region of ACTL6A encoding the full length of protein was inserted into the pcDNA3.1 vector to generate the ACTL6A expression vector. The coding sequences of YAP constitutive active form mutant (YAP S127A) were synthesized by the Site-Directed Mutagenesis Kit (TaKaRa, Beijing, China) and inserted into pcDNA3.1 vector to generate the mutant YAP S127A expression vector. Oligonucleotides and vectors were transiently transfected into cervical cancer cells using Lipofectamine 3000 transfection reagent (Invitrogen; Thermo Fisher Scientific Inc., Waltham, MA) following the standard protocols provided by the manufacturer.
2.4 RNA isolation, reverse transcription, and real-time quantitative polymerase chain reaction assays
Total RNA that contained miRNAs was extracted and enriched with the mirVana miRNA Isolation Kit (Invitrogen). For detecting mRNA expression, total RNA was reverse-transcribed into complementary DNA (cDNA) by using the TaqMan Advanced miRNA cDNA Synthesis Kit (Applied Biosystems; Thermo Fisher Scientific Inc). For detecting mRNA expression, total RNA was isolated by using the TRIzol RNA Isolation Reagent (Invitrogen) and converted into cDNA by using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystem). The synthesized cDNA was utilized as the template for real-time quantitative polymerase chain reaction (RT-qPCR), which was carried out using TaqMan Universal PCR Master Mix (Applied Biosystems) with appropriate primers. U6 and GAPDH were utilized as the internal controls for normalizing miRNA or mRNA expression, respectively.
2.5 Detection of cervical cell proliferation
The proliferative ability of cervical cancer cells was determined by using the Cell Counting Kit-8 (CCK-8) method. In brief, cervical cancer cells were plated into a 96-well cell tissue plate and incubated overnight. After overnight adherence, cells were transfected with miR-216a-3p mimics/inhibitor, ACTL6A siRNA, or ACTL6A expression vector. Transfected cells were cultured for 48 hr at 37°C. Thereafter, cells were treated with 10 μl/well CCK-8 reagent (Beyotime, Shanghai, China) and continually cultured at 37°C for 2 hr. Optical density values at 450 nm of the colorimetric solution were assessed with a microplate reader (Bio-Rad, Hercules, CA).
2.6 Measurement of colony-forming ability of cervical cancer cells
Transfected cervical cancer cells were trypsinized, counted, and resuspended into complete medium, followed by seeded into a six-well plate (1,000 cells/well). The medium was changed every 3 days and cells were allowed to grow for 2–3 weeks to form colonies. Then, the colonies were fixed and stained following conventional methods.
2.7 Assessment of the invasive potential of cervical cancer cells
Transfected cervical cancer cells were trypsinized, counted, and resuspended into 200 μl serum-free medium and then placed into the upper invasion chambers (BD Biosciences, San Jose, CA) precoated with Matrigel. At the same time, a complete medium containing 10% fetal bovine serum was loaded into the bottom chamber, serving as chemotaxis. Cells were allowed to pass through the membrane at 37°C for 24 hr. Invaded cells on the lower surface of the filter were fixed and stained following conventional methods. The invasion index was determined by counting the number of invaded cells under an inverted microscope.
2.8 miRNA-dependent dual-luciferase reporter assay
The cDNA fragment of ACTL6A 3′-UTR that contained the wild-type or mutant miR-216a-3p binding site was inserted into the miRNA reporter vector (Promega, Madison, WI). The 293T cells were cotransfected with recombinant vectors and miR-216a-3p mimics, followed by incubation at 37°C for 48 hr. Luciferase activity was determined after cells were lysed following the experimental protocols of the Dual-Luciferase Reporter Assay System (Promega). YAP/TAZ transcriptional activity was monitored by using the 8xGTIIC-luciferase reporter vector (Addgene, Cambridge, MA). In brief, cervical cancer cells were cotransfected with 8xGTIIC-luciferase vector, Renilla luciferase control reporter vector, and miR-216a-3p mimics/inhibitor, followed by incubation at 37°C for 48 hr. Luciferase activities were examined with the Dual-Luciferase Reporter Assay System (Promega).
2.9 Western blot procedure
After protein extraction and concentration determination, equal amounts of proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by transfer to a polyvinylidene difluoride membrane by the semidry method. For blocking nonspecific binding sites, the membrane was immersed in blocking buffer for 45 min at 37°C. Subsequently, primary antibodies against target proteins were diluted in blocking buffer and the membrane was immersed in the solution at 4°C overnight. The primary antibodies used in this study were as follows: anti-ACTL6A, anti-YAP1 (phospho-S127), and anti-GAPDH (Abcam, Cambridge, MA). After incubation with horseradish peroxidase-conjugated secondary antibody (Abcam), the membrane was subjected to enhanced chemiluminescent (ECL) procedures using Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific). The visualized protein bands were quantified with Image-Pro Plus 6.0.
2.10 Statistical analysis
Experimental data were presented as mean ± standard deviation. Student's t test or one-way analysis of variance followed by Bonferroni's posthoc test was performed to calculate the differences. Data were processed using the SPSS version 19.0 software or GraphPad Prism version 6.0 software. The p < .05 denoted the differences were statistically significant.
3 RESULTS
3.1 Low expression of miR-216a-3p was observed in cervical cancer
To understand the role of miR-216a-3p in the progression of cervical cancer, we first determined its expression pattern in tumor tissues of cervical cancer by RT-qPCR. Compared with normal tissues, significant lower expression of miR-216a-3p was detected in cervical cancer tissues (Figure 1a). Moreover, cervical cancer cell lines in vitro also showed decreased miR-216a-3p expression compared with normal H8 cervical epithelial cells (Figure 1b). Overall, these results suggest that miR-216a-3p is a downregulated miRNA in cervical cancer.
3.2 miR-216a-3p overexpression suppressed the proliferation and invasion of cervical cancer cells
To access the biological function of miR-216a-3p in regulating the malignant biological behaviors of cervical cancer cells, miR-216a-3p gain-of-function and loss-of-function experiments were performed in Ca_Ski and SiHa cells in vitro. Transfection of miR-216a-3p mimics resulted in a significant increase in miR-216a-3p expression (Figure 2a). Cell proliferation assay demonstrated that elevation of miR-216a-3p expression markedly decreased the proliferation of cervical cancer cells (Figure 2b). The results of colony formation assay further confirmed the inhibitory effect of miR-216a-3p on cell proliferation, since cells overexpressing miR-216a-3p formed fewer colonies (Figure 2c,d). Moreover, miR-216a-3p overexpression markedly restrained cervical cancer cell invasion (Figure 2e,f). On the contrary, miR-216a-3p inhibition had the opposite effects, showing an enhanced effect on the proliferation and invasion of cervical cancer cells (Figure 2a-d). Collectively, these data indicate that miR-216a-3p exerts antitumor effects in cervical cancer cells by inhibiting cell proliferation and invasion.

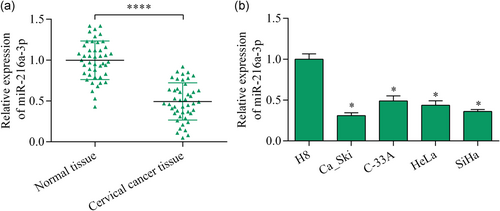
3.3 miR-216a-3p bound to the ACTL6A 3′-UTR and negatively modulated ACTL6A expression
To uncover the molecular mechanisms underlying miR-216a-3p-mediated effects, we investigated downstream miR-216a-3p targets. Interestingly, ACTL6A, a potential oncogene in multiple cancers, was predicted as a putative target of miR-216a-3p, as analyzed by the bioinformatics method. We next performed a dual-luciferase reporter assay to detect the direct interaction between miR-216a-3p and ACTL6A 3′-UTR (Figure 3a). The results demonstrated that cotransfection of miR-216a-3p mimics and wild-type ACTL6A 3′-UTR reporter vector decreased luciferase activity in 293T cells (Figure 3b), whereas miR-216a-3p overexpression did not affect luciferase activity of mutant ACTL6A 3′-UTR reporter vector (Figure 3b). To investigate whether miR-216a-3p exerts a direct effect on ACTL6A expression, we transfected miR-216a-3p mimics or inhibitor into Ca_Ski and SiHa cells and detected their effects on ACTL6A expression. The results demonstrated that miR-216a-3p upregulation significantly repressed ACTL6A expression, while its inhibition enhanced ACTL6A expression (Figure 3c-f). Collectively, these findings indicate that ACTL6A is a direct target gene of miR-216a-3p in cervical cancer.
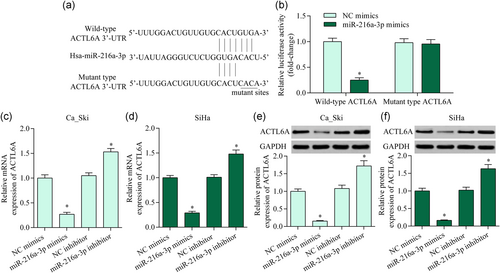
3.4 ACTL6A knockdown exhibited an antitumor effect in cervical cancer
To explore whether ACTL6A is involved in regulating cervical cancer progression, we examined ACTL6A expression in cervical cancer by RT-qPCR. ACTL6A mRNA was significantly upregulated in cervical cancer cell lines (Figure 4a). Correspondingly, the ACTL6A protein was also markedly elevated in cervical cancer cell lines (Figure 4b). Moreover, the silencing of ACTL6A markedly repressed the proliferative ability and invasive potential of cervical cancer cells (Figure 4c-e). Overall, these data imply that ACTL6A inhibition exhibits an antitumor effect in cervical cancer.
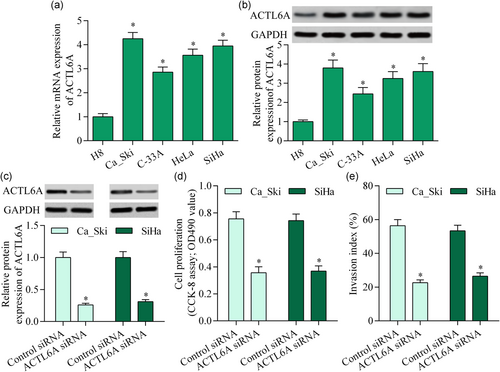
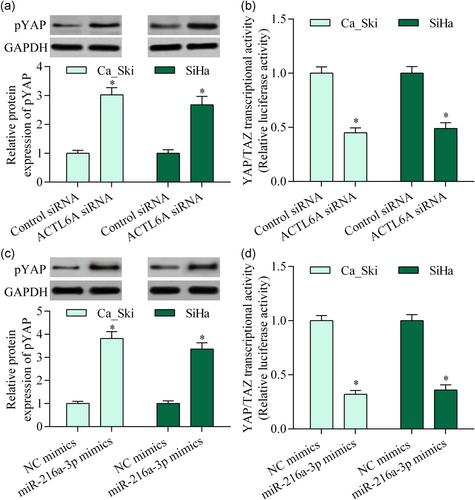
3.5 ACTL6A inhibition or miR-216a-3p overexpression inhibited YAP signaling activation
To elucidate the molecular mechanism of ACTL6A in cervical cancer, we measured the effect of ACTL6A inhibition on YAP activation, a well-known target of ACTL6A. Silencing ACTL6A significantly increased pYAP and downregulated YAP/TAZ-mediated transcriptional activity (Figure 5a,b). Moreover, miR-216a-3p overexpression showed a similar effect (Figure 5c,d). Collectively, these data suggest that miR-216a-3p/ACTL6A may contribute to YAP signaling regulation in cervical cancer.
3.6 ACTL6A restoration abrogated the miR-216a-3p-mediated antitumor effect
To determine whether miR-216a-3p exerts tumor-suppressive effect through the downregulation of ACTL6A, we restored the expression of ACTL6A by using an ACTL6A expression vector harboring no ACTL6A 3′-UTR. We showed that transfecting the ACTL6A expression vector into miR-216a-3p mimics-transfected cervical cancer cells significantly restored the expression of ACTL6A (Figure 6a). As expected, the restoration of ACTL6A expression markedly reversed the inhibitory effect of miR-216a-3p overexpression on the proliferation and invasion (Figure 6b,c). ACTL6A restoration significantly abolished the promotion effect of miR-216a-3p overexpression on phosphorylation of YAP (Figure 6d). Moreover, ACTL6A restoration partially reversed miR-216a-3p overexpression-mediated suppressive effect on YAP/TAZ-mediated transcriptional activity in cervical cancer cells (Figure 6e). Overall, these results suggest that miR-216a-3p exerts an antitumor function in cervical cancer cells by downregulating ACTL6A.

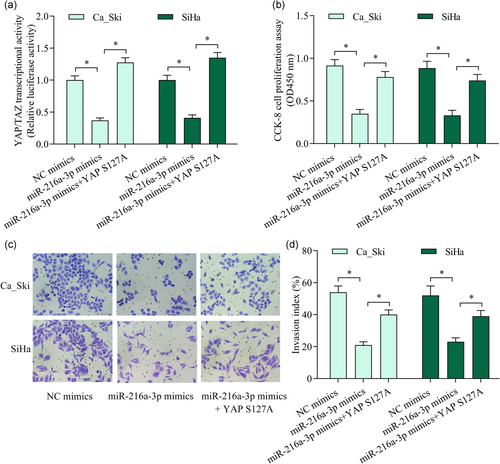
3.7 Reactivation of YAP reversed miR-216a-3p-mediated antitumor effect
To verify whether miR-216a-3p exerts the antitumor effect through the inactivation of YAP, we further investigated the effect of YAP reactivation on miR-216a-3p overexpression-mediated suppressive effect on cervical cancer cell proliferation and invasion. We reactivated YAP signaling in miR-216a-3p mimics-transfected cells by overexpression of constitutively active form mutant (YAP S127A; Figure 7a). As expected, YAP reactivation markedly reversed miR-216a-3p overexpression-mediated suppressive effect on cervical cancer cell proliferation (Figure 7b) and invasion (Figure 7c,d). Overall, these data confirm that miR-216a-3p represses cervical cancer cell proliferation and invasion through the inactivation of YAP.
4 DISCUSSION
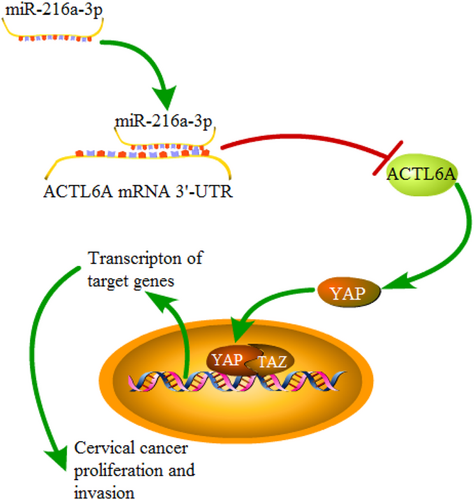
In the current study, we revealed a key role for miR-216a-3p in cervical cancer. Our results confirmed miR-216a-3p was a decreased miRNA in cervical cancer and suggested a tumor-suppressive role of miR-216a-3p in cervical cancer. The inhibitory effect of miR-216a-3p on cervical cancer cell proliferation and invasion was associated with its regulatory effect on its target gene ACTL6A. Notably, miR-216a-3p regulated YAP signaling activation via targeting ACTL6A, a finding that highlights the critical involvement of the miR-216a-3p/ACTL6A/YAP axis in regulating the malignant progression of cervical cancer (Figure 8).
miR-216a-3p is a tumor-associated miRNA in multiple cancer types. miR-216a-3p, mapped on human chromosome 2p16.1, is decreased in pancreatic ductal adenocarcinoma (Yonemori et al., 2017). Low miR-216a-3p expression is detected in colorectal cancer, which is related to shorter overall survival in patients (Wang et al., 2018). Moreover, miR-216a-3p overexpression restricted the growth of colorectal cancer cells through the downregulation of cyclooxygenase-2 and 5-lipoxygenase (Wang et al., 2018). In gastric cancer cells, upregulation of miR-216a-3p resulted in an inhibitory effect on the proliferation, migration, and invasion via targeting runt-related transcription factor 1 (Wu et al., 2018). Notably, decreased miR-216a-3p expression in cancer tissues is associated with promoter methylation (Song et al., 2019; Su et al., 2019). Overall, these findings support the notion that miR-216a-3p is a tumor-suppressive miRNA. However, whether miR-216a-3p is involved in cervical cancer remains unknown. Here, our study revealed a significant low expression of miR-216a-3p in cervical cancer. In vitro functional experiments demonstrated an inhibitory effect of miR-216a-3p on cervical cancer cell proliferation and invasion, confirming the tumor-suppressive function of miR-216a-3p.
ACTL6A is a critical tumor-related gene in a wide range of cancer types. Elevated ACTL6A expression is detected in colon cancer and hepatocellular carcinoma and contributes to regulating tumor growth and metastasis (S. Xiao et al., 2016; Zeng et al., 2018). ACTL6A promotes the progression of glioma associated with YAP/TAZ signaling activation (Ji et al., 2018; Meng et al., 2017). Moreover, ACTL6A is essential for driving YAP activation in promoting the tumorigenesis of head and neck squamous cell carcinoma (Saladi et al., 2017). Notably, ACTL6A contributes to oncogenic transformation by affecting c-Myc and p53 activity (Park et al., 2002; Zhong et al., 2019). These studies implicate ACTL6A as a potential oncogene. Interestingly, a recent study reported that ACTL6A regulates E6 and E7 gene expression from HPV integrants in cervical cancer, data that suggest the potential role for ACTL6A in cervical cancer progression (Lee, Lee, Kwon, & Kwon, 2011). To date, the extract biological function of ACTL6A in cervical cancer remains undermined. Herein, our study showed that ACTL6A expression was significantly elevated in cervical cancer, and ACTL6A knockdown restricted the proliferation and invasion of cervical cancer cells. These findings indicate that ACTL6A exerts a key role in regulating the malignant behavior of cervical cancer cells and may serve as an attractive anticancer target.
ACTL6A dysregulation in tumor tissues remains unclear. Interestingly, ACTL6A expression is posttranscriptionally regulated by miRNAs, including miR-9*, miR-124, and miR-206 (Taulli et al., 2014; Yoo, Staahl, Chen, & Crabtree, 2009). In this study, we identified ACTL6A as a new target gene of miR-216a-3p. We found that the upregulation of miR-216a-3p decreased ACTL6A expression, while its inhibition upregulated ACTL6A expression in cervical cancer cells. Therefore, decreased miR-216a-3p may contribute to the elevated ACTL6A levels in cervical cancer tissues. These results of our study indicate that the miR-216a-3p/ACTL6A axis may represent a critical regulation mechanism underlying the molecular pathogenesis of cervical cancer.
YAP/TAZ upregulation is a common feature in cervical cancer (C. He et al., 2015; Lorenzetto et al., 2014; H. Xiao et al., 2014), and it likely functions as an oncogenic factor. Multiple factors contribute to the regulation of YAP/TAZ activation. Our study confirmed ACTL6A as a critical regulator for YAP/TAZ in cervical cancer. Our findings are consistent with previous studies that revealed ACTL6A is essential for driving the activation of YAP/TAZ-mediated transcription (Ji et al., 2018; Saladi et al., 2017). ACTL6A collaborates with p63 to decrease pYAP and enhance YAP/TAZ-mediated transcriptional activity (Saladi et al., 2017). Moreover, ACTL6A suppresses ubiquitin-mediated YAP degradation and enhances YAP/TAZ stabilization (Ji et al., 2018). Collectively, these findings indicate ACTL6A as a positive regulator of YAP/TAZ activation. Our study suggests that the ACTL6A/YAP/TAZ regulation axis contributes to cervical cancer progression.
In conclusion, these findings uncovered that miR-216a-3p upregulation represses the proliferation and invasion of cervical cancer, phenomena that were associated with the downregulation of YAP activation via targeting ACTL6A. Our study highlights the critical involvement of miR-216a-3p/ACTL6A/YAP signaling in regulating the malignant growth and metastasis of cervical cancer. miR-216a-3p and ACTL6A may serve as candidate anticancer targets for cervical cancer.
ACKNOWLEDGMENT
This study was supported by the Scitech Program Foundation of Shaanxi Province (Grant/Award No. 2017SF-016 and 2017SF-126).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
J. Z. conducted experiments and wrote the manuscript. L. L. contributed to the interpretation of data. T. Y. designed this study and revised the manuscript. All authors reviewed and passed the final version of the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author upon request.




