The role of HSP90 molecular chaperones in hepatocellular carcinoma
Abstract
Misfolded proteins have enhanced formation of toxic oligomers and nonfunctional protein copies lead to recruiting wild-type protein types. Heat shock protein 90 (HSP90) is a molecular chaperone generated by cells that are involved in many cellular functions through regulation of folding and/or localization of large multi-protein complexes as well as client proteins. HSP90 can regulate a number of different cellular processes including cell proliferation, motility, angiogenesis, signal transduction, and adaptation to stress. HSP90 makes the mutated oncoproteins able to avoid misfolding and degradation and permits the malignant transformation. As a result, HSP90 is an important factor in several signaling pathways associated with tumorigenicity, therapy resistance, and inhibiting apoptosis. Clinically, the upregulation of HSP90 expression in hepatocellular carcinoma (HCC) is linked with advanced stages and inappropriate survival in cases suffering from this kind of cancer. The present review comprehensively assesses HSP90 functions and its possible usefulness as a potential diagnostic biomarker and therapeutic option for HCC.
1 INTRODUCTION
Liver cancer is the sixth most common cancer and the fourth cause of deaths due to cancer worldwide in 2018 and also hepatocellular carcinoma (HCC) accounts for 85% of the primary liver cancer (Bray et al., 2018). Because of the late diagnosis and limited effective treatment options, HCC is considered as a poor prognostic carcinoma (Hajiasgharzadeh, Somi, Shanehbandi, Mokhtarzadeh, & Baradaran, 2019). Most of the times, HCC is asymptomatic and the symptoms appear in the advanced stages of HCC (S. Chen, Cao, Wen, & Wang, 2019). Currently, surgical resection, radiofrequency ablation, transarterial chemoembolization, and transplantation are the common therapeutic options available for these patients. Unfortunately, the drug resistance toward HCC and undesirable side effects resulted in limited effectiveness. Therefore, the recurrence rate is high and the survival rate is low in these therapeutic approaches (Ferenci et al., 2010; Montomoli, Erichsen, Norgaard, & Hansen, 2011; Tampaki, Doumba, Deutsch, & Koskinas, 2015).
Molecular chaperones are involved in folding nascent polypeptides and the right accumulation/disassembly of protein complexes (Jackson, 2013; Mack & Shorter, 2016). Heat shock proteins (HSPs) are major types of chaperones that express against the elevated temperature or several cellular stresses (Ge, Yan, Guo, Tian, & Wu, 2018; Stetler et al., 2010). The classification of HSPs is based on their monomer's molecular weight (HSP100, HSP90, HSP70, HSP60, HSP40, and small HSP families; Rappa et al., 2012). These proteins seem fundamental to the prevention of protein misfolding diseases. Their expression occurs before the appearance of misfolded proteins to ensure the correct protein production (Condelli et al., 2019; Prodromou, 2017). Referring to functional characteristics, HSPs are important molecules for deregulation of cellular production and performance that leads to several diseases, such as neurodegenerative disorders, cardiovascular diseases, and cancers (Ciocca & Calderwood, 2005; Penke et al., 2018). Particularly, through malignant transformation and cancer progression, tumor cells with high proliferation produce several mutated and/or aberrant proteins, which needed folding (Tian et al., 2014). Among the chaperone proteins, HSP90 is a chaperone against cell stress and increases the malignancy and angiogenesis of tumor cells (Mabjeesh et al., 2002; H. Zhang & Burrows, 2004). Chaperone activity of HSP90 is associated with the phenotypic plasticity (Zabinsky, Mason, Queitsch, & Jarosz, 2019) and also mediates epigenetics via interaction with chromatin regulators and epigenetic effectors. HSP90 makes the mutated oncoproteins able to avoid misfolding and degradation and permits the malignant transformation (Barrott & Haystead, 2013; Wu et al., 2017). HSP90 promotes the growth and inhibits apoptosis in multiple types of cancers, such as bladder carcinoma (Lebret et al., 2003), medulloblastoma (Alexiou et al., 2013), renal (Zhu, Zhu, Qi, & Qiu, 2015), lung (Esfahani & Cohen, 2016), colorectal (Moser et al., 2007), and acute leukemia (Tian et al., 2014) as well as HCC (Dong, Xue, et al., 2018; C. Wang, Zhang, et al., 2016). More recently, it was shown that the HSP90 is a potential biomarker of HCC, and the diagnostic value of its expression for HCC detection was reported (Wei et al., 2020). In this review, we have attempted to summarize the molecular structure and expression of HSP90, the mechanism of action, the potential diagnostic role, and the possible therapeutic value of HSP90 in the HCC.
2 THE MOLECULAR STRUCTURE OF HSP90
HSP90 and its homologs have three structural domains, such as an N-terminal domain (NTD), a middle domain (MD), and a C-terminal domain (CTD). An NTD contains the ATP-binding site attached to an MD through the variable charged linker; MD contributes to the interaction sites for client proteins and some co-chaperones, and CTD is involved in HSP90 as a dimer (Genest, Wickner, & Doyle, 2019; Prodromou et al., 1997). The CTD of the cytosolic homologs has a MEEVD motif, which can be recognized via tetratricopeptide repeat domains related to many co-chaperones. Some co-chaperones attach the HSP90 system into the HSP70 system by Hsp70-HSP90 Organizing Protein (HOP) as well as the proteasome (using CHIP as the E3 ligase; Scheufler et al., 2000). More E3 ligases are associated with HSP90, such as Ubr1 and Cul5, which are involved in the quality control or degradation of different client proteins (Ehrlich et al., 2009). However, the HSP90 system has known to be simply a spacer between the NTD and MD, it has recently shown that the sequence of this binding area has a crucial role in regulation of the activity of HSP90 in vivo (Tsutsumi et al., 2012; R. Zhao & Houry, 2007). In eukaryotes, the charged binding region includes certain regulatory areas, which act as a “rheostat” and tunes the HSP90 chaperone machine (Tsutsumi et al., 2012). The co-chaperone FNIP1 has also been reported to function as a rheostat with respect to HSP90 (Sager et al., 2019).
3 HSP90 EXPRESSION IN HCC
HSP90 was upregulated in HCC clinical samples and was associated with clinical features (Albrethsen, Miller, Novikoff, & Angeletti, 2011; Dong, Xue, et al., 2018). HSP90α can express both inside and outside of different metastatic HCC, and the level of expression was consistent with metastasis potentials (W. Liu et al., 2019; Weihua et al., 2014). Extracellular Hsp90 (eHSP90) was shown to be critical for the regulation of tumor invasiveness and metastasis, central processes associated with cancer lethality (Wong & Jay, 2016). Low levels of gp96 (a member of the HSP90 family) show poor prognosis among cases with early-stage HCC following hepatectomy and is considered as an independent causative agent regarding poor postoperative survival in HCC cases (Ji et al., 2019). Independently, the negative expression of HSP90 improves the survival of patients with HCC (X. Liu, Chen, Tu, Cai, & Xu, 2016).
The expressions of HSPs have shown to be upregulated in HBV-caused HCCs (Lim et al., 2005). In addition, HSPA12A and HSP90B1 overexpression are possibly linked to poor survival due to the hepatitis B virus (HBV)-related early-stage HCC (Z. Yang et al., 2015). The gp96 expression in HCC tissues has shown to affect tumor differentiation level as well as tumor size. A high gp96 expression has been reported in many HCC cases with HBV DNA-positive (Yao et al., 2006). Hepatitis B virus X protein (HBx) can induce HSP90α expression in the transcription stage. HSP90α overexpression in HBx-transfected cells increases the invasion of cancer cells (Li et al., 2010).
HSP90 high expression promotes the epithelial–mesenchymal transition (EMT), improves Vimentin expression, reduces E-cadherin expression, and inhibits cancer stem cells apoptosis that all increase the liver cancer cells invasion (Gao, Geng, & Xiang, 2015). HSP90β has been shown to be involved in vasculogenic mimicry and EMT marker proteins in HCC tissues and also enhanced tube production, cell migration, and invasion (Meng et al., 2019). Tumors with high HSP90 expression have remarkably more microvessel density. The human HCC angiogenesis is possibly moderated via HSP90 (Cheng et al., 2015). HSP90β promoted vascular endothelial growth factor receptors (VEGFRs) and CD31 expression in HCC tissues were associated with increased tumor microvessel density (Meng et al., 2017). Zhao et al. reported that hepatic tumorigenesis and differentiated adipocytes may modulate both global histone deacetylase (HDAC) expression and specific class I HDAC genes in the tumor microenvironment (TME). In this context, the HDAC inhibitors in combination with HSP90 inhibitors may provide a promising strategy for HCC patients by targeting cellular communication within the TME (J. Zhao, Gray, Wabitsch, Greene, & Lawless, 2018).
As another study, the expression of carbamoyl-phosphate synthase 1 (CPS1) was significantly decreased in HCC tissues, and patients with low CPS1-IT1 expression had poor survival outcomes. CPS1-IT1 significantly reduced cancer-associated properties via reduced HSP90 binding to and activation of HIF-1a, thereby suppressing the EMT (T. H. Wang, Yu, et al., 2016). The known upregulated P16INK4A (an oncosuppressor gene) is linked to the HSP90 mild overexpression (Pascale et al., 2005). Besides, B-cell lymphoma 2 (Bcl-2)-associated transcription factor 1 (Bclaf1) is frequently upregulated in HCC, and its upregulation is associated with poor prognosis and decreased survival (X. Zhou et al., 2019). To exert its effect, Bclaf1 must interact with the HSP90α and it was reported that in HCC tissue samples, Hsp90α–Bclaf1 interaction was enhanced (X. Zhou et al., 2019). As another evidence about HSP90 expression in HCC, it was shown that following radiofrequency ablation, the cellular expression of HSP90 in HCC tissue increased (G. Schueller et al., 2004).
4 MECHANISM OF ACTION
HSP90 expression upregulates in HCC and it has two cytosolic isoforms: (a) Major form is Hsp90α (stress-inducible), which has two members of HSP90α1 and HSP90α2, (b) Minor form is HSP90β (constitutive; Powers & Workman, 2007). HSP90α is an essential factor for modulating MYC proto-oncogene (c-Myc) mRNA stability by Bclaf1, and interaction of HSP90α-Bclaf1 elevates HCC in the tissue (X. Zhou et al., 2019). In HepG2 cell line, hepatitis B virus X (HBx) protein upregulates c-Myc expression by using Ras/Raf/ERK1/2 signaling cascades, thus leading to an increased HSP90α expression (Li et al., 2010).
HSP90β interaction with Twist1 causes progressive vasculogenic mimicry, it promotes its deubiquitination and fixation to nuclear translocation and enhances the vascular endothelial-cadherin (VE-cadherin) promoter function in HCC (Meng et al., 2019). In addition, HSP90β, triosephosphate isomerase, and vimentin are possibly associated with the lnc-ELF209 tumor-suppressed activity. The lnc-ELF209 is an heterogeneous nuclear ribonucleoprotein AB (HNRNPAB)-regulated long-noncoding RNA, which is crucial to inhibit HCC progression (Y. Yang et al., 2019).
Inhibition of HSP90 reduces cell growth of HCC by increasing p53 and lowering survivin, cyclin D1, and nuclear factor-κB (NF-κB) expression (Leng et al., 2012).
Blocking stress-induced phosphoprotein 1 (STIP1) suppresses apoptosis and cell growth, whereas STIP1 is positively linked to HCC and contributes to a poor clinical prognosis (Y. Chen et al., 2016). In return, STP1 activates P13/AKT signaling pathway (Z. Chen et al., 2017). STIP1 is an adaptor protein that acts as a bridge between HSP70 and HSP90 client protein complexes, forming STIP1-HSP90 machinery shuttles snails transcriptional factor into the nucleus and regulates mesenchymal gene-expression pattern (Su et al., 2018).
15-Hydroxyeicosatetraenoic acid enhances development of HCC cells associated with interaction between AKT and HSP90 complex (Ma et al., 2013). During numb and proline-rich region exclusion, short isoform (PRRS) negatively regulates AKT phosphorylation and c-Myc expression. AKT antagonism and c-Myc silencing diminish proliferation caused by knocking down of the Numb-PRRS using small interfering RNAs. Furthermore, HSP90 directly regulates SR protein-specific kinase 2 (SRPK2) subcellular localization, whereas knockdown of SRPK2 causes accumulation of Numb-PRRS. Also HCC cells expressing PRRL appears sensitive to HSP90 inhibition (Lu et al., 2015).
HSP90 enhances cell proliferation and glycolysis, and suppresses apoptosis of HCC cells by binding to PKM2 threonine 328 phosphorylation (Xu et al., 2017).
HSP90 is vital for stability and function of glycogen synthase kinase 3β (GSK3β; Banz et al., 2009), hence GSK3β and AKT are client proteins of the HSP90 in HCC. High levels of diaphanous-related formin 3 in HCC cells regulate the development, migration, and metastasis; it can bind to HSP90 through β-Catenin-TCF signaling, which disrupts the interaction of HSP90-GSK3β (Dong, Li, et al., 2018).
There is a clear correlation between the HSP90 levels and the necrotic cell subpopulation in heat-shocked HCC cells (G. Schueller et al., 2001). An overexpression of Heat shock factor 1 (HSF1) in HCC patients has been reported (Y. Chen et al., 2013). This factor promotes HSP90 inhibitors activity through the DEDD2 gene and it might provide a therapeutic approach for the treatment of HCC (Y. Chen et al., 2013).
HSP90 regulates hypoxia-inducible factor (HIF-1α) protein redundancy through inhibition of the HIF-1α ubiquitination and proteasomal degradation in HCC cell lines (X. Liu et al., 2016). CPS1-IT1 remarkably reduces cell proliferation, migration, and invasive behavior by lowering HSP90 attachment and activating HIF-1α, leading to the suppressed EMT (T. H. Wang et al., 2016).
PIWIL2 (a gene with tumorigenesis role) interacts with HSP90 for preventing formation of HSP90–TβR complexes leading to TβR degradation and suppression of TGF-β signaling (Y. Chen et al., 2014). HSP90 regulates mevalonate pathway in the progress of HCC by interacting with 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR; Dong, Xue, et al., 2018). A combined collaboration of anaplastic lymphoma kinase (ALK), fibroblast growth factor receptor 2 (FGFR2), and ephrin type-A receptor 5 (EphA5) kinases is required for management of HCC cells growth. Their mechanisms of action are vigorously controlled by HSP90. A poorer overall survival exist in their co-activation (X. Wang et al., 2019).
HSP-expressing Tex is a stimulatory machine that mediates NK cell activity (Lv et al., 2012). HSPs expression is usually upregulated in HBV-caused HCCs and GRP78 is possibly essential in HBV-caused hepatocarcinogenesis stepwise progression (Lim et al., 2005). Translocase of the outer mitochondrial membrane 34 (TOM34) and HSP90α are probably applied as a possible biomarker to monitor the hepatitis C virus (HCV)-induced HCC progression (TOM34 represents a scaffolding cochaperone of HSP90/HSP70 complex through protein folding; Toraih et al., 2019).
A sensitive or resistant carcinogenic phenotype is recognized by HSP90/Cdc37 and E2f4/Crm1 (Pascale et al., 2005). HCC progression is correlated with cell cycle protection from p16INK4A inhibition via CDC37, HSP90, and CRM1 upregulation (Calvisi, Pascale, & Feo, 2008).
Gp96 (also called grp94) is a pro-oncogenic chaperone that its genetically missing can promote the adaptive assembly of long-chain ceramide, along with steatotic reformation of residual gp96+ hepatocytes (Rachidi et al., 2015).
5 HSP90 AS A BIOMARKER IN HCC
HSP90 gene showed great potential as screening or prognostic markers for human hepatocarcinogenesis (Hass, Jobst, Scheurlen, Vogel, & Nehls, 2015). Hepatocellular HSP90 may be positively involved in HCC development, and it is likely a possible biomarker for monitoring advanced HCC (Qin et al., 2019). It is also regarded as a probable biomarker for early detection, prognosis, and supervision in HCC treatment (Y. Sun et al., 2010). It has also been shown as a marker in the prognosis of HBV-caused HCC, which greatly suggests vascular invasion as well as intrahepatic metastasis (Lim et al., 2005).
HSP90α suggests for the screening and diagnosis of HCC (Y. Zhou et al., 2015). Plasma HSP90α is a diagnostic biomarker for liver cancer and is employed for evaluating the treatment outcome of liver cancer cases undergoing operation, or interventional treatment (Fu et al., 2017). HSP90α is overexpressed in human HCC and might be used as an indicator to judge the differentiation and prognosis of HCC (Chatterjee & Burns, 2017). Gene HSP90β was detected and partly described for the first time as potential discrimination markers (Hass, Vogel, Scheurlen, & Jobst, 2018). The validated proteins contain the reported biomarker candidates HSP90β, which demonstrated the robustness of the strategy (Mustafa, Larry, Petersen, & Elferink, 2015).
Overexpression of HOP in HCC tissues was confirmed, suggesting their potential as protein tumor markers (W. Sun et al., 2007). Gp96 has been reported as a possible and accurate predictive biomarker for cancer recurrence in HCC cases following curative resection (Ji et al., 2019). Bclaf1 expression can indicate an appropriate biomarker in HCC and possibly in other cancer cases (X. Zhou et al., 2019). Alternative splicing of the Numb (Numb has been detected in Drosophila as an important factor for cell fate) can be an effective predictive biomarker in HCC and is also tractable (Lu et al., 2015). Coactivation of ALK, FGFR2, and EphA5 (HSP90 Client proteins) can be a suitable marker to diagnose HCC cases (X. Wang et al., 2019).
6 HSP90 AS A THERAPEUTIC TARGET FOR HCC
6.1 Geldanamycin analogs (17-AAG and 17-DMAG)
6.1.1 17-AAG
Watanabe and colleagues have shown that HCC cells treatment with 17-(demethoxy), 17-allylamino geldanamycin (17-AAG) lead to decreased HCC cells viability and increased apoptosis. Also, this treatment leads to an increased proportion of the cells in the G2/M phase an associated decrease in cdc2 protein degradation (Watanabe, Behrns, Kim, & Kim, 2009). VEGF-C mRNA and HSP90 enhanced amounts are remarkably improved by using 17-AAG or Cisplatin in HCC ascites (Cui et al., 2016). 17-AAG as an HSP90 inhibitor induces GRP75 (HSP70 family) expression, leading to attenuating the growth-inhibitory impact of HSP90 blockade on cancer cells. GRP75 inhibitor MKT-077 can enhance 17-AAG-caused apoptosis in HCCs and increase p53-induced inhibiting of cancer development. Affecting both GRP75 as well as HSP90 is probably effective to treat HCCs (Guo et al., 2014). 17-AAG has tumor-associated assemblage with antineoplastic effect with no impact on hepatotoxicity (Breinig et al., 2009). Combination of Resminostat (class I HDAC inhibitor) and 17-AAG can be a “smart” approach for HCC cases by affecting cellular communication through the TME (J. Zhao et al., 2018).
SNX-2112 (an HSP90 inhibitor) shows a high inhibitory effect on cell development compared with 17-AAG via caspase-associated apoptosis (B. Wang et al., 2014). SNX-2112 decreases the calnexin and immunoglobulin-binding protein (BiP) expressions. In addition, SNX-2112 inhibits the ER stress sensors, including inositol-requiring gene 1 (IRE1), PKR-like ER kinase (PERK), and activating transcription factor 6 (ATF-6). Tunicamycin induces ER and greatly enhances SNX-2112-caused apoptosis (B. Wang et al., 2014).
6.1.2 17-DMAG
Specific HSP90 inhibitor 17-DMAG (17-dimethylaminoethylamino-17-demethoxy geldanamycin) is used to treat HCC through reduction in survivin, cyclin D1, and NF-kB protein levels and an elevated p53 protein level (Leng et al., 2012). 17-DMAG has tumor-associated assembly and also an antineoplastic effect with no remarkable hepatotoxicity (Breinig et al., 2009). The combined heat shock and 17-DMAG therapy of HCC cells weakens CDK1, cyclin B1, and CDC25C and also HSP90α interaction with CDC37 and CDK1 along with the reduced soluble CDK1. Hyperthermia sensitizes HCC to 17-DMAG (Huang et al., 2016). Cyclin B1 low levels are associated with chemoresistance against HSP90 inhibitor 17-DMAG under hypoxic conditions (J. Zhang et al., 2016). Cells were treated with DMAG-N-oxide (a small molecule cell-impermeant HSP90α inhibitor), the average migratory cell numbers had a significant reduction (W. Liu et al., 2019). ALK, FGFR2, and EphA5 decreased after treatment with 17-DMAG (X. Wang et al., 2019).
6.2 Bcl-2 target therapy
Treatment with HSP90 inhibitor (SNX-2112) showed effectively reduced cell growth, and proliferating cell nuclear antigen, Bcl-2-positive cell counts (Qin et al., 2019). Inhibiting HSP90 can be regarded as a novel approach to sensitize Bcl-2-targeted chemotherapies in HCC (X. Wang et al., 2014). Bclaf1 interacts with the HSP90α C-terminal domain, which disrupts via novobiocin (NB), a ;C-terminal inhibitor of HSP90, leading to proteasome-associated degradation of Bclaf1. Besides, the disrupted HSP90α-Bclaf1 interaction diminishes the mature c-MYC mRNA synthesis and weakens tumor cell development (X. Zhou et al., 2019). Melatonin treatment increased the expression of p21, p53, and poly ADP ribose polymerase (PARP1)/2, a higher Bax/Bcl-2 ratio, and decreased expression of HSP90 and GRP78 proteins in HCC (Sánchez et al., 2018).
6.3 Phosphatidylinositol-3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) signaling pathway target therapy
HSP90/CDC37 antagonists (celastrol, pristimerin, cel-D2, and cel-D7) are widely used agents possibly effective to treat heterogeneous subtypes of HCC via induction of cell apoptosis. They promote degradation and inhibit protein kinases phosphorylation in the Raf/MEK/ERK and PI3K/AKT/mTOR signaling routes. Also, cel-D7 exhibit lower toxicity (Wei et al., 2014). STIP1 promotes HCC development via PI3K–AKT-associated antiapoptotic route (Z. Chen et al., 2017). Downregulation of PI3K/AKT growth signaling pathway and also HSP90 and PARP has been reported following GALK1 (galactokinase) and galactose-1 phosphate uridylyltransferase gene expression knockdown in HCC (Tang, Etokidem, & Lai, 2016). Abrus agglutinin (AGG) obtained from the Abrus precatorius seeds (a medicinal herb from India), belongs to the class II ribosome-inactivating protein family, which decreases HSP90 expression and suppresses Akt phosphorylation as well as NF-κB expression in HCC (Mukhopadhyay et al., 2014).
SCP-0-1 prevents cell growth and elevates mitochondrial apoptosis more than autophagy in HCC. Inducing apoptosis in mitochondria is associated with the HSP90/AKT signaling pathway inhibition (Y. Chen et al., 2016). 8u is an acridine derivative and a pro-apoptosis/cell cycle arrest agent that can suppress invasion and metastasis by HSP90α downregulation and PI3K/Akt signaling route inactivation in HCC cells (N. Wang et al., 2018). Autophagy decreases in incomplete thermal ablation, and its possible inverse correlation with HSP expression has been shown. The HSP90/Akt/mTOR route has been reported, which is associated with signal transmission between autophagy and HSPs (F. Chen, Bao, Xie, Tian, & Jiang, 2019). Dual inhibition of mTOR and HSP90 (Hsp90 inhibitors improve mTOR inhibitor) results in beneficial oncogenic signaling cascades disruption and significantly increases growth inhibition (Lang et al., 2009).
6.4 HIF-1α
Upregulated HIF-1α expression relatively cancels HSP90 siRNA-induced HCC cell cycle arrest as well as apoptosis (X. Liu et al., 2016). Hypoxia induces HCC cells migration and invasion. HSP90 regulates HIF-1α in HCC tissues and cells. Of note, HSP90 elevates tumor growth rate in HCC partially through upregulation of HIF-1α. Proanthocyanidin B2 (a flavonoid) prevents expression and nuclear translocation of PKM2, which modulates interaction of PKM2 with HSP90 and HIF-1α. This mechanism of action inhibits proliferation and aerobic glycolysis of HCC in vivo and in vitro (Feng et al., 2019).
The weakening of the signaling through vorinostat (suberoylanilide hydroxamic acid (SAHA), a class I/IIb/IV histone deacetylase inhibitor confirmed by FDA, strongly suppresses HIF-α nuclear displacement through direct acetylation of HSP90. An increased degree of acetyl-HSP90 has been shown in the existence of SAHA as well as a reduced interaction between acetyl-HSP90 and HIF-α is decreased, nuclear/cytoplasmic HIF-α expression is decreased, HIF-α association with its nuclear karyopharyin Importin is absent, and markedly HIF-α transcriptional activity is decreased. These changes were associated with the downregulation of downstream hypoxia molecules such as endothelin 1, erythropoietin, glucose transporter 1, and vascular endothelial growth factor. Findings were associated with significant decreases in tumor size (C. Zhang et al., 2017). Exosomes of the HepG2 cells treated with HCC cell resistant to anticancer medications have higher immunogenicity to induce HSP-specific NK cell reactions and are effective to find a useful approach for HCC immunotherapy (Lv et al., 2012). Some of the other HSP90-related therapeutic agents and their related mechanisms are summarized in Table 1.
| Drug/inhibitor | Mechanism | References |
|---|---|---|
| 17-Demethoxy-reblastatin (17-DR) | Downregulation of myeloid cell leukemia-1 (Mcl-1) | Zhao et al. (2015) |
| Quercetin | Inhibitory effect on whole expression of HSP | Zhou et al. (2011) |
| Pomegranate | HSP90 and NF-κB suppression | Bishayee et al. (2013) |
| Panobinostat | Increasing histone H3 and HSP90 acetylation, downregulated BIRC5 (survivin) and upregulated CDH1 | Lachenmayer et al. (2012) |
| Geldanamycin (GA) | Transcriptional increase of N-myc downstream-regulated gene 1 (NDRG1) protein | Banz et al. (2009) |
| Inhibition the phosphorylation of NDRG1 by serum- and glucocorticoid-induced kinase 1 (SGK1) and glycogen synthase kinase 3β (GSK3β) targeting | ||
| Geldanamycin (GA) and synchronous thermal ablation | Simultaneous destruction of the HCC ablative margins and tumor core | Chen, Youn, and Furgeson (2011) |
| Lovastatin | Inhibitor of 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) | Dong, Xue, et al. (2018) |
| 15-Lipoxygenase-1 (15-LO-1) inhibitor or siRNA | Blockade of the 15-LO | Ma et al. (2013) |
| NEN (ethanolamine salt) | Binds to cell division cycle 37 (CDC37) protein and disrupt its interaction with HSP90 | Z. Chen et al. (2017) |
| NVP-BEP800 (HSP90β-specific inhibitor) | Downregulated vascular endothelial growth factor (VEGFR) expression | Meng et al. (2017) |
| NVP-BEP800 | Suppresses vasculogenic mimicry (VM) formation by releasing HSP90β and Twist1 interaction | Meng et al. (2019) |
| Ganetespib (STA-9090) | Downregulates VEGFR, c-MET, HER2, IGF-IR, EGFR, and other HSP90 client proteins involved in hepatocarcinogenesis | Goyal et al. (2015) |
| G-TPP (Gamitrinib variant containing triphenylphosphonium) | Induces cell death and causes dynamin-related protein 1 (Drp1)-mediated mitochondrial elongation in HCC cells by increasing the reactive oxygen species (ROS) level | Yoo et al. (2015) |
| Lexatumumab (Lexa) and cycloheximide (CHX) combination treatment | Induced ROS increase and apoptotic death | Zhao et al. (2011) |
| AUY922 (luminespib) | Led to the upregulation of HSP70 and the simultaneous depletion of HSP90 client proteins | Augello et al. (2019) |
| AUY922 | Angiogenesis inhibition | Cheng et al. (2015) |
| PU-H71 (8-(6-iodobenzo[d][1,3]dioxol-5-ylthio)-9-(3-(isopropylamino)propyl)- 9H-purin-6-amine), a non-quinone HSP90 inhibitor, | Exhibit tumor-specific accumulation and exert potent antineoplastic activity | Breinig et al. (2009) |
| Novobiocin (NB) | Reduced the invasive potential of HCC cells | Su et al. (2018) |
| STIP1 neutralization antibody (STIP1-NA) | Reduced tumor growth | Z. Chen et al. (2017) |
| NVP-AUY922 and NVP-HSP990 | Heat shock factor 1 (HSF1)-target gene DEDD2 is involved in attenuating the effect of HSP90 inhibitors. | Y. Chen et al. (2013) |
| 10058-F4 (c-Myc-specific inhibitor) | siRNA-mediated c-Myc knockdown in hepatitis B virus X protein (HBx)-transfected cells significantly suppressed HSP90α expression | Li et al. (2010) |
| lncRNA-PRAL (p53 regulation-associated lncRNA) | Inhibited HCC growth and induced apoptosis through p53 (enhancement p53 stability) | Zhou et al. (2016) |
| Protein engineered triblock biopolymer-geldanamycin conjugates | HSP90 inhibition and sustained tumor suppression | Y. Chen et al. (2014) |
| Ganetespib and NVP-AUY922 | Decrease the protein level of anaplastic lymphoma kinase (ALK), fibroblast growth factor receptor 2 (FGFR2), and ephrin type-A receptor 5 (EphA5) (client proteins of hSP90) | X. Wang et al. (2019) |
| Short-hairpin RNA (shRNA) lentiviral transduction/albumin promoter-driven cre recombinase-mediated disruption of gp96 gene, hSP90b1 | Perturbs multiple growth signals, and attenuates their proliferation and expansion | Rachidi et al. (2015) |
7 CONCLUSION
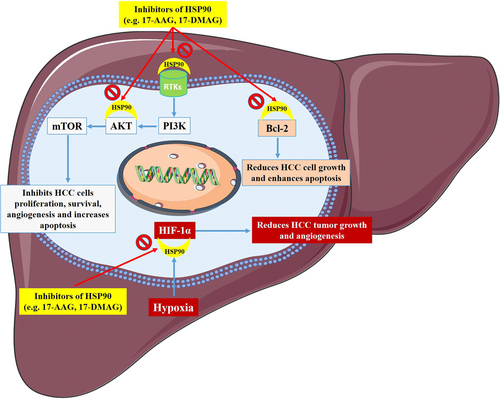
The HSP90 comprises relatively novel factors whose biological functions have been emerging during the last decades, although most of the roles in the cell are still to be discovered. HSP90 plays an important role in signaling routes due to its chaperoning effect on crucial proteins effective in cellular regulation (Figure 1). Therefore, HSP90 chaperones have important cellular activities, such as intracellular signaling, metabolism, and epigenetics. Their deregulation is also shown in HCC. A more profound comprehension of HSP90′s effect in HCC cells will be challenging for developing and/or improving treatments, specific against HCC.
ACKNOWLEDGMENTS
The authors would like to thank the Immunology Research Center, Tabriz University of Medical Sciences for their support. They also acknowledge all those researchers who have performed investigations identifying the involvement of HSP90 in hepatocellular carcinoma.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
M. N. V. and B. B. devised the main conceptual ideas. M. N. V. and K. H. wrote the initial draft of the manuscript. K. H. prepared the figure. B. B., L. A., A. M., and M. H. reviewed the manuscript and edited it critically for important intellectual content. B. B. supervised the study.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




