Radiosensitivity enhancement by Co-NMS-mediated mitochondrial impairment in glioblastoma
Abstract
We investigated the radiosensitizing effects of Co-NMS, a derivative of nimesulide based on a cobalt carbonyl complex, on malignant glioma cells. In the zebrafish exposed to Co-NMS ranging from 5 to 20 μM, cell death and heat shock protein 70 expression in the brain and neurobehavioral performance were evaluated. Our data showed that Co-NMS at 5 μM did not cause the appreciable neurotoxicity, and thereby was given as a novel radiation sensitizer in further study. In the U251 cells, Co-NMS combined with irradiation treatment resulted in significant inhibition of cell growth and clonogenic capability as well as remarkable increases of G2/M arrest and apoptotic cell population compared to the irradiation alone treatment. This demonstrated that the Co-NMS administration exerted a strong potential of sensitizing effect on the irradiated cells. With regard to the tumor radiosensitization of Co-NMS, it could be primarily attributed to the Co-NMS-derived mitochondrial impairment, reflected by the loss of mitochondrial membrane potential, the disruption of mitochondrial fusion and fission balance as well as redox homeostasis. Furthermore, the energy metabolism of the U251 cells was obviously suppressed by cotreatment with Co-NMS and irradiation through repressing mitochondrial function. Taken together, our findings suggested that Co-NMS could be a desirable drug to enhance the radiotherapeutic effects in glioblastoma patients.
1 INTRODUCTION
Glioblastoma multiforme (GBM) is associated with poor prognosis and rapid invasion, even with aggressive multimodality treatment (Nie et al., 2017; Z. Zhang et al., 2014). Radiation therapy (RT) evolved to be a primary adjuvant treatment to eliminate residual GBM tumor tissue after surgery, particularly for patients with tumors of invasion in key functional areas (Inaba et al., 2011; X. Zhang et al., 2018). However, radiation-induced side effects and radioresistance remains a major challenge in the treatment of glioma (He & Fan, 2018). As a matter of fact, the irradiated surviving cells can obtain more aggressive and invasive abilities (Kegelman et al., 2017; Wild-Bode, Weller, Rimner, Dichgans, & Wick, 2001). Therefore, new strategies to enhance the therapeutic effect of radiotherapy are urgently required.
Cyclooxygenase 2 (COX-2) is usually overexpressed in the most brain tumors, including glioblastoma, astrocytoma, meningioma, medulloblastoma, craniopharyngioma, oligodendroglioma, ependymoma, and neurinoma, which directly correlate with the clinical pathogenesis, progression and prognosis of GBM (Lin, Subbaramaiah, Shah, Dannenberg, & Boyle, 2002; New, 2004; Qiu, Shi, & Jiang, 2017). Some literatures reported that selective COX-2 inhibitors can enhance radiation sensitivity through suppressing cell proliferation, cell migration, angiogenesis, and DNA repair, as well as inducing apoptosis and cell cycle arrest (Bieniek, Childress, Swatski, & Yang, 2014; Kuipers et al., 2007; Saha, Pyo, & Choy, 2003; Salehifar & Hosseinimehr, 2016). Additionally, COX-2 inhibitors can intercept radiation-induced oxidative stress and inflammation in normal tissue to be as desirable radioprotectors (Laube, Kniess, & Pietzsch, 2016). Nimesulide (NMS), which we are going to try out in our study, a highly preferential COX-2 inhibitor, is a classical nonsteroidal anti-inflammatory, analgesic and antipyretic drug (Suleyman, Sener, Kurt, Comez, & Yapanoglu, 2015). Some experiments have been displayed the potent anticancer of NMS (Jian et al., 2017; Shaik, Chatterjee, & Singh, 2004; Zhong et al., 2012). But in head-and-neck cancer cells, the paradox role of NMS (50–600 mM) was reported to decrease the effect of RT (Czembirek et al., 2009). Thus, it is should be developed for making new NMS analogs to target more effective and less toxic radiosensitizers.
Recently, the development of nanoparticles (Yu et al., 2019; Yu, Zhang, Liu, Xu, & Liu, 2018) and nonplatinum metal complexes as diagnostic tools and anticancer drugs has been rapidly widening, such as copper (II) (Fei et al., 2019), palladium (II) (Vojtek, Marques, Ferreira, Mota-Filipe, & Diniz, 2019), ruthenium (II), and gold (III)-based complexes (King, Marker, Swanda, & Woods, 2019; Ma, Wu, Cheng, & Lee, 2019). Among them, cobalt complexes containing cobalt–Schiff base (Bajema, Roberts, & Meade, 2019), [Co2 (CO)6] moiety (Gong et al., 2016), cobalt (III)-polypyridyl (Abe, Eskandari, & Suntharalingam, 2018), cobalt nanowire (Zhu et al., 2019), and cobalt chloride (H. Y. Wang & Zhang, 2015) have attractive tumor cell inhibiting effects. Moreover, the anticancer bioactivities of these cobalt-based complexes depend on their structures of the ligands, and some structural fragments of nonsteroidal anti-inflammatory can increase anticancer activities and display significant cancer cell selectivity (Rubner et al., 2010). Furthermore, Co (III) complex as a promising drug was activated by ionizing radiation to release a very potent cytotoxin in hypoxic regions of tumors (Ahn, Ware, Denny, & Wilson, 2004). Klein et al. (2018) have also revealed that cobalt ferrite nanoparticles were employed as reactive oxygen species (ROS) formation enhancer in irradiated breast cancer cells. However, no data are available on the killing effects of cobalt complexes in combination with COX-2 inhibitors in radiation cancer therapy.
Here, we synthesized cobalt complexes bearing NMS ligand, a derivative of NMS (Co-NMS), to explore the toxicity on normal brain tissue and the enhanced radiosensitizing effects on human brain glioma cells.
2 MATERIALS AND METHODS
2.1 Synthesis of Co-NMS
Co-NMS was obtained in accordance with our previous work (J. Li et al., 2018). NMS (308 mg, 1.0 mmol) and anhydrous potassium carbonate (207 mg, 1.5 mmol) were dissolved in acetone solution, 3-bromoproparyne (94 μl, 1.2 mmol) was added to the mixture and reacted overnight at room temperature. After filtration, the filtrate was evaporated under reduced pressure to obtain the crude product of intermediate. The intermediate crude product and octacarbonyldicobalt (410 mg, 1.2 mmol) were dissolved in anhydrous tetrahydrofuran, and the reaction was carried out at room temperature overnight. Then, the solvent was removed in vacuo, and the residue was separated and purified by column chromatography (eluent: ethyl acetate/petroleum ether = 1:6, Rf = 0.42) to obtain 456 mg of red powdery product with a yield of 62%. IR (KBr, cm−1): 2,096(vs), 2 058(vs), 2,021(vs). 1H NMR (400 MHz, chloroform-d) δ 7.98 (dd, J = 8.6 and 2.4 Hz, 1H), 7.73 (d, J = 8.7 Hz, 1H), 7.65 (d, J = 2.4 Hz, 1H), 7.48 (t, J = 7.8 Hz, 2H), 7.26 (s, 1H), 7.06 (d, J = 7.9 Hz, 2H), 5.82 (s, 1H), 5.22 (s, 2H), 3.05 (s, 3H). ESI-HRMS: calc. for C22H14Co2N2O11SNa [M + Na]+ 654.8880 found 654.8926.
2.2 CO-release assay
The amount of CO release from Co-NMS was monitored by a myoglobin (Mb) assay (Gong et al., 2016). Mb was dissolved in phosphate buffer saline buffer and added sodium dithionite to reduce Mb to deoxy-myoglobin (deoxy-Mb). Co-NMS was added to deoxy-Mb solution and the ultraviolet absorption spectrum was recorded at a wavelength of 500–600 nm. Deoxy-Mb (absorption peak: 560 nm) binds with CO released from Co-NMS to form carbonyl Mb (absorption peak: 540 and, 578 nm). Finally, the alteration in the absorption peak at 540 nm calculated the released CO.
2.3 Zebrafish rearing and maintenance and cell culture
The zebrafish (AB strain) were kept in our standard laboratory procedures (S. Li et al., 2019). Human U251 glioma cells were purchased from the BeNa Culture Collection (Beijing, China), and grown in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (Hyclone, Logan, UT) under 37°C of 5% CO2 condition.
2.4 Experimental design and irradiation procedure
Embryos at 4 hr postfertilization (hpf) were divided into five groups as follows: (a) control group, (b) dimethyl sulfoxide group, (c–e) three Co-NMS treated groups, which exposed to Co-NMS at 5, 10, and 20 μM up to 144 hpf, respectively. For in vitro experiment, we added 5 μM Co-NMS to the U251 cells before X-ray irradiation. A radiation dose employed 4 Gy dose using the X-ray irradiation system (PXi, North Branford, CT).
2.5 COX-2 activity assessment
After U251 cells were treated with different concentrations of Co-NMS, cellular COX-2 level was measured by enzyme-linked immunosorbent assay kits (MEIMIAN, Jiangsu, China), according to the manufacturer's instructions.
2.6 Spontaneous movement and behavioral assay
At 24 hpf, spontaneous movement was recorded using Media Cruiser recording software (Canopus Corporation, Kobe, Japan). At 144 hpf, swimming activity was evaluated by Noldus tracking device and EthoVision XT 10.0 software (Noldus Information Technology, Wageningen, the Netherlands).
2.7 Growth inhibitory analysis
The growth curve was detected in the U251 cells after irradiation in the presence or absence of Co-NMS using Ki67 staining (Abcam, San Francisco, CA) and an iCELLigence RTCA system (ACEA Bioscience, San Diego, CA).
2.8 Colony formation assay
Colony forming ability was performed in the U251 cells receiving radiation with or without Co-NMS. Cells at a density of 2 × 103/ml were seeded and incubated at 37°C for 10 days and cloning forming efficiency was determined.
2.9 Cell cycle assay
According to the manufacturer’ s protocols, U251 cells treated with or without Co-NMS were performed using cell cycle staining kit (MultiSciences, Hangzhou, China). Cell cycle distribution was detected by BD Accuri C6 (Becton Dickinson, San Jose, CA).
2.10 Apoptosis analysis
The zebrafish embryos were stained with acridine orange (AO) at 48 hpf according to the method of Zhou et al. (2015). U251 cell apoptosis was measured using caspase 3/7 commercial assay kit (Promega, Madison, WI).
2.11 Mitochondrial morphology assessment
After irradiation in the presence or absence of Co-NMS, U251 cells were stained with Mitotracker Green (100 nM; M7514; Invitrogen) for 30 min. Fluorescent images were captured by a confocal laser microscope (Carl Zeiss AG, Germany) and analyzed by Fiji/the ImageJ software.
2.12 Mitochondrial membrane potential assay
Mitochondrial membrane potential (Δψm) after Co-NMS and irradiation treatment were determined by JC-1 staining kit (Beijing Solarbio Science & Technology Co., Ltd).
2.13 Redox statues detection
According to the manufacturer's instructions, mitochondrial ROS yield was assessed using the Amplex Red probe (Thermo Fisher Scientific, Waltham, MA). NADPH and total NADP were detected by the NADP/NADPH-Glo kit (Promega) and the ratio of GSH and GSSG was measured by an assay kit from the Jiancheng Bio (Nanjing, China).
2.14 Detection of mitochondrial enzyme activity
Treated cells were collected and mitochondrial respiratory chain complex I and complex III were measured by the reagent kits (Suzhou Comin Biotechnology, China).
2.15 Adenylates determination
Total ATP content was measured by the Mitochondrial ToxGlo Assay Kit (Promega). After irradiation at 4 Gy with or without Co-NMS, the ratio of ADP/ATP was determined by high-performance liquid chromatography analysis (Beijing Solarbio Science & Technology Co., Ltd).
2.16 Statistical analysis
Data were expressed as the mean ± standard error of mean, n = 3. Statistical analysis was performed with SPSS 16.0 (SPSS Inc., Chicago, IL). Baseline differences between groups were analyzed by using Student's t test and statistical significant was considered when p < .05.
3 RESULTS AND DISCUSSION
3.1 Syntheses and properties of Co-NMS
The intermediate was synthesized by a substitution reaction of NMS with bromopropyne, and then the addition reaction of the intermediate with dicobalt octacarbonyl was successfully prepared the Co-NMS, as evidenced by Figure 1a. The reaction conditions of Co-NMS were mild and the yield of each step was above 70%. The Co-NMS is soluble in most organic solvents and insoluble in water. The structure of Co-NMS was characterized by nuclear magnetic resonance, mass spectrometry, and infrared (IR) spectra. In the IR spectrum, there was some strong terminal carbonyl stretching absorption bands in the range of 2,096–2,021 cm−1. Higher absorption bands were attributed to the coordination of Co atoms with terminal carbonyl groups.
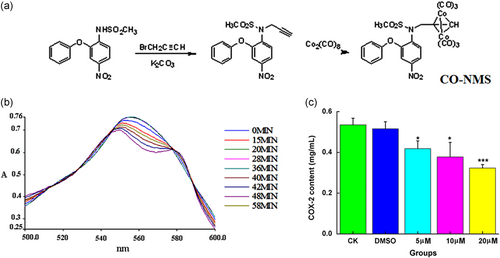
Recently, carbon monoxide releasing molecule (CORM)-derived CO has attracted attention as a potential therapeutic agent with versatile beneficial functions for tumor therapy and anti-inflammation (S. B. Wang et al., 2019). Figure 1b displayed both the total amount of CO released and the rate of CO release. The CO releasing half-life of Co-NMS was 26.8 min. Compared with CORMs cas CORM2 (∼1 min; Qureshi et al., 2016) and CORM 3 (∼3.6 min; Foresti et al., 2004), the rate of CO release of Co-NMS made CO liberating slower, which is a major benefit for the delivery of controlled amounts of CO to a target tissue. In terms of the mechanism, the structures of non-CO ligands mostly determined the rate of release of CO (J. Li et al., 2018).
In GBM, reducing the expression of overactivated COX-2 has been an effective approach to blockade glioma progression (Qiu et al., 2019; Won, Deshane, Leavenworth, Oliva, & Griguer, 2019). COX-2 levels in the U251 cells treated with different concentrations of Co-NMS were assessed. Figure 1c illustrated that Co-NMS abundantly inhibited COX-2 expression activities at 5 (p < .05), 10 (p < .05), and 20 μM (p < .001) with respect to the control group.
3.2 Alterations of brain response following Co-NMS treatments
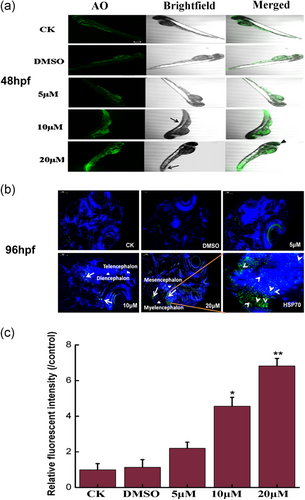
We evaluated the neurotoxicity of Co-NMS exposure in the brain of zebrafish model. Zebrafish are increasingly utilized as a systems toxicology model in vivo neurotoxicity screening, because they emerge a high degree of similarity with humans in structural features, gene, behavior, and development processes of the nervous system (Guo, 2009; Nishimura et al., 2015). Fluorescence images indicated that AO-positive cells were distinctly present in the brain of Co-NMS-treated embryos at 20 μM than in the control embryos (Figure 2a). This suggested higher doses of Co-NMS led to the brain impairment in zebrafish embryos. Heat shock protein 70 (HSP70) as a sensitive marker for general stress or genotoxicity protect cells by restoring damaged proteins to maintain their functional three-dimensional structure (Hartl, Bracher, & Hayer-Hartl, 2011). When embryos were exposed to 10 or 20 μM Co-NMS in our study, the localization of HSP70 expression has expanded to include cells in the telencephalic, diencephalic, mesencephalic as well as the myelencephalic regions of zebrafish. In particular, a greater abundance of HSP70 was visibly condensed in the dorsal midbrain of 96 hpf zebrafish, as evidenced by Figure 2b. Elevated HSP70 can facilitate the transfer of misfolded proteins to the proteasome for degradation to defense against neurodegenerative disorders (van Noort, Bugiani, & Amor, 2017). Moreover, fluorescence intensity at the HSP70 loci is apparent in the 10 μM Co-NMS-treated group (p < .05) and 20 μM Co-NMS-treated group (p < .01; Figure 2c). Although the used of Co-NMS at higher doses induced the acute stress in brain tissue, hematoxylin and eosin staining of histopathological sections of zebrafish displayed no obvious morphological abnormalities in all the Co-NMS-treated groups, compared to the control group at 144 hpf (Figure S1).
3.3 Changes in neurobehavior capacity of Co-NMS
We further investigated the neurotoxicity induced by Co-NMS during embryogenesis in zebrafish by means of the behavioral endpoints. Spontaneous tail movements at 24 hpf and swimming activities at 144 hpf were assessed in the present study. Data showed that a significant decrease in the cumulative duration of 24 hpf zebrafish was observed in the highest dose of Co-NM-treated group relative to the control group (p < .05). Moreover, the frequency of tail contraction in 20 μM Co-NMS-exposed group was prominently reduced by 71.4% than that of in the control group (Figure 3b). These data suggested that the higher doses of Co-NMS dramatically led to the behavioral abnormality at the earliest stages, which were in line with our previous outcomes (S. Li et al., 2019).
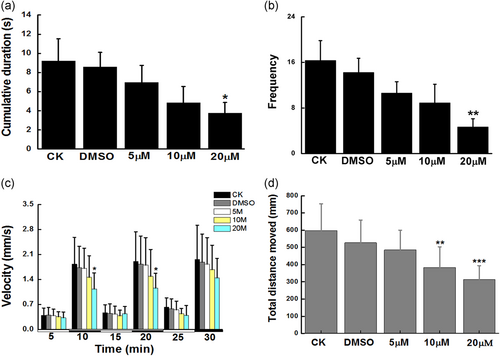
By 144 hpf, the swimming mode upon light-to-dark stimulation was consistent in all tested group. There was no significant differences locomotive behavior during visible light in the case of all treatment groups. Whereas, embryos added with 20 μM Co-NMS showed significant reductions of swimming velocity in the first (p < .05) or second (p < .05) dark phase respectively, when compared with control embryos (Figure 3c). The results of total distance moved were seen in Figure 3d, displaying a profound hypoactivity in the Co-NMS-treated groups at 10 μM (383.16 ± 120.43) and 20 μM (312.91 ± 80.88), as compared to the control group (597.45 ± 155.27). Generally, these findings indicated that cognitive development and neurological function were vulnerable to Co-NMS exposure, and the behavioral deficits were concentration-dependently.
However, exposure of 5 μM Co-NMS to zebrafish embryo/larvae did not cause the distinct impairment compared to the normal brain. Hence, we selected Co-NMS at 5 μM to investigate the radiosensitive influences in glioma cells.
3.4 Cell growth inhibition induced by Co-NMS and irradiation
The Ki67 labeling index is a strong correlation with diagnosis and prognosis among the glioblastoma patients (Chen, He, Tang, Ren, & Chen, 2015). After supplementing with Co-NMS at the dose of 5 μM before irradiation, the florescence accumulation of Ki67 was dramatically weakened in the U251 cells (Figure 4a), indicating the better antiproliferative effects.
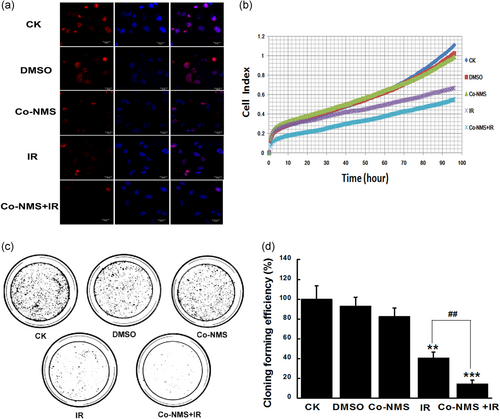
To verify the consecutive cell growth, survival curve was determined in the U251 cells exposed to irradiation with or without Co-NMS within 96 hr. A time-dependent inhibition of survival of U251 cells was significantly caused by irradiation alone and in combination with Co-NMS. The efficient kinetics of killing cells were quantitatively assessed in all treatment groups (Figure 4b). At 96 hr, the killing of target tumor cells was approximately increased by 39.9% and 50.4% in the irradiation group and Co-NMS combination with irradiation group than that of the control group, respectively. But the growth inhibition was less affected by the supplement with 5 μM Co-NMS. After 10 days, wwe found that the number of colonies formed was notably reduced by irradiation (p < .01) and irradiation combination with Co-NMS (p < .001), compared to the control group (Figure 4c,d). Moreover, in the combination group, the number of colonies formation was 64.3% lower than in that of the irradiation-only group. Collectively, this indicated that Co-NMS promoted U251 cells sensitivity to irradiation and consequently displayed a strong antitumor activity. Some cobalt complexes have been reported to serve as radiosensitizers (Klein et al., 2018; Teicher et al., 1987). In addition, the in vivo and vitro results found that COX-2 selective inhibitor increased radiosensitizing effect and decreased radioresistant forms of glioblastoma (Petersen, Petersen, Milas, Lang, & Tofilon, 2000; Karim et al., 2005). Thus, the underlying reasons for enhancing the radiosensitivity of U251 cells through Co-NMS administration could be closely related to the COX-2 inhibition and cobalt complexes structure.
3.5 Cell cycle arrest and apoptosis induced by Co-NMS and irradiation
To elucidate the mechanism of the radiosensitizing effect of Co-NMS on U251 cells, the cell cycle redistribution was measured using flow cytometry. Our data showed that the percentage of cells in G2/M phase was significantly elevated in the U251 cells at 24 hr after irradiation alone (p < .05) and in combination with Co-NMS (p < .001), when compared with the control group (Figure 5a,b). Normally, the induced G2/M cell cycle arrest can repair the damaged DNA and prevent cell division (Ouyang et al., 2009). Moreover, G2/M phase arrest is thought to be the major contribution to cell death caused by antitumor and radiosensitizing agents (Liu et al., 2013). These findings implied that the combination of Co-MNS with radiation could regulate the cell cycle progression and consequently suppress the tumor growth and cellular proliferation.
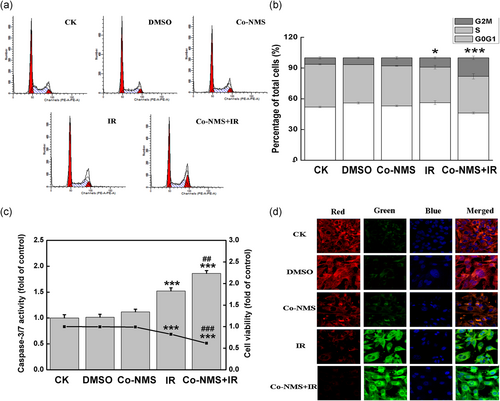
Subsequently, U251 cells treated with irradiation in the presence and absence of Co-NMS (5 μM) were subjected to caspase 3/7 activity and Cell Counting Kit-8 analysis at 48 hr after administration. Figure 5c showed that caspase 3/7 activities in the irradiated cells and the cotreated cells had 1.5-fold and 1.68-fold higher than that of in the control cells. Moreover, when U251 cells were treated with Co-NMS and irradiation, caspase 3/7 activity was increased by 18.1% than that of irradiated-only cells, indicating that the combination treatment enhanced an early apoptosis response. On the contrary, irradiation alone (p < .001) and Co-NMS combined with irradiation (p < .001) were promptly inhibited cell viability in comparison to the control group. Furthermore, cells treated with Co-NMS and irradiation promptly blocked the cell survival (25.2% reduction compared to the irradiation alone group). It was believed that caspases 3 and 7 as the critical mediators to regulate mitochondrial events in the apoptotic pathway (Lakhani et al., 2006). Mitochondrial apoptosis was associated with alterations of reduced inner mitochondrial membrane transmembrane potential (Δψm; Castedo et al., 2002). In our study, the fluorescence micrographs in Figure 5d clearly exhibited a weak intensity of red fluorescence and a distinct intensity of green fluorescence in the Co-NMS combination with irradiation group, compared with control groups. This result suggested that Co-NMS enhanced the loss of Δψm in irradiated U251 cells, which were consistent with the upward trend in the activity of caspase 3/7. Given all this, these data demonstrated that the cell death induced by the combination treatment was closely mitochondrial-dependent action.
3.6 Mitochondrial toxicity induced by Co-NMS and irradiation
We evaluated whether treatment with Co-NMS and irradiation could affect mitochondrial features. In the case of normal condition, mitochondria showed the thin filamentous structures by confocal microscopy. However, in U251 cells following irradiation and Co-NMS administration, the mitochondria lost these filamentous structures. Meanwhile, enrichment of punctiform mitochondrial phenotype was induced in the irradiation-exposed cells with Co-NMS supplementation (Figure 6a). The observation of mitochondrial network skeleton suggested that enhanced fragmentation of mitochondria was caused by the combination treatment, accompanied by the lowered mitochondrial membrane potential (Figure 5d). These are from insufficient fusion, excessive fission or both. More direct evidence demonstrated that mitochondrial fusion and fission can be in the control of apoptosis (Perfettini, Roumier, & Kroemer, 2005; Sugioka, Shimizu, & Tsujimoto, 2004). Furthermore, such morphological changes in the mitochondria were quantified by measuring the branch length of mitochondria in each condition. Mitochondria network with at least a single node and three branches (Martin-Maestro, Gargini, Garcia, Perry, & Avila, 2017). We revealed that the mean branch length was remarkably decreased after the cells exposing to Co-NMS and irradiation (p < .05, 42.5% reduction), compared to the control cells.
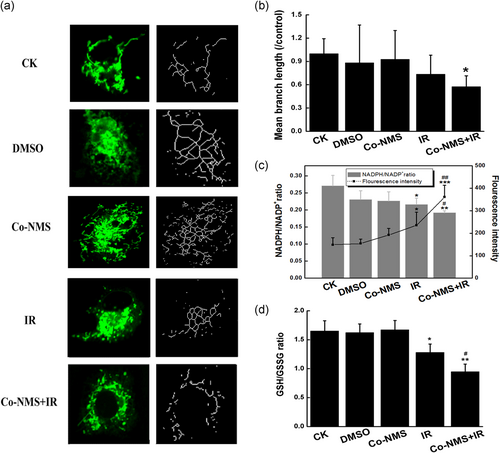
Accumulating evidence stated that there is a close link between redox homeostasis and mitochondrial morphology (Willems, Rossignol, Dieteren, Murphy, & Koopman, 2015). In this study, NADPH/NADP+ ratio showed a significant reduction in the radiation alone group (p < .05) and in the combination treatment group (p < .01) with respect to the control group. Further analysis revealed that NADPH/NADP+ level was 29.2% less in cotreatment cells than that of in the irradiated-only cells, and ROS generation in the Co-NMS plus irradiation group was higher than in the irradiation alone group (p < .01; Figure 6c). In our study, notable decreases in mitochondrial GSH/GSSG ratio were observed in the cells treated with irradiation alone (p < .05) and 5 μM Co-NMS combined with irradiation (p < .01; Figure 6d). Moreover, the ratio of GSH:GSSG in the cotreatment group was less than in the irradiation group (p < .05). These observations indicated that Co-NMS potently destroyed the redox defense and resulted in the obvious oxidative stress in the irradiated cells. Data from Wu, Zhou, Zhang, and Xing (2011) found that mitochondrial oxidative stress was able to cause mitochondrial fragmentation via disrupting the mitochondrial fission-fusion balance in human lung adenocarcinoma cells. Collectively, our findings suggest that the vulnerability of mitochondria was significantly augmented in the combination treatment of Co-NMS and irradiation.
3.7 Depletion of mitochondrial bioenergetic activity by Co-NMS and irradiation
Alteration in redox homeostasis and mitochondrial phenotype can initiate a cascade of events, such as cell death pathway and cellular metabolism (Arismendi-Morillo, 2009). Here, we investigated whether treatment with Co-NMS and irradiation could affect the energy metabolic capacity in U251 cells. The mitochondrial respiratory chain can adjust cellular energy demand. The enzymatic activities of redox-sensitive complex I and complex III that are mitochondrial free radical generation sites (Vyatkina, Bhatia, Gerstner, Papaconstantinou, & Garg, 2004) were obviously downregulated by irradiation alone exposure (p < .05) and Co-NMS combined irradiation treatment (p < .01), compared to the control group (Figure 7a,b). More important, inhibited activity of mitochondrial complex I was substantially decreased by 10.3% in the combined group compared with the irradiation-only group.
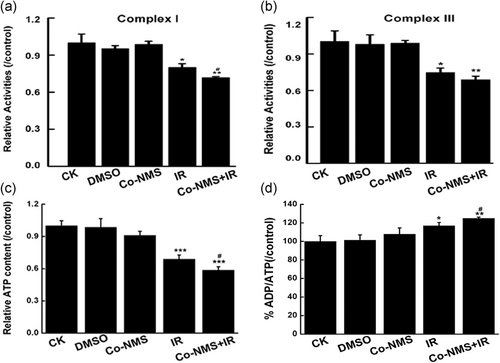
Next, ATP production in the U251 cells was determined by Mitochondrial ToxGlo assay. In our data, the significant decreases of cellular ATP content were found in the cells treated with irradiation (p < .001) and Co-NMS in combination with irradiation (p < .001) compared with the control group (Figure 7c). Moreover, 14.6% reduction of ATP yield was in the cotreatment group compared with the irradiation alone group, indicating that Co-NMS prominently resulted in the ATP depletion in the irradiated cells. Furthermore, the similar trend was noted in the ADP/ATP ratio (Figure 7d), and the significant decrease was in the combination treatment group compared with the irradiation group (p < .05).
4 CONCLUSION
Generally, our findings demonstrated that Co-NMS could disrupt the mitochondrial function, and subsequently leading to severe impairments in the irradiated glioma cells under the condition that there is not any obvious neurotoxicity in normal brain tissue. In summary, our data suggested that Co-NMS could be a desirable drug in improving the radiotherapeutic efficacy in glioblastoma patients.
ACKNOWLEDGMENTS
This study was supported by grants from the National Natural Science Foundation of China (Grant no. 11575262), Scientific Technology Research Projects of Gansu Province (18JR3RA377), and Lanzhou Talent Innovation and Entrepreneurship Project (2018-RC-36).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Y. L. conceived the idea, executed the research, and wrote the manuscript. T. F. Z. acquired the data and analyzed the data. G. L., S. R. L., J. L. L., X. L. H., Q. Y. Z., Q. F. W., and D. X. executed the research and interpreted the data. L. W. Z., Q. L., H. Z., and B. L. reviewed the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
All data generated or used during the study appear in the submitted article.




