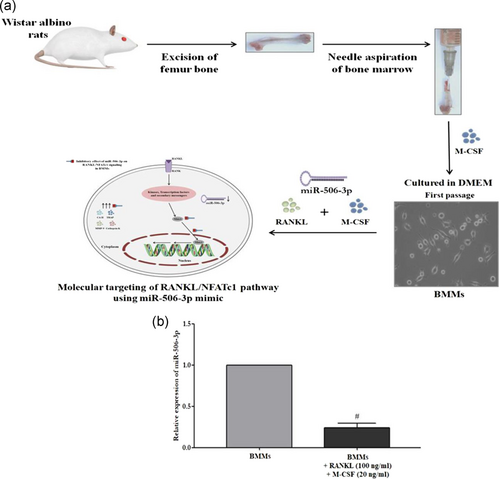
miR-506-3p alleviates uncontrolled osteoclastogenesis via repression of RANKL/NFATc1 signaling pathway
Palani Dinesh and Sowmiya Kalaiselvan equally contributed to this work.
Abstract
Bone erosion is the major cause of deformities in autoimmune disease conditions such as osteoporosis and rheumatoid arthritis. Aberrant receptor activator of nuclear factor kappa B ligand (RANKL) secretion in bone disorders have been implicated to promote uncontrolled osteoclast differentiation through the regulation of nuclear factor of activated T cells 1 (NFATc1) transcription factor. This phenomenon is governed by several molecular factors including microRNAs, which are under-expressed during disease progression. This report focuses on elucidating the molecular mechanism of miR-506-3p towards the RANKL/NFATc1 pathway. miR-506-3p showed high binding affinity towards NFATc1 (ΔG = −22.4 kcal/mol). Bone marrow-derived macrophages (BMMs) isolated from rats stimulated with RANKL (100 ng/ml) showed active expression of NFATc1 which differentiated into mature osteoclasts. Moreover, NFATc1 activation resulted in downstream secretion of various bone resorptive enzymes (cathepsin K, carbonic anhydrase II, tartarate acid phosphatase, and matrix metalloproteinase 9) which lead to active bone resorption. However, transfection of miR-506-3p resulted in selective repression of NFATc1 inside the cells. This further resulted in the diminished release of bone resorptive enzymes that were essential for the degradation of the bone. Overall, we predict that miR-506-3p can be used as a molecular intervention for RANKL/NFATc1 mediated osteoclastogenesis.
1 INTRODUCTION
Bone remodeling is an intricate cellular process that essentially sustains the formation of bone and maintains the skeletal architecture (Brylka & Schinke, 2019; Ragipoglu et al., 2020). Nonetheless, this process gets altered during bone degenerative autoimmune disorders including rheumatoid arthritis (RA), ankylosing spondylitis, and osteoarthritis (Boyce, Li, Xing, & Yao, 2018; Mohammadi et al., 2018; Pourakbari, Khodadadi, Aghebati-Maleki, Aghebati-Maleki, & Yousefi, 2019). In clinical perspectives, these deformities deal with uncontrolled bone erosion leading to dysfunction and diminished locomotion (Hardy & Fernandez-Patron, 2020; Vlot et al., 2018). The aberrant bone loss or osteoclastogenesis is mediated through macrophage-derived osteoclast cells that are magnified than the threshold level during an autoimmune condition (Coury, Peyruchaud, & Machuca-Gayet, 2019; Madel et al., 2019).
Macrophage-derived osteoclasts are large multinucleated cells that degrade the essential components of the bone by massive secretion of acids and proteinases that dissolve the organic matrix (Ponzetti & Rucci, 2019; Zhao, 2018). During normal physiological conditions, bone resorption is reverted and new segments are formed through specialized bone-forming cells called as osteoblasts (Dirckx, Moorer, Clemens, & Riddle, 2019). An imbalance between the osteoclast/osteoblast population forms the crux of various bone mediated deformities (Li, Chen, Lu, & Yu, 2020; Terashima & Takayanagi, 2019). For the osteoclasts to thrive in the microenvironment, two essential extracellular secretions namely macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor kappa B ligand (RANKL) are required (de Vries et al., 2019; Souza & Lerner, 2019). M-CSF is majorly released by circulating/adherent macrophages and RANKL is provided by cells of the leukocyte lineage and fibroblast-like synoviocytes (Kim, Kim, Kim, Cho, & Lee, 2015). Macrophages designated as bone marrow-derived macrophages (BMMs) originating from the bone marrow undergo osteoclast differentiation in the presence of M-CSF and RANKL (Han, You, Xing, Zhang, & Zou, 2018; Zhan et al., 2019). However, this phenomenon is controlled through various cellular checkpoints that are eluded during an autoimmune disease condition (Di Munno & Ferro, 2019). Moreover, RANKL involvement in promoting uncontrolled osteoclastogenesis is mediated through the regulation of various signaling pathways (Kovacs, Vajda, & Nagy, 2019).
RANKL binds with the RANK receptor expressed on the surface of differentiated macrophages through a series of intermediate signaling to convert it into osteoclasts (Maruotti, Corrado, Rotondo, & Cantatore, 2019). Several well-established signaling pathways are prone to promote osteoclast differentiation (Park-Min, 2019; Sobacchi, Menale, & Villa, 2019). One such signaling pathway is mediated through calcium efflux inside the cells leading to activation of a key transcription factor called as nuclear factor of activated T cells 1 (NFATc1) (Yang et al., 2018). The regulation of NFATc1 is controlled by kinases (PI3K/Akt and mitogen-activated protein kinases [MAPKs]), transcription factors (NFκB and STAT3) and secondary messengers inside the cells (Cheon et al., 2016; Jia et al., 2019; Nakanishi & Tsukamoto, 2015; Xu et al., 2019). Continuous sequestration of NFATc1 inside mature osteoclasts leads to the release of acidic enzymes in the synovium such as tartarate acid phosphatase (TRAP), matrix metalloproteinases (MMPs), carbonic anhydrase II and Cathepsin K that degrade the bone and matrix (Chaweewannakorn et al., 2019). Uncontrolled release of these enzymes results in erosion and deformities to the bone (Yang et al., 2018). Studies have shown that molecular intervention of this mechanism results in diminished bone erosion leading to modification of the disease pathology (Boyce, 2013). Small molecular transcriptional regulators designated as microRNAs (miRNAs) have been recently elucidated to play a potential role in the alteration of osteoclast differentiation and resultant bone erosion (Inoue, Nakano, & Zhao, 2019; Lozano, Duroux-Richard, Firat, Schordan, & Apparailly, 2019; Yuan et al., 2017).
miRNAs are indigenous small molecular RNA complexes that have been well studied to attenuate the disease outcome by targeting key elements that promote the pathogenesis (Ye, Xu, Tian, Cai, & Zeng, 2019). Several classes of miRNAs discovered in the recent years alter various aberrant conditions including inflammation, metastatic cancers, and autoimmune disorders (Niu & Schulert, 2019; Roncarati, Lupini, Shankaraiah, & Negrini, 2019). Moreover, miRNAs that are exclusively illustrated in current reports are said to diminish bone erosion via targeting key molecules that induce uncontrolled osteoclast formation (Hesse & Taipaleenmaki, 2019). Initially, a report provided by Mizoguchi et al. (2010) showed strong evidence that the NFATc1 transcription factor plays a central role in osteoclast resorptive activity, and targeting this will control the aggravated differentiation. In par with this report, several studies have illustrated the role of various miRNA clusters to alter NFATc1 expression and diminish uncontrolled osteoclastogenesis. The cluster spanning between miR-17-92 has been exclusively studied to interact with NFATc1 and control osteoclast differentiation (Mizoguchi, Murakami, Saito, Miyasaka, & Kohsaka, 2013; Rossi et al., 2013; Sun et al., 2015; Yin, Tang, Chen, & Lu, 2017). Moreover, other classes of miRNAs including miR-124, miR-218, and miR-222-3p showed promising effects in controlling PI3K/Akt/MAPK dependent NFATc1 induction in RANKL mediated osteoclast differentiation (Lee et al., 2013; Nakamachi et al., 2016; Qu et al., 2015; Takigawa et al., 2016). To explore this phenomenon, we checked for the molecular effect of miR-506-3p towards RANKL mediated NFATc1 activation. Moreover, the role of miR-506-3p towards RANKL/NFATc1 in osteoclast differentiation will be explored in this study.
2 MATERIALS AND METHODS
2.1 Reagents and chemicals
TRIzol was purchased from Sigma chemicals Co. (St. Louis, MO). Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum, trypsin, antibiotics (penicillin and streptomycin) were obtained from HIMEDIA (Mumbai, India). RANKL and M-CSF were procured from PeproTech (NJ). High capacity cDNA reverse transcriptase kit was purchased from Applied Biosystems (Foster City, NY). TRAP staining kit was purchased from Sigma-Aldrich (St. Louis, MO). Osteo Assay plates were obtained from Corning Life Sciences (MA). EvaGreen Master Mix was purchased from G-Biosciences (St. Louis, MO). Rat specific primers depicted in Table 1 were purchased from Sigma-Aldrich (St. Louis, MO). Primary antibodies for NFATc1, TRAP, and cathepsin K were obtained from ABclonal Technology (Woburn, MA). Antibody against β-actin was purchased from Bioss Antibodies (Woburn, MA). Secondary horseradish peroxidase (HRP) conjugated antibody and fluorescent-tagged antibody (Alexa Fluor 488) were purchased from Cell Signaling Technology (Danvers, MA). Transfection reagents (X-fect™ transfection reagent) were purchased from Takara Clonetech Laboratories (Mountain View, CA). miR-506-3p mimic and inhibitor were obtained from Guangzhou Ribobio Co. Ltd. (Guangzhou, China). Dual-luciferase expression vectors (pmiRGLO) and dual-luciferase reporter assay systems were procured from Promega (Madison, WI).
| Gene | Forward | Reverse |
|---|---|---|
| Carbonic anhydrase II | 5′-AAGAGCAACGGACCAGAGAA-3′ | 5′-GGCAGGTCCAATCTTCAAAA-3′ |
| Cathepsin K | 5′-AGTAGCCACGCTTCCTATCC-3′ | 5′-GAGAGGCCTCCAGGTTATGG-3′ |
| MMP9 | 5′-CTCTGCTGCCCCTTACCAG-3′ | 5′-CACAGCGTGGTGTTCGAATG-3′ |
| TRAP | 5′-CACCTCAAGAGTCGAAGGCG-3′ | 5′-CTGAGTCCAGAGTTGCAGAGG-3′ |
| β-actin | 5′-ACCACCATGTACCCAGGCATT-3′ | 5′-CCACACAGAGTACTTGCGCTCA-3′ |
- Abbreviations: MMP9, matrix metalloproteinase 9; TRAP, tartarate acid phosphatase.
2.2 Target prediction using bioinformatics tools
The target prediction of the miR-506-3p towards NFATc1 was performed using TargetScan 7.2 (http://www.targetscan.org/; Agarwal, Bell, Nam, & Bartel, 2015). Moreover, the binding affinity and spontaneity were predicted using the Sfold web server for miRNAs (http://sfold.wadsworth.org/starmirDB.php; Rennie et al., 2019).
2.3 Isolation of BMMs and osteoclast differentiation
BMMs were isolated from Wistar albino rats of 6–8 weeks as described previously with minor modifications (Kwok et al., 2012). In brief, the thigh bones of the experimental rats were surgically removed and bone marrow was taken out by rapidly flushing with DMEM medium using a 5 ml syringe. For separating the red blood cells, red blood cell lysis buffer was added for 30 s at 37°C following centrifugation at 1,000 rpm for 5 min. Further, the acquired pellet was rinsed in ice-cold phosphate-buffered saline (PBS) solution and resuspended in complete DMEM for 12 hr. Nonadherent cells were removed and the adherent cells were cultured in the presence of 30 ng/ml M-CSF for 3 days under similar culture conditions. Differentiated BMMs were converted to mature osteoclast cells using RANKL (100 ng/ml) and M-CSF (20 ng/ml) stimulation for 5 days and were used for all the experiments.
2.4 Transfection of miR-506-3p mimic/inhibitor
Transfection of miR-506-3p mimic/inhibitor to BMMs was performed using X-fect™ RNA transfection reagent following the manufacturer's protocol. BMMs were plated at a density of 1.5 × 106 cells/well in a six-well culture plate followed by stimulation with RANKL (100 ng/ml) and M-CSF (20 ng/ml) for 5 days before transfection. Next, 880 μl of serum-free media and 120 μl of transfection cocktail were added to make up the final volume. miR-506-3p mimic (50 pMol) was used for enhancing the expression of miRNA alongside a miR-506-3p inhibitor (50 pMol) as a negative control. After incubation for 4 hr, the serum-free media was discarded and DMEM complete medium was added to the cells and incubated for 48 hr.
2.5 Quantitative RT-PCR
Total RNA was isolated using TRIzol reagent (Sigma Chemicals Co.) and it was reversed transcribed into complementary DNA (cDNA) using a high capacity cDNA reverse transcription kit (Applied Biosystems, CA) according to manufacturer's protocol. Gene-specific primers were designed manually using the online NCBI Primer-BLAST tool and were purchased from Sigma-Aldrich (Table 1). The gene expression levels of these factors were quantified using EvaGreen PCR Master Mix (G-Biosciences) following the manufacturer's instruction. Thermal cycling conditions were as follows: Denaturation at 94°C for 15 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s. The fold change in gene expression levels of target genes was calculated with normalization to β-actin values using the 2−ΔΔCt comparative cycle threshold method.
2.6 Estimation of bone resorptive enzymes
The release of bone resorptive enzymes (Carbonic anhydrase II, MMP9, cathepsin K, and TRAP) by RANKL (100 ng/ml) stimulated BMM cells were assayed using rat specific ELISA kits (Peprotech, RockyHill, NJ) as per manufacturer's instruction. Briefly, BMMs were plated at a density of 1.5 × 106 cells/well into six-well plates and stimulated with RANKL (100 ng/ml) and M-CSF (20 ng/ml) for 5 days with/without miR-506-3p mimic (50 pmol) or inhibitor (50 pmol) transfection. Subsequently, the supernatant of the cells were collected and used for measuring the enzyme levels. The absorbance was measured at a wavelength of 450 nm using a microplate reader (Biotek, Winooski). The standard curves were used to quantify their levels released by the cells. All experiments were performed in triplicates.
2.7 Cellular expression of NFATc1
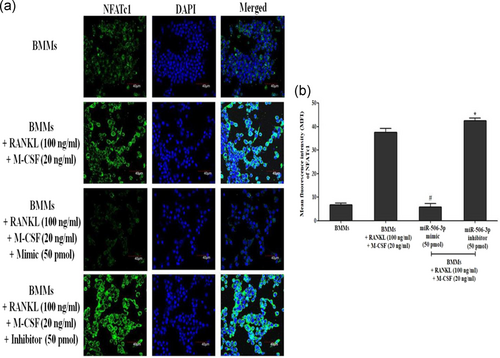
Immunofluorescence was performed as previously reported with minor alterations (Wang et al., 2019). Briefly, BMMs were seeded on a gelatin-coated round glass coverslips into six-well tissue culture plates followed by stimulation with RANKL (100 ng/ml) and M-CSF (20 ng/ml) for 5 days with/without miR-506-3p mimic (50 pmol) or inhibitor (50 pmol) transfection. After transfection, the media was discarded and the cells were washed with 1× PBS. Immediately the cells were fixed with 4% formaldehyde for 15 min at 37°C. Thereafter the cells were rinsed with 1× PBS and were subjected to permeabilize with 0.1% Triton X-100 in 1× PBS for 5 min. The coverslips were stained with rabbit polyclonal antibodies of NFATc1 overnight at 4°C. Subsequently, the cells were incubated with a secondary goat-anti-rabbit antibody labeled with Alexa Fluor 488 (Cell Signaling Technology, Beverly, MA) at 37°C for 2 hr in the dark. The nuclei were counterstained with 4′6-diamindino-2-phenylindole (1 μg/ml; Sigma-Aldrich) for 5 min at 37°C and examined using an Olympus confocal microscope (Olympus America, Melville, NY). Mean fluorescence intensity was measured using the ImageJ software.
2.8 Protein isolation and western blot analysis
The western blot analysis was performed as previously described with certain modifications (Wang et al., 2019). Whole-cell lysates were obtained by homogenization in ice-cold radioimmunoprecipitation assay buffer containing protease inhibitor cocktail and centrifuged at 14,000 rpm for 15 min at 4°C. The protein concentration in the cell lysates was estimated using the Bradford method (Bio-Rad, Hercules, CA). The cell lysates (40 µg/ml) were separated on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electro-transferred onto polyvinylidene fluoride membrane (Amersham Pharmacia Biotech, Uppsala, Sweden). The transfer was confirmed using Ponceau S staining method. After destaining, the membranes were blocked with 5% (wt/vol) bovine serum albumin overnight at 4°C. Subsequently, the membranes were incubated with rabbit polyclonal antibody against NFATc1, cathepsin K and TRAP overnight at 4°C; washed and then probed for 2 hr at room temperature with HRP conjugated secondary antibodies (1:10,000). Protein bands were visualized using the enhanced chemiluminescence detection system (Bio-Rad Laboratories, Mississauga, Canada). The blots were stripped and reprobed with β-actin to confirm equal protein being loaded. Each protein blot is representative of three similar independent experiments.
2.9 TRAP staining and osteo assay
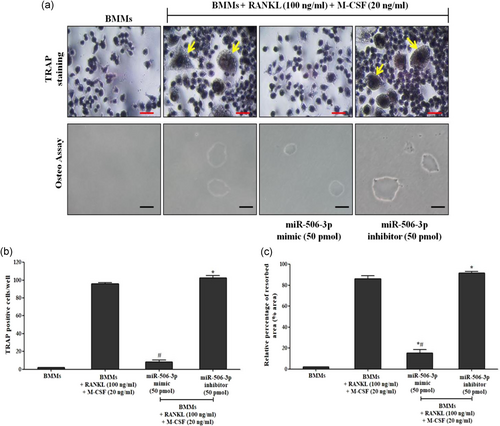
To assess the formation of mature osteoclasts, TRAP staining analysis was performed using acid phosphatase, TRAP kits as per the manufacturer's protocol (Sigma-Aldrich). In brief, BMMs were cultured in six-well plates with RANKL (100 ng/ml) and M-CSF (20 ng/ml) for 5 days. Stimulated cells were transfected with miR-506-3p mimic/inhibitor (50 pmol). Posttransfection, cells were washed with 1× PBS and fixed with 4% formaldehyde at room temperature for 15 min. Further, the plates were stained for TRAP expression with repeated rounds of PBS wash. Then it was stained positive for TRAP expression. The cells containing three or more nuclei were considered to be osteoclasts.
Osteo assay was performed for in vitro assessment of miR-506-3p towards the functional activity of osteoclasts as per manufacturer's protocol. BMMs were seeded on Corning Osteo-assay surface 24-well plates (Corning Life Sciences) that provides a smooth mineralized surface which mimics bone material. Further, cells were transfected with/without miR-506-3p mimic/inhibitor (50 pmol) followed by RANKL (100 ng/ml) and M-CSF (20 ng/ml) stimulation for 5 days. After the incubation period, cells were suspended with 100 µl bleach solution (10%) for 5 min at room temperature. Following bleaching, the plate was washed with 1× PBS for two times and air-dried for 3–5 hr. The resorbed area formed by mature osteoclasts was captured using an inverted microscope (Olympus, Tokyo, Japan) at ×40 magnification.
2.10 Plasmid construct
Dual-luciferase miRNA target expression vectors (pmirGLO) were obtained from Promega. The primers were designed for NFATc1 to amplify the target region of the coding sequence and were digested with XbaI and DraI restriction sites. The primer sequences of wild-type 3′-untranslated region (UTR) of NFATc1 (Sense: 5′-AAATGGGCGGCCGCGGTACCGGTTCAATACTGGAAGTGCCTTATT-3′; antisense: 5′-TTTACCCGCCGGCGCCATGGCCAAGTTATGACCTTCACGGAATAAGATC-3′) and mutant 3′-UTR of NFATc1 (sense: 5′-AAATGGGCGGCCGCGGTACCGGTTCAATACTGGAAAATATAATTT-3′; antisense: 5′-TTTACCCGCCGGCGCCATGGCCAAGTTATGACCTTTTATATTAAAGATC-3′) were chemically synthesized. After restriction enzyme digestion, the PCR product containing fragments of the NFATc1 3′-UTR region with the predicted target site of miR-506-3p was cloned into the pmirGLO expression vector. A point mutation was induced using 3′-base substitution of the target sequence (Philippe et al., 2013).
2.11 Luciferase assay
Luciferase assay was performed as previously described with certain modifications (Philippe et al., 2013). In brief, BMMs were seeded on six-well plates with/without RANKL (100 ng/ml) and M-CSF (20 ng/ml) stimulation for 5 days followed by miR-506-3p mimic (50 pmol) or inhibitor (50 pmol) treatment. The construct containing pmirGLO-NFATc1 luciferase vector (wild-type and mutant) were co-transfected into cells using X-fect™ transfection reagent (Takara Clontech Laboratories, Mountain View, CA). The dual-luciferase assays were performed 24 hr after transfection using a dual-luciferase reporter assay kit after harvesting using a passive lysis assay buffer (Promega). For every sample, firefly luciferase activity was normalized to that of Renilla luciferase activity as the relative fluorescence intensity.
2.12 Statistical analysis
The data were presented as mean ± standard error mean. The statistical analysis between the experimental groups was carried out using a one-way analysis of variance by Bonferroni's posttest using GraphPad 5.0 for windows. The *#p < .05 implies statistical significance.
3 RESULTS
3.1 Bioinformatics analysis
RANKL/RANK interaction in macrophages helps differentiate them into mature osteoclast cells. This phenomenon is regulated by a key transcription factor designated as NFATc1. We initially predicted the far end regions of miRNA clusters which showed a high binding affinity towards NFATc1. TargetScan and SFold data predicted that miR-506-3p elicited a high binding affinity towards NFATc1 with ΔG = −22.4 kcal/mol. We further performed various molecular studies to support this initial analysis.
3.2 miR-506-3p attenuates NFATc1 mediated osteoclast formation
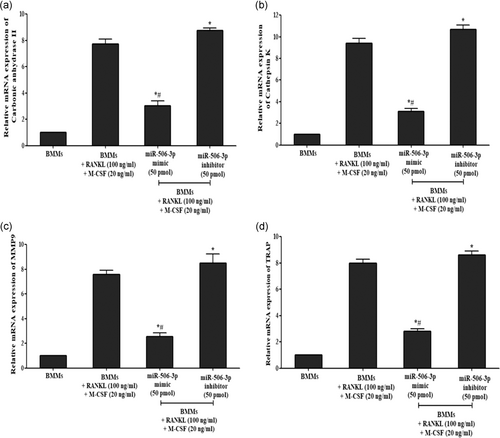
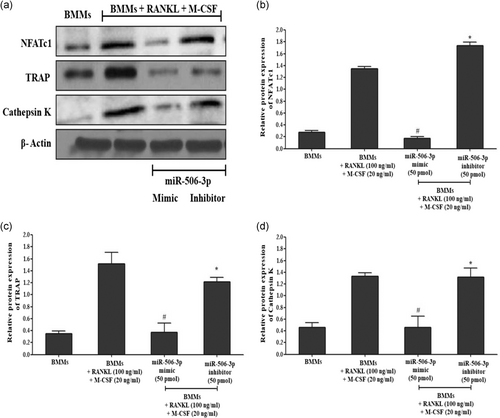
RANKL/RANK interaction which promotes osteoclast differentiation has been showcased to be controlled by active NFATc1 expression inside the cells. With our initial bioinformatics analysis eliciting that miR-506-3p showing a high binding affinity towards NFATc1 transcription factor, we wanted to explore its molecular mechanism against this pathway. BMMs stimulated with RANKL for a period of 5 days showed diminished expression of miR-506-3p with active NFATc1 transcription factor inside the cells (Figure 1a,b). Moreover, active osteoclasts depicted elevated levels of bone resorptive enzymes such as MMP9, cathepsin K, carbonic anhydrase II, and TRAP at the gene/protein level which degraded the bone surface and this was more pronounced in the miR-506-3p inhibitor group (Figures 2a–d and 3a,b). However, the transfection of miR-506-3p counteracted this mechanism through selective inhibition of NFATc1 and diminished the levels of bone resorptive enzymes (Figures 4a–c and 5a–d). These molecular studies elicit that miR-506-3p acts as a selective inhibitor for the NFATc1 transcription factor.
3.3 miR-506-3p suppresses bone resorptive enzyme release
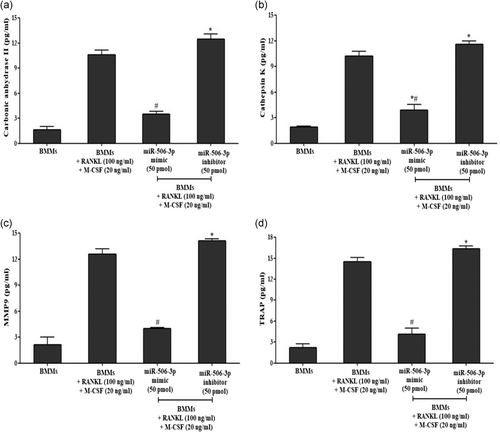
Release of various bone resorptive enzymes (Cathepsin K, MMP9, TRAP, and carbonic anhydrase II) in RANKL (100 ng/ml) stimulated BMMs transfected with miR-506-3p mimic/inhibitor treatment were estimated using sandwich enzyme-linked immunosorbent assay. As witnessed from Figure 6a–d after RANKL stimulation for 5 days, mature osteoclasts secreted high volumes of bone resorptive enzymes compared to the unstimulated group which was more pronounced after transfection with miR-506-3p inhibitor (50 pmol). However, after transfection with miR-506-3p mimic (50 pmol); there was a significant reduction in the levels of these bone resorptive enzymes which were controlled by NFATc1 transcription factor.
3.4 Direct targeting of NFATc1 by miR-506-3p
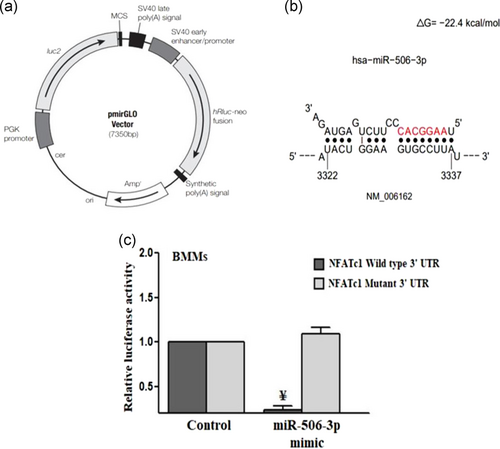
When BMMs were stimulated with RANKL (100 ng/ml) for 5 days, there was a diminished expression level of miR-506-3p with increased NFATc1 transcription factor, which led to uncontrolled osteoclast formation. To further substantiate this initial finding, we performed the dual-luciferase reporter assay (Figure 7a–c). The pmiRGLO construct containing a dual-luciferase expression system (firefly luciferase and Renilla luciferase) was utilized for the cloning of NFATc1 3′-UTR. The regions of NFATc1 which showed high repressive strength against miR-506-3p were cloned into the vector system. The levels of Firefly luciferase in the vector system was expressed constitutively and normalized with that of Renilla luciferase activity. The vector construct containing the wild/mutant type NFATc1 3′-UTR downstream to that of the Firefly luciferase gene was co-transfected with control miRNA (50 pmol) and miR-506-3p mimic (50 pmol). After cotransfection, the relative luciferase activity was measured. The results depicted that there was a significant reduction in the luciferase activity when BMMs were co-transfected with the vector containing NFATc1 3′-UTR wild-type alongside with miR-506-3p mimic. The mutant NFATc1 3′-UTR did not alter the luciferase activity when co-transfected with control miRNA/miR-506-3p mimic, elucidating the potential inhibitory effect of miR-506-3p towards NFATc1 expression resulting in regulation of RANKL/NFATc1 signaling pathway (Figure 8).
4 DISCUSSION
Bone erosion is a clinical complication witnessed in bone-related disorders such as osteoporosis and RA (Boyce et al., 2018; Mohammadi et al., 2018; Pourakbari et al., 2019). Aberrant bone erosion is correlated with the uncontrolled release of RANKL from several cell subtypes involved in the pathogenesis of bone disorders (Kim et al., 2015). RANKL interacts with RANK receptors uninterruptedly without any checkpoints, which results in the upregulation of an important transcription factor essential for survival/proliferation of osteoclasts designated as NFATc1 (Yang et al., 2018). Recent studies have illustrated that small molecular RNA molecules depicted as miRNAs to possess inhibitory effect towards uncontrolled osteoclast formation in bone disorders (Hesse & Taipaleenmaki, 2019). This study showcases such a phenomenon by exploring the molecular inhibitory capacity of miR-506-3p towards the RANKL/NFATc1 pathway in osteoclast-derived from BMMs.
Several reports have elicited the central role of NFATc1 in promoting aberrant osteoclastogenesis, which resulted in various bone-related clinical complications (Jia et al., 2019; Xu et al., 2019). Such complications can be counteracted through intervention by small molecular miRNA molecules (Ye et al., 2019). Studies have exclusively focussed on targeting NFATc1 pathways to overcome the aberrant bone erosion (Niu & Schulert, 2019; Roncarati et al., 2019). For instance, reports have showcased that the cluster of miRNA spanning from miR-17-92 possesses anti-bone resorptive activity of osteoclast via targeting the NFATc1 transcription factor (Mizoguchi et al., 2013; Rossi et al., 2013; Sun et al., 2015; Yin et al., 2017). Moreover, other classes of miRNAs including miR-124, miR-218 and miR-222-3p have been exclusively studied to diminish osteoclast differentiation via posttranscriptional gene silencing of NFATc1 (Lee et al., 2013; Nakamachi et al., 2016; Qu et al., 2015; Takigawa et al., 2016). Recently, it has been shown that miR-23a diminishes osteoclast formation through selective inhibition of glycogen synthase kinase 3 beta (GSK3B) phosphorylation which possesses direct control over NFATc1 activation (Sujitha & Rasool, 2019).
In par with these reports, our study aimed at illustrating the molecular inhibitory effect of miR-506-3p towards the RANKL/NFATc1 pathway in BMMs/osteoclast lineage. miR-506-3p has been previously described in various studies to possess anti-cancer activities by targeting key molecular mechanisms that promote cellular proliferation/survival (Han et al., 2019; Hu, You, Zhang, Zhang, & Jiang, 2019). Moreover, miR-506-3p possesses an anti-proliferative effect on osteosarcoma cells that are involved in the metastatic condition of the bone (Jiashi et al., 2018). Similarly, our initial bioinformatics prediction elicited that miR-506-3p shows a strong binding affinity towards NFATc1 with a ΔG = −22.4 kcal/mol. Moreover, there was reduced expression of miR-506-3p under RANKL stimulation in comparison with an unstimulated control group.
These initial outcomes led to the conclusion that miR-506-3p and NFATc1 elicit opposite action towards each other. To illustrate this theory, we performed various molecular mechanistic detection of important factors involved in the RANKL/NFATc1 pathway. RANKL which interacts with the RANK receptor alongside M-CSF promotes the conversion of macrophages into multinucleated osteoclast cell subtypes (Souza & Lerner, 2019). During disease conditions such as osteoporosis and RA, the number of osteoclasts circulating in the bone region is more pronounced than the threshold value alongside with diminished levels of osteoblasts (bone-forming cells) (Dirckx et al., 2019). This uncontrolled proliferation is mediated through continuous expression of NFATc1 transcription factor. NFATc1 expression is driven through RANKL/RANK interaction and calcium efflux inside the cells (Yang et al., 2018). This was the case witnessed in our study, where RANKL stimulation promoted differentiation of macrophages to osteoclasts through continuous expression NFATc1 transcription factor. However, miR-506-3p mimic transfection inhibited NFATc1 through posttranscriptional gene silencing, which resulted in reduced number of osteoclasts. Mature osteoclasts under the influence of aberrant NFATc1 expression leads to uncontrolled bone erosion and matrix degradation. This process is carried out through secretion of acidic enzymes including TRAP, cathepsin K, carbonic anhydrase II, and MMPs (predominantly MMP9) (Chaweewannakorn et al., 2019). Similar conditions were observed under continuous RANKL stimulation to BMMs which resulted in degradation of the bone surface via secretion of these acidic enzymes. This aberrant release of bone degrading enzymes were diminished after direct targeting of NFATc1 by miR-506-3p. Overall, it is evident that miR-506-3p possess anti-bone resorptive activity towards osteoclasts showcased through direct targeting and inhibition of NFATc1 transcription factor.
5 CONCLUSION
This report elicits the anti-osteoclastogenic effect of miR-506-3p towards RANKL/NFATc1 pathway. miR-506-3p which showed a high binding affinity towards NFATc1, diminished its function in RANKL stimulated BMMs through posttranscriptional gene silencing. Specific targeting of NFATc1 by miR-506-3p resulted in the regulation of osteoclast differentiation and suppression of various bone resorptive enzymes that are essentially required for its function. Overall, it is evident that miR-506-3p specifically targets the NFATc1 transcription factor and may play a crucial role in regulating uncontrolled osteoclastogenesis.
ACKNOWLEDGMENTS
Palani Dinesh would like to thank Council of Scientific and Industrial Research for providing funds in the form of Senior Research Fellowship [acknowledgment no.: 112290/2K17/1; File no: 09/844(0059)/2018].
Sali Sujitha would like to thank University Grants Commission for Maulana Azad National Fellowship for providing funds in the form of Junior Research Fellowship [file no.: 2017-18/MANF-2017-18-KER-82149].
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Palani Dinesh—conceptualization, investigation, methodology, writing—original draft (equal); Sowmiya Kalaiselvan—investigation, methodology, formal analysis (equal), Sali Sujitha—investigation; Mahaboobkhan Rasool—funding acquisition, reviewing and editing, supervision.
Open Research
DATA AVAILABILITY STATEMENT
Research data are not shared.




