STAT3 inhibition reduced PD-L1 expression and enhanced antitumor immune responses
Abstract
Colon cancer is one the most common diagnosed cancers in America and Europe. Signal transducer and activator of transcription 3 (STAT3) in colon cancer is associated with proliferation of the tumor cells and suppression of immune responses. STAT3 activation upregulates the transcription of many suppressor genes, including programmed death-ligand 1 (PD-L1). This study was aimed to investigate the effect of STAT3 inhibition in a colon cancer cell line, HCT-15, and particularly in presence of samples obtained from the patients suffering from colon cancer. In this project, the expression of PD-L1 and apoptosis-related proteins were assessed following STAT3 inhibition, using FLLL32, in HCT-15 cells. To evaluate the effects of STAT3 inhibition on immune response, lymphocytes from 20 men with Stage III colon cancer and 20 healthy donors were cocultured with HCT-15 cells in presence or absence of STAT3 inhibitor. Then, T regulatory (T-reg) cell evaluation and intracellular cytokine staining (ICS) were performed using flowcytometry to assess the T-reg and T helper (Th) subset cytokines following STAT3 inhibition. STAT3 inhibition suppressed PD-L1 expression and induced apoptosis in HCT-15 cells. The population of T-reg cells in patients with colon cancer significantly decreased after treatment with STAT3 inhibitor. ICS revealed that STAT3 inhibition promotes Th1 protective immune responses. These findings suggest that STAT3 inhibition through either induction of apoptosis in the colon cancer cells and/or activation of efficient immune responses can lead to overcome cancer-induced immune tolerance.
1 INTRODUCTION
Colon cancer is the third most common cancer in both men and women, and the second leading cause of cancer death in the United States (Tian et al., 2019). According to global cancer statistics, each year about 1.2 million new cases are diagnosed worldwide (Böckelman, Engelmann, Kaprio, Hansen, & Glimelius, 2015; Watanabe et al., 2018). Although several chemotherapeutic agents have been developed, unwanted toxicity effects to bone marrow, lungs, and gastrointestinal tract, and also new drug resistances, cause chemotherapy failures (Tang et al., 2018). In recent years, the discovery and development of new immunotherapeutic agents have represented a valuable milestone in cancer medicine (Tang et al., 2018).
Recently, cancer-associated molecular signaling gained enormous attention worldwide (Pandurangan et al., 2018). Several signaling pathways play critical roles in colon cancer. Signal transducer and activator of transcription 3 (STAT3), as a transcription factor, regulates several cell functions, including proliferation, apoptosis, and immune system response (Galoczova, Coates, & Vojtesek, 2018; Huynh, Chand, Gough, & Ernst, 2018). Studies revealed that STAT3 activated by tyrosine phosphorylation at position 705, which leads to the expression of a variety of genes and transcription factors, plays a potential role in normal cellular processes, such as proliferation and apoptosis. However, it has been reported that phosphorylated STAT3 (p-STAT3) is highly expressed in several human malignancies including colon cancer. Persistent activation of STAT3 in colon cancer is associated with poor prognosis and adverse clinical outcome, that consistently proposes STAT3 as a potential therapeutic target (Ji et al., 2016). STAT3 is proved to play a key role in induction of tumor-specific immune tolerance. STAT3 upregulates immune-suppressive molecules, such as programmed death-ligand 1 (PD-L1) on tumor cells and induce antiapoptotic molecules in tumor cells (Chung, Giehl, Wu, & Vadgama, 2014).
Recent investigations revealed that FLLL32 is a novel small molecule, which specifically inhibits STAT3 phosphorylation (but not other STAT family members) and revealed growth-repressive activity in constitutively activated STAT3 tumor cells (Wu et al., 2016). However, the main mechanisms played by FLLL32 to control the colon cancer progression are yet to be clarified.
Based on the fact that p-STAT3 is a major factor for induction of immune tolerance against tumor cells, it has been hypothesized that FLLL32 may overcome immune tolerance through downregulation of antiapoptotic and anti-inflammatory molecules in cancer cells and also alter T helper (Th) differentiation to enhance antitumor immune responses. Therefore, this project was designed to explore the in-vitro effects of FLLL32 on the expression of PD-L1, as the main molecule to induce antigenic tolerance, and to evaluate the apoptosis-related molecules in the HCT-15 colon cancer cell line. Another aim of this project was to assess the ex-vivo effects of FLLL32 on the adaptive immune cell populations, including T regulatory (T-reg) lymphocytes and cytokines of Th subsets.
Hereupon, considering the importance of cytokine networks in distinct Th subsets and as a result, shaping the response type (Ehsani et al., 2019; Kagina et al., 2015; Kaiko, Horvat, Beagley, & Hansbro, 2008; Mobini et al., 2015), the intracellular cytokine staining (ICS) by flowcytometry technique was used to determine the Th cytokine expression after treatment with FLLL32.
2 MATERIALS AND METHODS
2.1 Cell culture
Human colon cell line, HCT-15, was purchased from the Iranian Biological Resource Center (Tehran, Iran) and maintained in monolayer culture in Dulbecco's modified Eagle's medium (Sigma-Aldrich, Darmstadt, Germany) supplemented with 10% fetal bovine serum (FBS; Gibco, Karlsruhe, Germany), 50 U/ml penicillin, and 50 mg/ml streptomycin at 37°C in an atmosphere of 5% CO2. FLLL32 (Sigma-Aldrich), as STAT3 inhibitor, was dissolved in dimethyl sulfoxide, and added to the selected cell culture medium at 2.5 μM (IC50 determined by MTT assay [data not shown]). The cells were used for analyzing the expression of PD-L1 and apoptotic and antiapoptotic molecules.
2.2 Cocultures
Blood sample was collected according to Medical Ethic Committee (Code: IR.AJAUMS.REC.1398.183), and written informed consent for this study was obtained from all patients and healthy donors. Peripheral blood mononuclear cells (PBMCs) were separated by FICOLL gradient (Sigma-Aldrich) from 20 men who had Stage III colon cancer (according to the tumor node metastasis classification) and 20 healthy donors. Criteria for eligible patients and healthy donors included mean age of 60.24 ± 5.35, no history of prior rectal tumors or surgery for colon, and no known hereditary cancer, Crohn's, or ulcerative colitis disease, and inflammatory bowel disease.
PBMCs were cocultured with HCT-15 cells in six-well plates at a ratio of 3:1 in Roswell Park Memorial Institute 1640 medium supplemented with 10% autologous serum, 2 mM/Ul l-glutamine, 1:100 penicillin/streptomycin, 150 U/ml IL-2 (PeproTech, London, UK), and FLLL32. Half of the medium was gently replenished every 2 days and after 2 weeks of culture, cells were collected for flowcytometry analysis.
2.3 Western blot analysis
Apoptotic and antiapoptotic molecules were evaluated using western blot analysis technique. Accordingly, HCT-15 cells were lysed using 100 μl of radioimmunoprecipitation assay buffer (Thomas Scientific, Leeds, UK). After centrifugation at 1,500g, the protein concentration of supernatant was measured using Thermo Scientific BCA Protein Assay (Thermo Fisher Scientific, Dreieich, Germany). Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and transferred to a polyvinylidene difluoride membrane (GE Healthcare Life Sciences) and probed with primary specific Abs against STAT3, phosphorylated STAT3 (Tyr705) (Biolegend, London, UK), Bcl-2, Bcl-xl, cleaved caspase 3, cleaved PARP1, vascular endothelial growth factor (VEGF; Thermo Fisher Scientific), P53 (Biolegend), and β-actin (Sigma-Aldrich), followed by horseradish peroxidase-conjugated secondary antibodies (Thermo Fisher Scientific). Reactive bands were visualized using an ECL Western Blot Analysis Detection Kit (Thermo Fisher Scientific).
2.4 Flowcytometry
To evaluate the PD-L1 expression on tumor cells, single cell prepared using trypsin-ethylenediaminetetraacetic acid (EDTA; Sigma-aldrich), and washed with FACS buffer (phosphate buffered saline + 5 mM EDTA + 1% FBS). Then, the cells were stained with PE-conjugated anti-PD-L1 (anti-CD-274 29E.2A3; Biolegend) and the respective isotype control.
To assess T-reg population after 2 days of coculture, nonadherent cells were washed twice with FACS buffer and stained with anti-CD3-PE (UCHT; Biolegend), anti-CD4-FITC (PRA-T4; Biolegend), anti-CD25-APC (M-A251; BD Bioscience, Eysins, Switzerland), and anti-Foxp3-PE-CF594 (236A/E7; BD Bioscience).
ICS was used to evaluate cytokines of Th subsets in ex-vivo condition. Accordingly, interferon-γ (IFN-γ) and interleukin-18 (IL-18) were considered as Th1 markers, IL-4, IL-13, IL-9, and IL-10 were considered as Th2 markers and IL-17A was used as the main marker of Th17 subset. Furthermore, in our experiment, IL-2 stood as a marker for proliferation of all Th subsets and tumor necrosis factor-α (TNF-α) as the main marker of proinflammatory response. ICS was used after 2 weeks of coculturing PBMCs with HCT-15 cells. Golgi-Stop (BD Bioscience), as an inhibitor of cytokine secretion, was added to all cultures, 8 hr before measurements. Then, the nonadherent cells were washed twice with FACS buffer and stained with anti-CD3-PE (UCHT; Biolegend) and anti-CD4-FITC (PRA-T4; Biolegend) and incubated for 30 min and then washed twice with FACS buffer. For intracellular staining, cells were permeabilized using fixation/permeabilization buffer (00-5523; eBioscience) and stained with fluorescent conjugated antibodies against IL-2-APC (MQ1-17H12; BD Bioscience), IFNγ-V450 (XMG1.2; BD Bioscience), IL-18-APC-Cy7 (TC11-18H10; BD Bioscience), IL-4-APC (8D4-8; BD Bioscience), IL-13-BV421 (JES10-5A2; BD Bioscience), IL-9-PerCP-Cy5.5 (MH9A3; BD Bioscience), IL-10-BV421 (JES10-5A2; BD Bioscience), TNF-α-PE-Cy7 (Mab11; BD Bioscience), and IL-17A-PerCP-Cy5.5 (TC11-18H10; BD Bioscience). Respective isotype controls were performed for all fluorochromes. Flowcytometry analysis was done using BD FACSCantoTM II flowcytometry system.
2.5 Statistical analysis
Statistical evaluation was done using Student's t test and two-way analysis of variance and p < .05 was considered statistically significant. To plot the data and statistical analysis, GraphPad Prism was used and the results were presented as mean ± SD.
3 RESULTS
3.1 STAT3 inhibition induced apoptosis in HCT-15
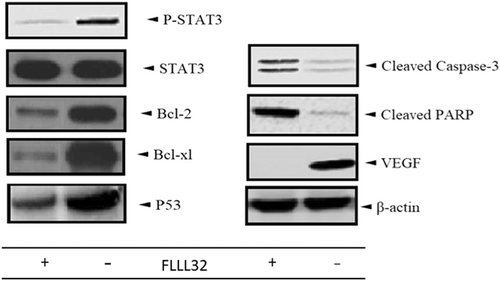
Western immunoblot analysis revealed that HCT-15 cells exhibited high levels of STAT3, p-STAT3, and antiapoptotic molecules expression, while treatment with FLLL32 resulted in downregulation of p-STAT3, the antiapoptotic molecules (Bcl-2 and Bcl-xl), and VEGF, but not STAT3. Treatment with FLLL32 also led to increased expression of cleaved caspase 3, P53, and cleaved PARP1 (Figure 1).
3.2 PD-L1 decreased on HCT-15 cells after STAT3 inhibition
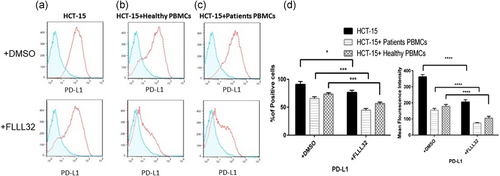
HCT-15 cells were cocultured with PBMCs from Stage III colon cancer patients and healthy donors in presence or absence of FLLL32. As shown in Figure 2, the flowcytometry analysis revealed that HCT-15 expresses high levels of PD-L1. STAT3 inhibition not only significantly decreased PD-L1 expression in HCT-15, but also reduced PD-L1 in coculture groups.
3.3 STAT3 inhibition provokes immune response toward colon cancer
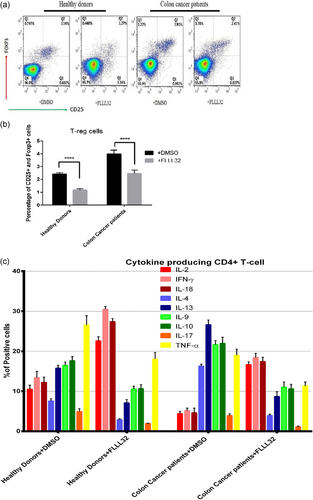
The results showed that the population of T-reg cells significantly (p < .05) decreased after STAT3 inhibition in both patient (3.99 ± 1.01% vs. 2.36 ± 0.98% after p-STAT3 inhibition) and healthy (2.56 ± 0.86% vs. 1.12 ± 0.69% after p-STAT3 inhibition) groups. The data demonstrated that IFN-γ and IL-18 cytokines are the predominant T-cell products after p-STAT3 inhibition. FLLL32 application has also declined the TNF-α production by the immune cells of the patients and healthy controls in a significant manner. FLLL32 treatment resulted in downregulation of Th2 and Th17 cytokines in either healthy donors or patients with colon cancer (Figure 3).
4 DISCUSSION
The findings demonstrated that FLLL32 diminished PD-L1 expression either on the HCT-15 cells or on the patients PBMCs/HCT-15 cocultures. Due to the results, it appeared that p-STAT3 is a main inducer of PD-L1, as inhibition of STAT3 led to downregulation of PD-L1 on both HCT-15 and HCT-15/PBMCs cocultures. Moreover, the results confirmed the roles of p-STAT3 in the induction of apoptosis, and demonstrated that inhibition of STAT3 was associated with downregulation of Bcl-2, Bcl-xl, and P53, the main antiapoptotic molecules, and upregulation of cleaved caspase 3, and cleaved PARP1, the main molecules involved in the induction of caspase-dependent and extrinsic apoptosis pathways. Therefore, it appeared that STAT3 inhibition can be associated with decrease in PD-L1 expression and increased apoptosis. This augmented apoptosis of cancer cells and enhanced immune responses stimulated via downregulation of anti-inflammatory molecules and PD-L1, can reflect one of the most pre-eminent goals for cancer treatment.
Patients with Stage III colon cancer have exhausted lymphocytes and inefficient immune responses. To stimulate the weakened immune response toward tumor cells, the immune cells need to be activated and promoted the effective Th1 responses. The results showed that p-STAT3 inhibition was associated with increased IFN-γ and IL-18 producing cells. Interestingly, Th1 immune cells also upregulated IL-2, which acts as the main inducer of clonal expansion of adaptive immunity. Additionally, the results demonstrated that the levels of TNF-α, the main innate immunity cytokine participating in the tumor associated inflammation, was increased after the inhibition of p-STAT3. Therefore, it appeared that inhibition of p-STAT3 by FLLL32 can be considered as a potential strategy to improve Th1 immune responses against colon cancer and constrain tumoral innate immunity–associated inflammation. Moreover, FLLL32 treatment in ex-vivo condition led to downregulation of IL-17A and Th2-related cytokines, including IL-4, IL-9, IL-10, and IL-13. It has been reported that IL-17A plays key roles in the induction of fibrosis in inflamed tissues and suppression of Th1 immunity (Arababadi, Bidaki, & Kennedy, 2014). Th2-related cytokines are also the main Th1 suppressor molecules during cancers (Karimabad et al., 2013). Therefore, based on the results, it may be concluded that FLLL32 can elicit an appropriate immune response against colon cancer by reinforcing the Th1 populations. In addition, it has been documented that in the tumor microenvironment, T-regs inhibit the antitumor immune response and induce immune tolerance toward tumor antigens (Verma et al., 2019). Our data suggest that p-STAT3 inhibition resulted in a reduction in T-reg populations and function, by decreasing the IL-10 production (Tatsumi et al., 2002). Therefore, a decreased immune tolerance to tumor antigens is another effect of p-STAT3 inhibition. Our previous investigation on the MCF-7 cancer cell lines revealed that STAT3 inhibition decreased expression of hypoxia-inducible factor 1-α, VEGF, IL-10, and transforming growth factor-β, the critical molecules participated in the tumor development (Jahangiri, Dadmanesh, & Ghorban, 2018). Another study by Lin et al. (2011) proved our results and revealed that FLLL32 increases apoptosis in the stem cell-like human colon cancer cells via suppression of p-STAT3. Although there are not many studies toward application of FLLL32 in colon cancer, investigations regarding the roles of the p-STAT3 inhibitor on other types of cancers have been conducted by several researchers (Fletcher et al., 2019; Wang et al., 2016; Wu et al., 2016). Thus, it seems that p-STAT3 can be considered as a promising target for immune therapy against colon cancer.
Collectively, the present study suggested that STAT3 played an important role in the colon cancer immortality. STAT3 also had a pivotal role in the suppression of efficient antitumor immune responses via upregulation of proinflammatory molecules and Th2 and Th17, as the main suppressors of Th1 immune responses. This transcription factor also significantly participated in the development of T-reg cells. However, we demonstrated that FLLL32, a p-STAT3 inhibitor, has the capacity to act as a game changer. Hence, considering all these observations together, it can be addressed as a novel anticancer target for independent implementations and/or in combination with other therapeutic strategies.
ACKNOWLEDGMENT
This study was supported by a grant from Aja Medical University.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
K. G. and M. D. contributed to the conception of the study. A. J. contributed to the design, performance, and data analysis of the experiments. A. J. contributed to the collection and assembly of data. A. J. contributed to the writing of the manuscript. K. G. contributed to the critical revision and final approval of the article.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the Supporting Information Material of this article.




