Romina: A powerful strawberry with in vitro efficacy against uterine leiomyoma cells
Abstract
Uterine leiom yomas are benign tumors highly prevalent in reproductive women. In thecurrent study, initially, we aimed to screen five different strawberry cultivars (Alba, Clery, Portola, Tecla, and Romina) to identify efficient cultivars in terms of phytochemical characterization and biological properties by measuring phenolic and anthocyanin content as well as antioxidant capacity, and by measuring apoptotic rate and reactive oxygen species (ROS) production in uterine leiomyoma cells. Next, we focused on the most efficient ones, cultivar Alba (A) and Romina (R) as well as Romina anthocyanin (RA) fraction for their ability to regulate oxidative phosphorylation (oxygen consumption rate [OCR]) glycolysis (extracellular acidification rate [ECAR]), and also fibrosis. Leiomyoma and myometrial cells were treated with a methanolic extract of A and R (250 μg/ml) or with RA (50 μg/ml) for 48 hr to measure OCR and ECAR, as well as gene expression associated with fibrosis. In the leiomyoma cells, RA was more effective in inducing apoptosis and increasing intracellular ROS levels, followed by R and A. In myometrial cells, all strawberry treatments increased the cellular viability and decreased ROS concentrations. Leiomyoma cells showed also a significant decrease in ECAR, especially after RA treatment, while OCR was slightly increased in both myometrial and leiomyoma cells. R and RA treatment significantly decreased collagen 1A1, fibronectin, versican, and activin A messenger RNA expression in leiomyoma cells. In conclusion, this study suggests that Romina, or its anthocyanin fraction, can be developed as a therapeutic and/or preventive agent for uterine leiomyomas, confirming the healthy effects exerted by these fruits and their bioactive compounds.
1 INTRODUCTION
Uterine leiomyomas, or fibroids, are the most common benign, monoclonal, gynecological tumors in women’s uterus (myometrium; Walker & Stewart, 2005). Although the exact etiological mechanism remains unknown, recent reports suggest that stem cells, genetic and epigenetic factors, sex steroids, growth factors, cytokines, chemokines, and extracellular matrix (ECM) components are involved in the development and growth of leiomyomas (Islam, Protic, Stortoni, et al., 2013; Islam et al., 2016; Islam, Ciavattini, Petraglia, Castellucci, & Ciarmela, 2018).
Uterine leiomyomas affect about 77% of women of reproductive age, and 25% of them bear clinically apparent tumors causing significant morbidity, including heavy or abnormal uterine bleeding, pelvic pain or pressure, infertility, and reproductive dysfunction in rare cases (Cramer & Patel, 1990). Hysterectomy is the definitive treatment that removes the whole uterus for women with symptomatic uterine fibroids, and the main drawback of this surgical option is the loss of fertility (Buttram & Reiter, 1981). In addition, after surgery, it takes a long time to recover with the risk of significant complications such as hemorrhage, bowel or bladder injury, infection, and pain. Myomectomy via laparotomy, hysteroscopy, or laparoscopy is an alternative surgical option to remove uterine fibroids from symptomatic women that save their fertility without destroying their uterus. However, myomectomy is associated with significant morbidity including hemorrhage, adhesion formation, leiomyoma recurrence, blood transfusion, bowel injury, and rarely hysterectomy. Unfortunately, no medicines are currently available that effectively work for the management of uterine fibroids. Gonadotrophin-releasing hormone agonist (GnRHa) is effective medical treatment of shrinking the fibroid size up to 50% of their initial volume and temporary control of bleeding. This type of treatment is restricted to a 3 to a 6-month interval, but when treatment stops, usually fibroids come back their pretreatment size. In addition, side effects include menopausal symptoms and bone loss with long-term use (Islam, Protic, Toti, et al., 2013). Compared with GnRHa, ulipristal acetate is the latest medical treatment that has been approved by the European Medicines Agency (EMA) for the treatment of women associated with moderate to severe symptoms. This synthetic compound can effectively reduce fibroid size and its associated symptoms without causing serious side effects (Donnez et al., 2014, 2015).
Dietary phytochemicals are known to possess tumor preventive and/or tumor-destroying properties. They commonly found in plant parts, such as fruits, vegetables, cereals, legumes, herbs, spices, nuts, and seeds. The ability of dietary phytochemicals to regulate tumorigenic processes, including proliferation, inflammation, fibrosis, apoptosis, angiogenesis, and cell metabolism has made them consider an alternative therapeutic option for multiple pathological conditions (Islam et al., 2014). Consequently, dietary phytochemicals could be effective against uterine leiomyoma as well (Islam et al., 2014, 2017; Islam, Akhtar, Segars, Castellucci, & Ciarmela, 2017; Islam, Segars, Castellucci, & Ciarmela, 2017).
Strawberry, a common fruit of Mediterranean diet, a rich source of nutritive (sugar, fibers, minerals, and vitamins) as well as non-nutritive compounds or phytochemicals (anthocyanins, flavonols, flavanols, phenolic acids, and ellagitannins) (Afrin et al., 2016; Giampieri et al., 2012; Mazzoni et al., 2016). These compounds have a synergistic and cumulative role in health promotion and disease prevention. Their role is not only limited to neutralize oxidative stress but also involved in modulating cell metabolism, survival, proliferation, inflammation, and DNA damage (Forbes-Hernandez et al., 2016; Gasparrini et al., 2018; Giampieri et al., 2015; Wang, Chan, Chen, & Leung, 2005).
The content of nutritive compounds or micronutrients and phytochemicals may greatly vary from cultivar to cultivar (Diamanti, Mezzetti, et al., 2014; Tulipani et al., 2008). The genetic background plays an important role in the nutritional quality of strawberry fruits and the breeding programs can be used to improve strawberry nutritional value, also using wild germplasm (Alvarez-Suarez et al., 2011; Capocasa, Scalzo, Mezzetti, & Battino, 2008; Diamanti, Mazzoni, et al., 2014; Scalzo, Battino, Costantini, & Mezzetti, 2005). For example, Alba is a cultivar of strawberry selected for higher sensorial and nutritional parameters (Diamanti, Capocasa, et al., 2012). Since we have recently demonstrated that fruit extract of strawberry belonging to cultivar Alba is able to induce apoptosis and ROS concentration, and to decrease glycolysis and fibrosis in human uterine leiomyoma cells (Islam et al., 2017), we concerned also to expand the study to other cultivars (Clery, Tecla, Portola, and Romina).
In the current study, initially, we aimed to screen five different strawberry cultivars (Alba, Clery, Portola, Romina, Tecla) to identify efficient ones by measuring their phytochemical profile as well as by measuring the apoptotic rate and reactive oxygen species (ROS) production in myometrial and leiomyoma cells. Then, we focused on the most promising cultivars, with the highest phytochemical content and the most relevant properties, and on its anthocyanin fraction evaluating also their effect on the metabolic status and fibrosis in myometrial and leiomyoma cells. We decide to isolate anthocyanin fraction since they are the best known polyphenolic compounds and quantitatively the most important, being the main responsible for the biological activities of this fruit (Giampieri et al., 2012; Forbes-Hernandez et al., 2017)
2 MATERIALS AND METHODS
2.1 Plant material and sampling method
Whole fresh strawberries (Fragaria x ananassa, Duch.) of five cultivars, Alba, Clery, Portola, Romina, and Tecla were collected from the experimental fields of the Agriculture Faculty of the Università Politecnica delle Marche, Italy. Fruit samples were hand-picked on the same day–time for three harvests corresponding to the commercial ripening times of each cultivar and were selected for homogeneity, avoiding unripe, wounded, or shriveled samples. Within 2 hr after harvest, all strawberry samples were stored at −80°C until the analysis.
2.2 Methanolic extract preparation
The methanolic strawberry extract was obtained as previously defined (Giampieri et al., 2014). Briefly, 50 g of fruits of each cultivar were added to 100 ml of the extraction solution, consisting of methanol/Milli-Q (Merck, Milan, Italy) water/concentrated formic acid, and were homogenized using an Ultra-Turrax T25 homogenizer (Janke & Kunkel, IKA Labortechnik, Staufen, Germany). Extraction was maximized by stirring the suspension at 22g (ARE Magnetic stirrer, VELP Scientifica, Usmate, Italy) for 2 hr in the dark at room temperature (RT). The mixture was then centrifuged at 2400g for 15 min for two sequential times, to sediment solids. Supernatants were filtered through a 0.45-µm Minisart filter (PBI International, Milan, Italy) and transferred into an amber glass. Finally, the methanolic extract was concentrated and dried through a rotary evaporator. The final sample was stored in aliquots at −80°C.
2.3 Extraction of the anthocyanins fraction
The anthocyanin fraction was obtained as previously described by Alvarez-Suarez et al. (2011). Shortly, 50 g of strawberries, cultivar Romina, were homogenized in 100 ml of methanol containing 0.1% HCl, stirred overnight, and subsequently filtered through a Büchner funnel under vacuum. The solid residues were washed with methanol and the filtrates obtained were centrifuged (4000g, 15 min, 21°C). All supernatants were mixed, dried through a rotary evaporator, and resuspended in 50 ml of water. After that aliquots of the aqueous phase were carefully charged into C18 SepPaks Vac 6cc cartridges (Waters, Milan, Italy) for solid phase extraction. Sugars and more polar substances were removed by passing ultrapure water and anthocyanin pigments were further eluted with methanol/0.1% trifluoroacetic acid. The final methanolic extract was concentrated and dried again through a rotary evaporator. The sample was stored in aliquots at −80°C.
2.4 Fruit analysis
The total antioxidant capacity (TAC) was determined by the Trolox Equivalent Antioxidant Capacity Assay (TEAC; Re et al., 1999), while the total phenolic content (TPH) was determined using the Folin–Ciocalteu method (Total phenol analysis: automation and comparison with manual methods); the results were expressed as mmol Trolox equivalent (TE) and mg of gallic acid (GA) per kg of fresh weight (FW). Finally, the anthocyanins content (ACY) was performed using a modified pH differential method as previously reported (Giusti & Wrolstad, 2001), with some modifications, and the results were expressed as mg pelargonidin-3-glucoside (pel-3-glu) per kg of FW.
2.5 Primary cell cultures
The study included a sample of myometrial and leiomyoma tissue excised from women undergoing hysterectomy for fibroids. We included the most homogeneous sample, considering the possible high variability, due to a different age, race, hormonal milieu, tumor size, and location of tumors. All patients were Caucasian and in the proliferative phase of the menstrual cycle. The location of leiomyomas was intramural, and their size was 7–10 cm in diameter. We considered a patient who has not received exogenous hormones for the previous 3 months. All patients gave their informed consent and the permission of the Human Investigation Committee was granted. After surgery, the myometrial and leiomyoma samples were collected in Hank’s Balanced Salt Solution (HBSS; Euroclone, Milan, Italy), and immediately transported to the laboratory. The samples were washed several times with Dulbecco phosphate-buffered saline (PBS; Invitrogen, Thermo Fisher Scientific, Carlsbad, CA) to remove excess blood. After cutting tissue into small pieces, sample were mixed in 0.1% collagenase type 8 (Serva Electrophoresis GmbH, Heidelburg, Germany) in serum-free Dulbecco Modified Eagle Medium (DMEM; Lonza, Walkersville, MD), and incubated at 37°C for 3–5 hr in water bath with manual shaking. After digestion, the cell suspensions were centrifuged at 1200 rpm for 10 min and the collagenase activity was inactivated with fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific). Finally, the cell pellet was dispersed in DMEM containing 10% FBS, 1% antibiotic (penicillin–streptomycin; Euroclone), 1% Fungizone (Amphotericin B; Lonza), and 1% glutamine (Gibco, Thermo Fisher Scientific) in plastic dishes at 37°C in 95% air, 5% CO2. The growth medium was changed after 24 or 48 hr to remove unattached cells and subsequently twice a week. The purity of cells was assessed by staining with a specific smooth muscle cells marker (anti-α-smooth muscle actin; Sigma-Aldrich, Milan, Italy). The lower passage number (P0-P4) of cells was used for experiments to avoid changes in phenotype and gene expression.
2.6 Image analysis assay for apoptosis detection
Apoptosis was measured using the Tali® apoptosis assay kit and propidium iodide (Invitrogen™, Life Technologies, Milan, Italy) as previously reported (Islam et al., 2017). Concisely, on the first day of the assay 1.5 × 105 myometrial and leiomyoma cells were seeded in a 6-well plate and left to adhere for 18–20 hr. The day after seeding, the cells were treated for 48 hr with the different strawberry methanolic extracts (250 μg/ml) and with Romina anthocyanin fraction (50 μg/ml). At the end of each treatment, cells were centrifuged and resuspended in 100 μl of annexin binding buffer (ABB). After that, Annexin V Alexa Fluor® (Thermo Fisher Scientific, Foster, CA) 488 was added, mixed well, and the solution was incubated in the dark at RT. Then, cells were centrifuged, resuspended in ABB, and propidium iodide was added, mixed well, and incubated in the dark at RT. Samples were analyzed using the Tali® Image-Based Cytometer (Invitrogen™, Life Technologies) and the percentage of apoptotic nuclei, dead cells, and live cells were determined on the basis of the corresponding fluorescence histogram compared with an untreated control. Each treatment was performed three times and the final results were reported as fold increase compared with control.
2.7 Morphometric measurement of ROS level
The determination of intracellular ROS levels was performed using the probe CellROX® Orange reagent (Invitrogen™, Life Technologies) according to the manufacturer’s instructions, as previously reported (Gasparrini et al., 2017). Briefly, on the first day of the assay 1.5 × 105 myometrial and leiomyoma cells were seeded in a 6-well plate and allowed to adhere for 16–18 hr. The cells were treated, the day after seeding, with the different strawberry methanolic extracts at 250 μg/ml and with Romina anthocyanin fraction at 50 μg/ml. At the end of each treatment (48 hr), the medium was removed and collected, then CellROX® Orange Reagent was directly added to the medium. Samples were incubated at 37°C, centrifuged once to remove medium and excess dye, and then resuspended in PBS solution. After labeling with CellROX® Orange Reagent, cells were analyzed with the Tali® Image-Based Cytometer (Invitrogen™, Life Technologies) collecting 20-fields per sample. Control cells were used to determine baseline levels of intracellular ROS and to set the fluorescence threshold for the Tali® instrument. Each treatment was carried out in three replicates and final results were expressed as fold increase compared with control.
2.8 Bioenergetics analysis
XF24 Extracellular Flux Analyzer (Agilent, Milan, Italy) was utilized to detect in real-time OCR and ECAR, representing oxidative phosphorylation and glycolysis, respectively, as recently described (Gasparrini et al., 2017; Islam et al., 2017). In brief 3 × 104 myometrial and leiomyoma cells were seeded for 16 hr in the XF-24 plate and treated for 48 hr with strawberry Alba cultivar methanolic extract at 250 μg/ml, Romina cultivar methanolic extract at 250 μg/ml and Romina anthocyanin fraction at 50 μg/ml. At the end of the treatment the medium was replaced with 450 ml/well of XF-24 running media, supplemented with sodium pyruvate, glutamine, and glucose, without serum and preincubated in the XF Prep Station incubator (Agilent, Milan, Italy) in the absence of CO2. The plate was then transferred to the XF24 Extracellular Flux Analyzer and for each analysis, injections of different compounds, that modulate mitochondrial respiration or glycolysis, were injected sequentially: for OCR, 55 μl of oligomycin (2.5 μg/ml), 61 μl of 2,4-DNP (1 mM), and 68 μl of antimycin A/rotenone (10 μM/1 μM), while for ECAR, 55 μl of rotenone (3 μM), 61 μl of glucose (10 mM), and 68 μl of 2-deoxyglucose (2-DG; 100 mM). Each treatment was carried out in three replicates and the final results were expressed as pmol of O2 consumed per 105 cells per minute (pmol O2·min−1·10−5cells); in case of OCR, and as mpH unit change per 105 cells per minute (mpH·min−1·10−5cells) for ECAR.
2.9 RNA extraction and real-time polymerase chain reaction (PCR)
Total RNA was extracted using TRIzol® reagent (Invitrogen, Thermo Fisher Scientific) according to the manufacturer’s instructions. Samples were digested with a ribonuclease-free deoxyribonuclease (Promega Corporation, Madison, WI), and the RNA was cleaned up and concentrated using ReliaPrep™ RNA Cell Miniprep System (Promega Corporation). We performed the reverse transcriptase (RT) using the high-capacity complementary DNA (cDNA) reverse transcriptase kit (Applied Biosystems, Thermo Fisher Scientific, Foster, CA) with 1 μg RNA, and we performed the TaqMan real-time PCR (Applied Biosystems, Thermo Fisher Scientific) for all the genes analyzed.
We used the TaqMan gene expression assays (Applied Biosystems, Thermo Fisher Scientific): collagen 1A1(Hs00164004_m1), fibronectin (Hs00365052_m1), versican (Hs00171642_m1), activin A (Hs00170103_m1), and housekeeping genes, hypoxanthine phosphoribosyltransferase 1 (HPRT1; Hs99999909_m1) and β-actin (ACTB; Hs99999903_m1), performing the following thermal cycle protocol (initial denaturation at 95°C for 20 s, followed by 40 cycles of 95°C for 1 s and 60°C for 20 s) using 100 ng cDNA in a final reaction volume of 10 μl.
The blank for each reaction, consisting of amplifications performed in the absences of the reverse transcriptase enzyme, was performed.
2.10 Western blot analysis
At the end of treatment, cells were rinsed with PBS and solubilized using TRIzol® reagent (Invitrogen, Thermo Fisher Scientific). Proteins were extracted following the manufacturer’s instructions. Soluble protein was quantified using a Bradford protein assay (Bio-Rad, Milan, Italy), and equal amounts of proteins were loaded onto 4–12% NuPAGE gels (Invitrogen, Thermo Fisher Scientific) and resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis under reducing conditions. Proteins were transferred to 0.2 μm nitrocellulose membranes in an X-cell II apparatus (Invitrogen, Thermo Fisher Scientific) according to the manufacturer’s instructions. Ponceau S solution (Euroclone) was used for the detection of proteins on nitrocellulose membranes. After blocking the membrane with 5% (wt/vol) nonfat milk powder in Tris-buffered saline with Tween 20 (TBST; 50 mm Tris–HCl, pH 7.4, 150 mm NaCl, 0.05% Tween 20), membranes were incubated overnight with a primary antibody: 1:30,000 dilution for monoclonal mouse anti-human fibronectin (Sigma-Aldrich), and 1:3,000 dilutions for monoclonal mouse tubulin (Sigma-Aldrich). On the next day, membranes were washed four times in 5% (wt/vol) nonfat milk powder in TBST and incubated with 1:10,000 horseradish peroxidase-linked anti-mouse (Amersham) against anti-human fibronectin and tubulin for 2 hr. Membranes were washed four times with 5% (wt/vol) nonfat milk powder in TBST, and immunoreactive proteins were visualized using the Clarity™ Western ECL substrate (Bio-Rad). Protein levels were measured using ImageJ 1.49n software (National Institutes of Health, http://imagej.nih.gov/ij).
2.11 Immunocytochemistry
The purity of cells was assessed by immunocytochemical staining with a specific smooth muscle cell marker, monoclonal mouse anti-α-smooth muscle actin (α-SMA; Sigma-Aldrich). All cells were strongly positive for α-SMA (data not shown).
Myometrial and leiomyoma cells were seeded in chamber tissue culture slides and allowed to divide. Cells were washed three times with PBS, treated with 0.2% Triton X-100 in PBS for 5 min, and washed three times with PBS. To inhibit endogenous peroxidase activity, cells were incubated for 10 min with 3% hydrogen peroxide in deionized water. Cells were washed three times with PBS, and to block nonspecific background, cells were incubated for 20 min at RT with normal horse serum, diluted 1:75 in 1% bovine serum albumin in PBS. Cells were then incubated with fibronectin (Sigma-Aldrich) monoclonal mouse antibody at 1:600 for 1 hr at RT. After washing with PBS, cells were incubated with biotinylated anti-mouse immunoglobulin G made in horse diluted 1:200. The peroxidase Avidin-Biotin Complex method was performed for 1 hr at RT using 3,3′-diaminobenzidine as chromogen. Sections were counterstained in Mayer’s hematoxylin, dehydrated, and mounted with Eukitt solution (Bio-Optica, Milan, Italy).
2.12 Data analysis
Statistical analyses were performed using GraphPad Prism version 6.01 for Windows (GraphPad, San Diego, CA). The data were analyzed using nonparametric “Kruskal-Wallis” one-way analysis of variance, followed by post hoc “Dunn” test for multiple comparisons. Results are expressed as mean ± SD. Differences were considered significant when p < 0.05.
3 RESULTS
3.1 Screening of five different strawberry cultivars according to antioxidant capacity, and phenolic and anthocyanin content
The properties of five different cultivars are reported in Table 1. The cultivar Alba showed the higher antioxidant capacity, with values of 21.07 mmol TE/kg FW, followed by Romina (p < 0.05); Clery showed the statistically lower values of 12.97 mmol TE/kg FW (Table 1).
| Strawberry cultivar | TAC (mmol TE/kg FW) |
TPH (mg GA/kg FW) | ACY (mg pel-3-glu/kg FW) |
|---|---|---|---|
| Alba | 21.07 ± 0.63a | 1,685.63 ± 46.34a | 384.96 ± 4.03b |
| Clery | 12.97 ± 0.82c | 962.84 ± 26.27c | 355.65 ± 1.43c |
| Portola | 13.46 ± 0.85c | 880.53 ± 44.96c,d | 349.19 ± 3.95c |
| Romina | 18.31 ± 1.03b | 1,213.25 ± 92.68b | 698.38 ± 21.93a |
| Tecla | 13.79 ± 0.53c | 794.93 ± 23.07d | 252.22 ± 4.82d |
- Note. ACY: total anthocyanin content of five different strawberry cultivars (Alba, Clery, Portola, Romina, and Tecla); FW: fresh weight; GA: gallic acid; pel-3-glu: pelargonidin-3-glucoside; TAC: total antioxidant capacity; TE: trolox equivalent; TPH: total phenolic content.
- Data are expressed as mean values ± standard deviation.
- Columns belonging to the same set of data with different superscript letters are significantly different (p < 0.05).
The TPH measured in the five strawberries showed that Alba contained the higher amount (1,685.63 mg GAE/kg FW), followed by Romina (1,213.25 mg GAE/kg FW). Clery and Portola showed intermediate values (962.84 and 880.53 mg GAE/kg FW, respectively), while the lower TPH was detected in Tecla, with a value of 794.93 mg GAE/kg FW (Table 1).
Regarding the ACY content, Romina showed the statistically higher value, with 698.38 mg pel-3-glu/kg FW, respectively, followed by Alba (384.96 mg pel-3-glu/kg FW). Finally, as for TPH, Tecla showed the lower ACY value, with a value of 252.22 mg pel-3-glu/kg FW (Table 1).
3.2 Screening of five different strawberry cultivars according to their ability to induce apoptosis and ROS in myometrial and leiomyoma cells
Primary myometrial and leiomyoma cells were treated with the different strawberry methanolic extracts for 48 hr and intracellular ROS levels and apoptosis rate were assessed by Tali Image-Based Cytometer (ThermoFisher Scientific, Milan, Italy). We found that in myometrial cells all strawberry samples decreased the ROS concentration, Romina being the most effective cultivar, followed by Alba (p < 0.05), Clery, Tecla, and Portola. Otherwise, in leiomyoma cells, Romina was the most efficient cultivar in increasing the levels of intracellular ROS, followed by Alba (p < 0.05), Clery, Tecla, and Portola (Figure 1).
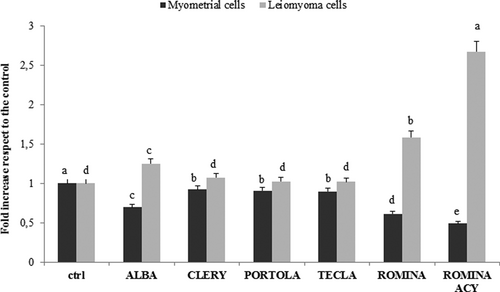
Effect of five cultivars of strawberry extracts on induction of ROS in myometrial and leiomyoma cells. Cell ROS level in myometrial and leiomyoma cells treated with five different strawberry cultivars (Alba, Clery, Portola, Romina, and Tecla) at 250 µg/ml for 48 hr. Data are expressed as mean values ± SD. Columns belonging to the same set of data with different superscript letters are significantly different (p < 0.05). ACY: total anthocyanin content; ROS: reactive oxygen species; SD: standard deviation
A similar trend was found for the apoptotic rate: in myometrial cells, strawberry treatments did not affect the cellular viability, whereas in leiomyoma cells Romina increased the number of apoptotic and dead cells, and decreased the number of live cells, followed by the cultivars Alba (p < 0.05), Clery, Tecla, and Portola (Figure 2).
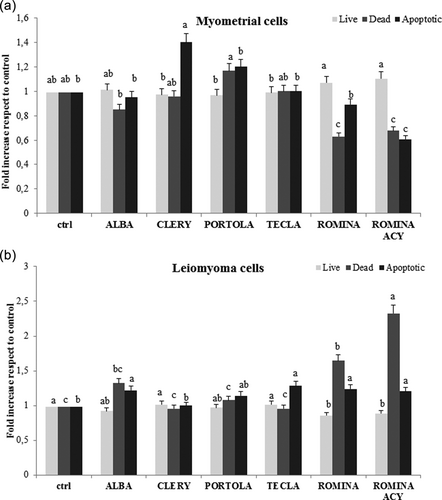
Effect of five cultivars of strawberry extracts on induction of apoptosis in myometrial and leiomyoma cells. (a) Live, dead, and apoptotic levels in myometrial cells treated with five different strawberry cultivars (Alba, Clery, Portola, Romina, and Tecla) at 250 µg/ml for 48 hr. Data are expressed as mean values ± SD. Columns belonging to the same set of data with different superscript letters are significantly different (p < 0.05). (b) Live, dead, and apoptotic levels in leiomyoma cells treated with five different strawberry cultivars (Alba, Clery, Portola, Romina, and Tecla) at 250 µg/ml for 48 hr. Data are expressed as mean values ± SD. Columns belonging to the same set of data with different superscript letters are significantly different (p < 0.05). ACY: total anthocyanin content; SD: standard deviation
According to our results, Romina was the most promising cultivar among those studied and for this reason, we isolated its anthocyanin fraction, evaluating its effects on intracellular ROS levels and apoptotic rate. As shown in Figure 1, in myometrial cells the treatment with the Romina anthocyanin fraction led to the lowest level of intracellular ROS (p < 0.05), while it induced the highest ROS concentration in leiomyoma cells.
Romina anthocyanin fraction was also very effective in promoting apoptotic death in leiomyoma cells (p < 0.05), while it did not affect cellular viability on myometrial cells (Figure 2).
3.3 Effect of Alba, Romina, and Romina anthocyanin fraction on metabolic status
Primary myometrial and leiomyoma cells were treated with Alba and Romina fruit methanolic extract (250 µg/ml) and Romina fruit anthocyanin fraction (50 µg/ml) for 48 hr and oxidative phosphorylation and glycolysis were measured with a Seahorse XF24 Extracellular Flux Analyzer (Agilent, Milan, Italy) was used.
As shown in Figure 3, both myometrial and leiomyoma cells presented an increase in OCR after strawberry treatment, though statistically not significant, while regarding the ECAR leiomyoma cells presented a significant decrease (p < 0.05) with respect to the controls. In particular, in leiomyoma cells, the effect was more evident for the anthocyanin fraction, followed by Romina and Alba cultivars.
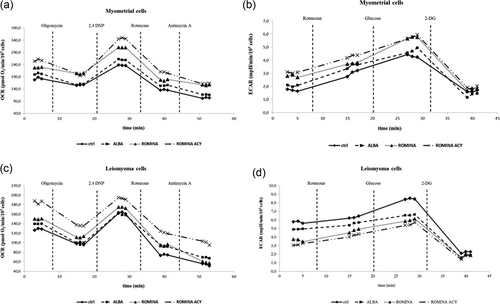
Metabolic characterization of myometrial and leiomyoma cells. (a) OCR values of myometrial cells treated with strawberry Alba cultivar extract at 250 μg/ml for 48 hr; Romina cultivar extract at 250 μg/ml for 48 hr; Romina anthocyanin fraction at 50 μg/ml for 48 hr. Data are expressed as mean values ± SD. (b) ECAR values of myometrial cells treated with strawberry Alba cultivar extract at 250 μg/ml for 48 hr; Romina cultivar extract at 250 μg/ml for 48 hr; Romina anthocyanin fraction at 50 μg/ml for 48 hr. Data are expressed as mean values ± SD. (c) OCR values of leiomyoma cells treated with strawberry Alba cultivar extract, at 250 μg/ml for 48 hr; Romina cultivar extract at 250 μg/ml for 48 hr; Romina anthocyanin fraction at 50 μg/ml for 48 hr. Data are expressed as mean values ± SD. (d) ECAR values of leiomyoma cells treated with strawberry Alba cultivar extract, at 250 μg/ml for 48 hr; Romina cultivar extract at 250 μg/ml for 48 hr; Romina anthocyanin fraction at 50 μg/ml for 48 hr. Data are expressed as mean values ± SD. ECAR: extracellular acidification rate; OCR: oxygen consumption rate
3.4 Effect of strawberry extracts on fibrotic genes
To determine the antifibrotic effect of strawberry extract, primary myometrial and leiomyoma cells were treated with strawberry methanolic extract in particular with cultivar Alba and Romina fruit extract (100 and 250 µg/ml) as well as Romina fruit anthocyanin enriched fraction (50 µg/ml) for 48 hr, and measured messenger RNA (mRNA) expression by real-time PCR. We found that extracts of strawberries, reduced fibronectin, collagen 1A1, activin A, and versican mRNA expression in both primary myometrial and leiomyoma cells compared with the untreated cells; however, Romina was more efficient than Alba, and Romina anthocyanin fraction had the highest response (Figure 4).
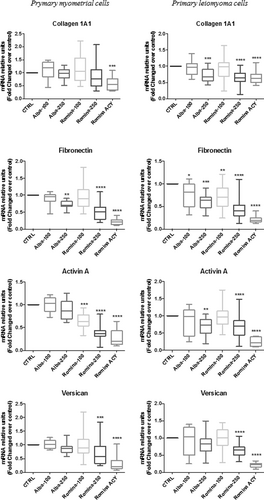
Effect of strawberry extracts on fibrotic genes expression in myometrial and leiomyoma cells. Results of real-time PCR for relative amounts of collagen 1A1, fibronectin, activin A, and versican in myometrial and leiomyoma cells in response to strawberry Alba cultivar extract at 100 μg/ml (Alba-100); Alba cultivar extract at 250 μg/ml (Alba-250); Romina cultivar extract at 100 μg/ml (Romina-100); Romina cultivar extract at 250 μg/ml (Romina-250); Romina anthocyanin fraction at 50 μg/ml (Romina-ACY). Data are expressed as mean ± SD; (n = 6 for RNA). *p < 0.05; **p < 0.01; ***p < 0.001. ACY: total anthocyanin content
3.5 Effect of strawberry extracts on fibronectin protein
Data from western blot analysis showed that protein expression of fibronectin was reduced with the treatment with strawberry, in particular, with Romina fruit methanolic extract and Romina fruit anthocyanin fraction, in primary leiomyoma and myometrial cells compared with untreated controls (Figure 5).
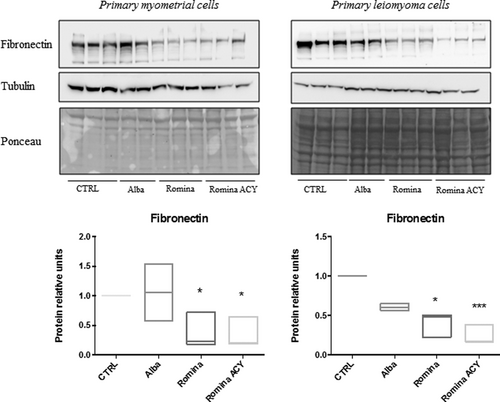
Effect of strawberry extracts on fibronectin protein expression in myometrial and leiomyoma cells. Western blotting showing the protein expression of fibronectin in myometrial and leiomyoma cells in response to strawberry Alba cultivar extract at 250 μg/ml (Alba-250); Romina cultivar extract at 250 μg/ml (Romina-250); Romina anthocyanin fraction at 50 μg/ml (Romina-ACY) or left untreated. The results showing the decrease expression of fibronectin after treatment with strawberry extract Romina and Romina anthocyanin fraction compared with negative control (untreated cells). Data are expressed as mean ± SD; (n = 3). *p < 0.05; **p < 0.01; ***p < 0.001. ACY: total anthocyanin content
Data from immunocytochemistry showed the decreased expression of fibronectin after treatment with Romina strawberry methanolic extract and Romina anthocyanin fraction compared with negative control (untreated cells; Figure 6).
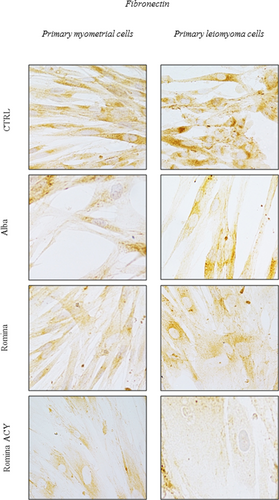
Effect of strawberry extracts on fibronectin protein expression in myometrial and leiomyoma cells. Immunocytochemistry showing the effect of the strawberry extract on fibronectin protein expression in myometrial and leiomyoma cells in response to strawberry Alba cultivar extract at 250 μg/ml (Alba-250); Romina cultivar extract at 250 μg/ml (Romina-250); Romina anthocyanin fraction at 50 μg/ml (Romina-ACY) or left untreated. The results showing decreased expression of fibronectin after treatment with the strawberry extract of Romina and Romina anthocyanin fraction compared with negative control (untreated cells). ACY: total anthocyanin content [Color figure can be viewed at wileyonlinelibrary.com]
4 DISCUSSION
Although uterine leiomyoma is a benign tumor, its associated symptoms, including menorrhagia, dysmenorrhea, chronic pelvic pain, infertility, recurrent abortion, premature delivery, and postpartum hemorrhage can substantially hamper women’s quality of life. Considering the burden of this tumor, medical treatments are still very limited. Therefore, our group focused on seeking preventive and/or therapeutic options (Ciarmela et al., 2011).
In our recent study, we showed the in vitro effect of the strawberry fruit of cultivar Alba on leiomyoma cells suggesting that strawberry extract would favor a method of prevention. In the current study, we were interested in evaluating the effects of other strawberry cultivars to identify the variability among the fruits of different cultivars and to identify the interaction within fruit composition and their effect on cell protection.
In recent years, the strawberry bioactive compounds with high antioxidant properties, such as polyphenol compounds, have received ample attention, especially for their potential health benefits on human health, stimulating a detailed phytochemical investigation to identify the phenolic compounds present in strawberries. The results obtained in this study outlined significant differences among cultivars in terms of TAC, TPH, and ACY contents; these differences were especially due to the genetic background that significantly affected all these parameters, giving that all the studied cultivars were grown on the same field and at the same time, with the same environmental conditions. From this point of view, Romina, that represents the results of the genetic improvement program of the Università Politecnica delle Marche, and Alba were the most promising cultivars among those studied, showing the higher content of TPH and ACY which explain the highest antioxidant capacity registered for these two cultivars.
The phytochemical content, in particular, the ACY concentration, and the total antioxidant capacity of strawberry are usually related to the health effects exerted by this fruit; the higher is the content the healthier is the strawberry. Our results showed that all the studied strawberry cultivars showed important biological effects on myometrial and leiomyoma cells, modulating the intracellular ROS concentration and cellular viability. In physiological condition, ROS exert a dual effect, depending on their concentration: at low concentration, they are essential for maintaining cellular homeostasis, acting as a redox messenger, whereas at high concentration they become harmful, damaging lipids, proteins, and DNA leading to cell death. Conversely, in cancer cells, high ROS levels, on one hand, play a role in tumor initiation but, on the other hand, they exert chemotherapeutic effects by inhibiting cancer growth and promoting apoptosis and cell death (Liou, & Storz, 2010). Our results confirm this dual behavior: in leiomyoma, Alba, Romina, and Romina anthocyanin fraction were the most effective in affecting cells, by increasing intracellular ROS levels and promoting apoptosis, with respect to the other strawberry cultivars; conversely, opposite effects were found in myometrial cells, by the decrease in ROS concentration and the improvement of cellular viability. Again, Alba, Romina, and Romina anthocyanin fraction were the most effective.
Oxidative phosphorylation (oxygen-dependent) and glycolysis (oxygen-independent) are common pathways to generate energy for maintaining normal cellular function. Although oxidative phosphorylation (38 adenosine triphosphate [ATP]) is the main process for ATP generation than glycolysis (2 ATP) in normal cells, such as myometrial cells, cancer or tumor cells, such as leiomyoma, can activate aerobic glycolysis (presence of adequate oxygen levels) as a main source of their energy supply that helps them to survive and subsequently their growth. This phenomenon is well known as the Warburg effect (Warburg, 1956). Therefore, interrupting energy balance through inhibition of tumor glycolysis represent a key therapeutic strategy.
In the current study, we found that both myometrial and leiomyoma cells showed no significant difference in OCR after strawberry treatment. On the other hand, strawberry extracts were able to decrease the glycolytic rate in leiomyoma cells. This observation suggests that strawberry extracts can be developed as a therapeutic option based on their ability to regulate glycolysis in leiomyoma cells.
ECM accumulation or fibrosis is the distinguishable feature of uterine leiomyoma. The common ECM components found in leiomyomas are collagens, fibronectin, and proteoglycan (versican; Islam et al., 2018) that exclusively make the fibroid harder and massive in size. The large size and rigid structures are believed to be associated with abnormal bleeding and pelvic pain. Therefore, inhibition of ECM accumulation could be a key therapeutic strategy to control fibroid growth as well as its associated major symptoms.
In the current study, we found that the Romina strawberry extract and its anthocyanin fraction were able to inhibit ECM components, including collagen 1A1, fibronectin, and versican expression in leiomyoma cells. This result suggests that Romina strawberry extract or its anthocyanin fraction can be developed as potential antifibrotic agents for the treatment and/or prevention of uterine leiomyomas.
The health benefits of strawberry consumption are justified by a plethora of studies (Afrin et al., 2016; Forbes-Hernandez et al., 2016; Giampieri et al., 2015). Strawberry exerts therapeutic effects against multiple diseases, including inflammatory disorders, obesity, cardiovascular diseases, neurodegenerative diseases, and cancers through combination effects of their bioactive compounds, such as anthocyanins, flavonols, flavanols, phenolic acids, and ellagitannins (Afrin et al., 2016; Giampieri et al., 2012, 2015). In the case of Romina fruit, its therapeutic effect against leiomyoma cells may be attributed due to its high levels of anthocyanin and flavonol content. The elevated levels of antioxidant capacity as well as good levels of vitamin C, and folic acids also supports its high health benefits (Forbes-Hernandez et al., 2017).
Overall, the in vitro data obtained, support the hypothesis that “powerful strawberries” can be used to develop therapeutics and preventive dietary or locally administrated agent for uterine fibroids. Powerful strawberries should have high antioxidant capacity (e.g., >15 mmol TE/kg FW), high TPH (e.g., >1000 mg GA/kg FW), high ACY (e.g., >350 mg pel-3-glu/kg FW). Potentially, all strawberries with specific characteristic are eligible to be used as a preventive and therapeutic agent for uterine leiomyoma. In addition to cultivar Alba, Romina possesses all the characteristics of “powerful strawberry” that support the use of this fruit for future preventive and/or agent for uterine leiomyoma. In conclusion, this study demonstrated that Romina and its anthocyanin fraction can induce apoptosis and increase intracellular ROS levels, as well as decrease glycolysis and fibrosis in leiomyoma cells. Interestingly, in myometrial cells, strawberry treatment increases the cell viability and decreases ROS concentrations. This result suggests that Romina or its anthocyanin fraction can be developed as a therapeutic and/or preventive agent for uterine leiomyomas.
ACKNOWLEDGEMENTS
This study was supported by the “Fondazione Cassa di Risparmio di Fabriano e Cupramontana” (to M. C. and P. C.). The Università Politecnica delle Marche has pending patent application (Italian patent application no. 102016000089627 which is suitable for extension abroad claiming priority).
CONFLICTS OF INTEREST
The authors have declared that there are no conflicts of interest.




