Amino acids stimulate glycyl-tRNA synthetase nuclear localization for mammalian target of rapamycin expression in bovine mammary epithelial cells
Abstract
Amino acids are required for the activation of mammalian target of rapamycin (mTOR) to increase cell growth, protein and lipid synthesis, and inhibit autophagy. However, the mechanism through which amino acids activate the mTOR signaling is still largely unknown. In our previous study, we discovered that glycyl-tRNA synthetase (GlyRS) is a key mediator of amino-acid-induced mTOR expression and activation in bovine mammary epithelial cells (BMECs). Here we show that amino acids stimulate GlyRS nuclear localization for mTOR expression in BMECs. Met stimulates GlyRS nuclear localization, and the nuclear GlyRS is cleaved into a C-terminus-containing truncated form. We prove that GlyRS has a bipartite nuclear leading sequences, and GlyRS is phosphorylated at Thr544 and Ser704 in the cytoplasm under the stimulation of amino acids (Met, Leu, and Lys). The nuclear GlyRS physically binds to nuclear factor kappa B1, triggers its phosphorylation, thereby enhancing mRNA expression of its target genes including mTOR, S6K1, and 4EBP1. We further demonstrate that GlyRS is required for the inhibition of autophagy by Met. Thus our work elucidates that amino acids trigger GlyRS phosphorylation and nuclear localization to enhance the mRNA expression of mTOR.
1 INTRODUCTION
Amino acids are required for the activation of the mammalian target of rapamycin (mTOR; Bröer & Bröer, 2017; Wolfson & Sabatini, 2017). mTOR is an evolutionarily conserved protein, a member of the phosphatidylinositol 3-kinase-related kinase superfamily. The active phosphorylated mTOR (p-mTOR) phosphorylates its downstream effectors ribosomal protein S6 kinase (S6K) and eukaryotic translation initiation factor 4E-binding protein (4EBP) to promote cell anabolic processes such as protein and lipid synthesis and cell growth, furthermore p-mTOR phosphorylates unc-51-like autophagy activating kinase 1 leads to the inhibition of autophagy (Efeyan, Comb, & Sabatini, 2015; Rabanal-Ruiz, Otten, & Korolchuk, 2017; D. Yang et al., 2018).
The mechanisms through which amino acids signal to mTOR have been partially elucidated. Amino acids including methionine (Met), leucine (Leu), lysine (Lys), glutamine, and arginine have been well documented as the main contributors to mTOR activation (Martin, Turner, Farrington, Player, & Lewis, 2017; Nie, He, Zhang, Zhang, & Ma, 2018; Zhang et al., 2018). These amino acids influence mTOR activity via cytoplasmic sensors, amino acid transporters, G-protein coupled receptors, v-ATPase on the lysosomal membrane, or Arf1 on the Golgi membrane (Wolfson & Sabatini, 2017; Zheng et al., 2016). These reports show the mechanism via which amino acids stimulate mTOR phosphorylation, but it is still largely unknown how amino acids stimulate the gene expression of mTOR for activation.
Aminoacyl-tRNA synthetases (aaRSs) have been found as a new kind of signaling proteins with essential noncanonical functions beyond their classic role to catalyze the aminoacylation reaction. During evolution, new domains and motifs were incorporated into aaRSs, and aaRSs have developed regulatory roles on cell proliferation, inflammation, amino acid sensing, tumorigenesis, and so forth (Fang & Guo, 2017; Yakobov, Debard, Fischer, Senger, & Becker, 2018; X. L. Yang, 2013). For example, leucyl-tRNA synthetase (LeuRS) is reported as an intracellular leucine sensor signaling to mTOR activation (Bonfils et al., 2012; Han et al., 2012). Recent reports also point that glycyl-tRNA synthetase (GlyRS) has noncanonical functions. GlyRS can be released by damaged cells and may act on cadherin-6 to activate multiple beneficial functions of mesenchymal stem cells (Park et al., 2018). Dominant mutations in GlyRS can cause one subtype of Charcot−Marie−Tooth neuropathy. GlyRS mutants aberrantly interact with neuropilin 1 and histone deacetylase 6, which leads to neurodegeneration and axonal transport deficits (Mo et al., 2018; Sleigh et al., 2017). GlyRS directly interacts with multiple components of the neddylation pathway and critically supports neddylation (Mo et al., 2016).
In our previous study, we found that Met triggers GlyRS nuclear localization (Lu et al., 2012), and we further demonstrate that GlyRS is a key mediator of amino-acid-induced milk protein and fat synthesis in bovine mammary epithelial cells (BMECs). GlyRS is required for Met-induced mRNA expression and activation of the mTOR-S6K1/4EBP1 signaling. Thus, we hypothesized that amino acids might stimulate GlyRS nuclear localization for mTOR expression. Here, we conducted structural and functional analyses to define this mechanism.
2 MATERIALS AND METHODS
2.1 Cell culture and treatments
Primary BMECs were separated from dairy cow mammary glands in midlactation period. Cells were maintained in dulbecco's modified Eagle medium: Nutrient Mixture F-12 (DMEM/F-12) medium (Invitrogen, Carlsbad, CA), supplemented with 10% fetal bovine serum (FBS; Gibco, Grand Island, NY) and 1× penicillin/streptomycin. The cell purity was identified by immunofluorescence observation of the expression of cytokeratin 18 (sc-51582; Santa Cruz, CA) and β-casein (CSN2; 251309; Abbiotec, San Diego, CA). To detect the effects of amino acids, cell medium was substituted with DMEM/F-12 medium without FBS 24 hr before adding additional amino acids, then cells were split into 24-well plates at densities of 1 × 105 cells·cm−2 and treated with Met, Leu or Lys (0.6 mM). Cells were harvested 24 hr after amino acid stimulation.
2.2 Nuclear and cytoplasmic fractionation
The cytoplasmic and nuclear fractions were extracted by using NE-PER Nuclear and Cytoplasmic Extraction Kit (P0028; Beyotime, Shanghai, China).
2.3 Constructs and transfection
All genes encoding related proteins or domains were amplified from BMECs total RNA by using PrimeScript Real-Time Polymerase Chain Reaction (RT-PCR) Kit (TaKaRa, Dalian, China). For overexpression test, GlyRS and nuclear factor kappa B1 (NFκB1) were individually subcloned into pGCMV/IRES/EGFP expression vector (GenePharma, Shanghai, China). For the subcellular localization of GlyRS and its three domains: the N-terminal (aa 1–116), CD (aa 117–610), ABD (aa 611–739), and CD-ABD (aa 117–739), they were subcloned into pCMV-N-Flag and pCMV-C-Flag (Beyotime). For fluorescence resonance energy transfer (FRET) analysis, GlyRS was subcloned into pEX-6(pGCMV/MCS/RFP/Neo expression vector (GenePharma), and NFκB1 was subcloned into pCMV-C-EGFP expression vector (GenePharma). All constructs were completely sequenced to insure their function. Cells were transfected with recombinant plasmids using Lipofectamine 2000 (11668027; Invitrogen).
2.4 Generation of GlyRS mutations
Point mutations in GlyRS were generated using a QuikChange Site-Directed Mutagenesis Kit (200519; Stratagene, La Jolla, CA), and the mutants were validated by DNA sequencing.
2.5 Small interfering RNAs and transfection
Small interfering RNAs (siRNAs) targeting GlyRS and NFκB1 and negative control RNA oligonucleotides (NC) were purchased from GenePharma. Transfection was performed using Lipofectamine 2000 according to the manufacturer’s protocol. The best effective siRNA from three siRNAs targeting different regions of GlyRS or NFκB1 mRNA was selected by transfection experiments and western blot analysis. Selected siRNA sequences: GlyRS-siRNA, 5′-AACACUCAUCACAAAUGGCTT-3′; NFκB1-siRNA, 5′-GCCACUACCAACAGCAGAUTT-3′.
2.6 Western blot analysis
Western blot analysis was used to detect the relative protein levels of GlyRS and other specific proteins or domains. Primary antibodies to GlyRS: GlyRS (sc-365311; Santa Cruz), a mouse monoclonal antibody raised against amino acids 440–732 mapping at the C-terminus of GlyRS. Other primary antibodies: S6K1(sc-230), p-S6K1 (Thr389) (sc11759), 4EBP1 (sc-6936), p-4EBP1 (Ser65/Thr70) (sc-12884), GCN2 (sc-66902), lamin B1 (sc-20682), β-tubulin (sc-9935), and β-actin (sc-47778), Santa Cruz; mTOR (#2983), p-mTOR (Ser2448) (#5536), Cell Signaling Technology, Danvers, MA; NFκB p105/p50 (ab32360), p-NFκB1 p105/p50 (S337) (ab28849), anti-phosphoSer/Thr/Tyr antibody (ab15556), p-GCN2 (Thr899) (ab75836), LC3B (ab48394), and Flag (ab49763), Abcam, Cambridge, MA; and CSN2 (251309), Abbiotec. Secondary antibodies: appropriate horseradish-peroxidase-conjugated anti-rabbit, goat or mouse IgG (ZSGB-BIO, Beijing, China). The reactive proteins were visualized using Super ECL plus (Applygen, Beijing, China).
2.7 Immunofluorescence observation
Immunofluorescence observation was performed to detect expression and subcellular localization of GlyRS and other specific proteins with corresponding primary antibodies. Briefly, cells were grown on coated glass coverslips and after treatments were fixed in 4% paraformaldehyde for 30 min and probed with primary antibodies and next detected with the appropriate Alexa Fluor 488 (bs-0368G-AF488, Bioss, Beijing, China) or Alexa Fluor 647 secondary antibody (bs-0369M-AF647; Bioss). Nucleus was stained with 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride or propidium iodide, then cells were visualized with a confocal microscopy (TCS-SP2 AOBS, Leica, Heidelberg, Germany). For the quantitative analysis of colocalization, we also used ImageJ colocalization finder plugin (US National Institutes of Health, MA). The indexes of colocalization were reported as mean ± standard error (SE) of the overlap coefficient (R)*100, more than 10 cells were imaged to obtain each colabeling. The ratio between green and red signals is ranged between 0.8 and 1.2.
2.8 Co-IP and mass spectrometry
Proteins were immunoprecipitated from cells using a Pierce Co-Immunoprecipitation (Co-IP) Kit (26149; Thermo Fisher Scientific, Rockford, IL), according to the manufacturer’s protocol, and antibodies against Flag or GlyRS were added to 200 mg of cell lysates. To detect phosphorylated proteins, phosphotate buffer saline (PBS) was substituted with tris buffered saline (TBS). The IP complexes along with the negative controls were subjected to matrix-assisted laser desorption/ionization-time of flight-time of flight (MALDI-TOF/TOF) peptide mass spectrometry analysis for identification.
2.9 Antibodies against p-GlyRS
The affinity-purified phospho-specific rabbit antibodies p-Thr544 and p-Ser704 were prepared by a company (AbMax Biotechnology, Beijing, China). The two synthetic phosphopeptides Thr544-P corresponding to 535CEKGEFTVE (pT) EGKT548 and Ser704-P corresponding to 695CRAEVSELP (pS) VVR707 were separately conjugated to ovalbumin (inject maleimide activated ovalbumin; 77126; Thermo Fisher Scientific, Shanghai, China) and used as the immunogens. The two phospho-specific antibodies were both used to recognize the phosphorylated form of GlyRS. The specificities of these antibodies were verified by western blot analysis of the reaction with the coprecipitates of wild type (WT) and Thr544/A or Ser704/A C-terminal Flag-tagged GlyRS mutants (Supporting Information Figure S1).
2.10 FRET analysis
FRET was performed according to a published method (Yu et al., 2014). Cells were cotransfected with the pEGFP-C1-NFκB1 FRET donor and pEX-7(pGCMV/RFP/MCS/Neo)-GlyRS FRET acceptor plasmids. Negative control cells were cotransfected with the pEGFP-C1 FRET donor and pEX-7 (pGCMV/RFP/MCS/Neo) FRET acceptor plasmids. Cells were imaged using a confocal microscope (Leica TCS-SP2 AOBS). FRET efficiency was determined by measuring acceptor photo-bleaching and calculated using Leica confocal software. Over 15 randomly chosen regions of interest were calculated. FRET efficiency =100*([donor postbleach – donor prebleach]/donor postbleach).
2.11 ChIP assays
Chromatin immunoprecipitation (ChIP) was performed using an EpiQuikTM ChIP Kit (#P-2002; Epigentek, Farmingdale, NY) with antibodies against p-NFκB1 (S337; ab28849; Abcam). An antibody against RNA polymerase was used as the positive control and IgG as the negative control. For ChIP-PCR, the oligonucleotide sequences of different primers to amplify p-NFκB1 target gene promoters were designed and sequenced, then the primers amplifying predicted sequence were used for next ChIP-qPCR. ChIP-qRT-PCR primers: mTOR, F: GAATGTTCAGACCCACGGCCAAT, R: CCAGAAACGCACGATAGGCTAAAGG; S6K1, F: TCAGTGGTATTGTAAAGCAGAAGC, R: ACTTGGCTCTTGACTATAGCTTTTT; 4EBP1, F: AAATAAAGTTCTTTTCTCTCCCCTC, R: CTTTGGGTTGGGTCCGTAG; GlyRS, F: GACCCCTTTCATTCCCTGG, R: TAAAATGGAAATGATGGAAAATGC; NFκB1, F: CAGCAGTACATGCATGGAATCTT, R: TTCAATGTAATACAATAAAATGGGG. For ChIP-qPCR, the data represent the percentage of input that is immunoprecipitated.
2.12 In vitro kinase assay
To obtain the nonphosphorylated NFκB1, NFκB1 were expressed in Escherichia coli as glutathione S-transferase (GST) fusion proteins and then purified on glutathione-sepharose beads. The purities of protein preparations were verified by SDS-PAGE. Protein concentration was determined by the method of Bradford. For affinity purification of nuclear p-GlyRS, the C-terminal Flag tagged GlyRS (GlyRS-C-Flag) was transfected into cells, and the nuclear fractions were extracted. The Flag M Purification Kit (CELLMM2; Sigma-Aldrich, St. Louis, MO) was used for the first step of purification. Then the Pierce Phosphoprotein Enrichment Kit (#90003, Thermo Fisher Scientific) was used for the second step of purification to remove nonphosphorylated proteins binding to GlyRS-C-Flag. The purities of protein preparations were verified by SDS-PAGE and western blot analysis with antiphosphoSer/Thr/Tyr antibody.
The in vitro kinase assays were carried out according to previously described (Yannay-Cohen et al., 2009). Nuclear GlyRS-C-Flag (20 ng) were incubated respectively with its substrate recombinant GST-NFκB1 (200 ng) in phosphorylation buffer (1 ml) for 15 min. Phosphorylation buffer: 20 mM Tris/HCl (pH 7.5), 25 mM β-glycerophosphate, 5 mM ethylene glycol tetraacetic acid (EGTA), 1 mM Na3VO4, 1 mM DL-dithiothreitol (DTT), 0.12 mM ATP, 2.5× protease and phosphatase inhibitor cocktail (P1045; Beyotime). The reaction mixture was then subjected to sodium dodecylsulphate polyacrylamide gel electrophoresis (SDS-PAGE) and western blot analysis.
2.13 Detection of autophagy
Cell lysates after treatments were collected to detect LC3-II/LC3-I ratio by western blot analysis with antibody against LC3B. Cells treated with rapamycin (8 mM) or autophagy activator trehalose (5 mM) for 24 hr to induce autophagy were used as controls. To observe the number of EGFP-LC3 puncta, cells were transfected with EGFP-LC3B plasmid 1 hr before treatment, and after treatment, accumulation of EGFP-LC3 in puncta was monitored using a confocal microscope (Leica TCS-SP2 AOBS). Quantitative analysis of EGFP-LC3 puncta was performed by using ImageJ. Ten cells were analyzed per sample.
2.14 Statistics
Data were expressed as mean ± SE of three independent experiments. One-way analysis of variance was used to determine significant differences of the means among groups. Values of p < 0.05, p < 0.01 were considered to be statistically significant.
3 RESULTS
3.1 Met stimulates GlyRS nuclear localization and GlyRS is cleaved into a C-terminus-containing truncated form in the nucleus
We first observed whether amino acids trigger GlyRS nuclear localization and detected the molecular form of GlyRS in the nucleus of BMECs. Our previous 2-DE and mass spectrometry data detected that the amino acid sequence of GlyRS in the nucleus might start from aa residue 73 and extend to the C-terminus, whereas the aa residues 1–72 were not detected (Figure 1a; Lu et al., 2012). Since the experiments were repeated for three times, it is more likely that nuclear GlyRS is truncated into a 73aa-C-terminus form and less likely that the exact 1–72 aa peptides always poorly fly in mass data. We thus transfected a C-terminal Flag-tagged GlyRS (GlyRS-C-Flag) vector into cells stimulated with Met to observe the molecular form of GlyRS in the nucleus. By western blot analysis, cytoplasmic GlyRS-C-Flag was detected as an approximately 80 kDa full length (FL) form, whereas only a truncated form (~70 kDa) was detected in the nucleus (Figure 1b). Met stimulation further detected the increase of cytoplasmic and nuclear localization of GlyRS (Figures 1b,c). To give further evidence and find the possible N-terminal form of GlyRS, we also transfected a N-terminal Flag-tagged GlyRS (Flag-N-GlyRS) vector into cells and observe the molecular form of GlyRS. Flag-N-GlyRS was detected as an approximately 80 kDa FL form in the cytoplasm and an approximately10 kDa form in the nucleus. The level of the approximately10 kDa form was dramatically lower than the FL (Figure 1d). These data demonstrate that Met trigger GlyRS nuclear localization and GlyRS in the nucleus is cleaved into two fragments, and the approximately 10 kDa form might be rapidly degraded in the nucleus.
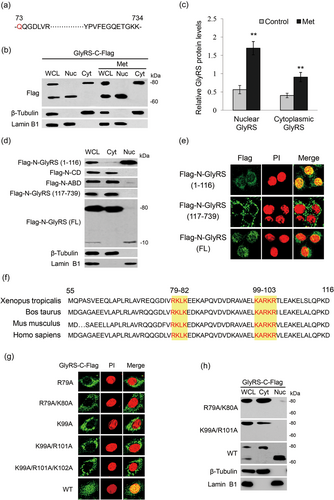
GlyRS translocates to nucleus with a bipartite NLS and is cleaved into two fragments in the nucleus. (a) The sequence of GlyRS in the nucleus of BMECs was shown, which was detected by 2-DE and mass spectrometry analysis in our previous study (Lu et al., 2012). Nuclear GlyRS started from aa residue 73 (red letter) and extended to the C-terminus. (b) Cells were transfected with a C-Flag-tagged GlyRS vector and stimulated by Met (0.6 mM) for 24 hr. The cytoplasmic and nuclear fractions were extracted by an extraction kit. The protein forms of GlyRS-C-Flag in whole cell lysate, cytoplasm and nucleus were analyzed by western blot analysis. β-tubulin and lamin B1 were used to show the purities of cytoplasm and nucleus. Figure shows one representative result from three independent experiments. (c) Quantification of cytoplasmic and nuclear protein levels (GlyRS-C-Flag/β-actin) from the western blots in (b) by gray scale scan. (d) Cells were transfected with indicated N-Flag-tagged full length or domains of GlyRS. Western blot analysis was performed to analyze the subcellular localization of these protein or domains. (e) Cells were treated as in (d), the nuclear localization of indicated protein or domains of GlyRS were observed by immunofluorescence analysis. Flag (green), nuclei were stained with PI (red). (f) Amino acid sequence comparison of the N-terminal WHEP domains (55–116 aa) of GlyRS isoforms. Conserved amino acids in the possible NLSs are highlighted in yellow. (g,h) Cells were transfected with indicated C-Flag-tagged GlyRS NLS mutants. Wild type GlyRS was used as a control. Immunofluorescence observation (g) and western blot analysis. (h) were performed to observe the nuclear localization of these mutants. BMECs: bovine mammary epithelial cells; GlyRS: glycyl-tRNA synthetase; NLS: nuclear localization signals; PI: propidium iodide [Color figure can be viewed at wileyonlinelibrary.com]
3.2 A bipartite type of NLS directs GlyRS nuclear localization
We then asked whether GlyRS contains certain nuclear localization signals (NLSs). To find possible NLSs in GlyRS, we first identified which domain of GlyRS is associated with GlyRS nuclear localization. GlyRS contains the N-terminal extension sequence (aa 1–116) containing a WHEP domain (aa 55–116), the catalytic domain (CD; aa 117–610), and anticodon binding domain (ABD; aa 611–739; Xie, Nangle, Zhang, Schimmel, & Yang, 2007). For this purpose, N-terminal flag-tagged vectors expressing these distinct domains were individually transfected into cells. Western blot analysis (Figure 1d) and immunofluorescence analysis (Figure 1e) detected Flag-N-GlyRS (aa 1–116) and Flag-N-GlyRS (FL) in the nucleus, whereas Flag-N-CD, Flag-N-ABD, and Flag-N-GlyRS (aa 117–739) were located to the cytoplasm. These data suggest that the N-terminal extension sequence is involved in GlyRS nuclear translocation.
We next tested whether the N-terminal extension contains certain NLSs. Bioinformatics analysis identified two potential NLSs in the WHEP domain as follows: RKLK (aa 79–82) and KARKR (aa 99–103; Figure 1f). We then introduced point mutations in these NLSs into GlyRS-C-Flag, and found by immunofluorescence observation that only the WT entered the nucleus but not the mutant proteins R79A, R79A/K80A, K99A, K99A/R101A, or K99A/R101A/K102A (Figure 1g). To give further evidence, we also observed by western blot analysis that only the WT entered the nucleus but not R79A/K80A and K99A/R101A (Figure 1h). Together, these results reveal that GlyRS contains a bipartite type of NLS.
3.3 GlyRS phosphorylation is required for its nuclear translocation
Since some aaRSs are phosphorylated before releasing from cytoplasm to enter nucleus (Arif, Jia, Halawani, & Fox, 2017; Ofir-Birin et al., 2013), and we found that Met stimulates GlyRS nuclear localization by comparative phosphoproteomics analysis (Lu et al., 2012), we speculated that GlyRS might also be phosphorylated before entering nucleus. To demonstrate this hypothesis, we transfected Flag-N-GlyRS and GlyRS-C-Flag into BMECs, and analyzed the molecular modifications of GlyRS in the nucleus by Co-IP and mass spectrometry analysis. We found that GlyRS in the nucleus was phosphorylated at Thr544 and Ser704 (Figure 2a, Supporting Information Tables S1 and S2). Inspection of Bos taurus GlyRS protein sequences showed that both Thr544 and Ser704 are conserved in mammals (Figure 2b).
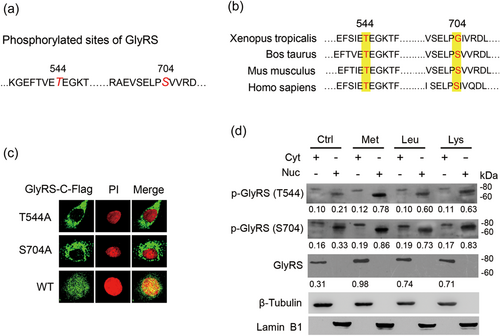
Amino acid-induced GlyRS nuclear localization requires phosphorylation at two sites in the cytoplasm. (a) Cells were transfected with a N-Flag-tagged or a C-Flag-tagged GlyRS expression vector. The cytoplasmic and nuclear fractions were extracted by an extraction kit. The phosphorylated sites (large, red italicized letters) were determined by using Co-IP of nuclear GlyRS with Flag antibody and mass spectrometry analysis, also see in Supporting Information Tables S1 and S2. (b) Comparison of the sequences near the two phosphorylation sites (highlighted in yellow) of GlyRS isoforms. Both Thr544 and Ser704 are conserved in mammals. (c) Cells were transfected with C-Flag-tagged GlyRS with mutations (Thr544/A and Ser704/A) in the phosphorylation sites. Immunofluorescence observation were performed to observe the nuclear localization of these mutants. Flag (green), nuclei were stained with PI (red). (d) Cells were treated with Met, Leu, or Lys (0.6 mM) for 24 hr. The cytoplasmic and nuclear fractions were extracted by an extraction kit. Western blot analysis was performed to detect the levels of indicated proteins in the cytoplasm and nucleus of BMECs. The specificities of the antibodies against p-GlyRS(Thr544) and p-GlyRS(Ser704) were verified by western blot analysis as shown in Supporting Information Figure S1. The ratio of indicated proteins/β-actin were shown in the bottom of each band. BMECs: bovine mammary epithelial cells; Co-IP: Co-Immunoprecipitation; GlyRS: glycyl-tRNA synthetase; PI: propidium iodide [Color figure can be viewed at wileyonlinelibrary.com]
We then developed two antibodies against the phosphorylated Thr544 and Ser704 sites, respectively, and observed whether these phosphorylation are required for the nuclear localization of GlyRS and the effects of amino acids on these phosphorylation. The specificities of these antibodies were verified as shown in Supporting Information Figure S1. We observed that the localization of GlyRS-C-Flag with mutated Thr544 or Ser704 to Ala were both undetectable in the nucleus, in contrast to WT (Figure 2c). We next observed that Met, Leu, and Lys all upregulated the protein levels of GlyRS (~80 kDa FL) in the cytoplasm and p-GlyRS (~70 kDa) in the nucleus. There were rather fewer 80 kDa p-GlyRS in the cytoplasm, suggesting that GlyRS might rapidly translocate to the nucleus after it is phosphorylated in the cytoplasm (Figure 2d). These observations suggest that GlyRS is phosphorylated in the cytoplasm in response to amino acid stimulation, which is needed for its nuclear translocation.
3.4 GlyRS triggers NFκB1 activation and downstream mTOR gene expression
We further speculated that nuclear p-GlyRS might interact with some nuclear factors to trigger gene expression of the mTOR signaling pathway. We first performed an interactome study to search the interaction partners of nuclear GlyRS. We transfected BMECs with GlyRS-C-Flag or Flag-N-GlyRS vector and conducted Co-IP and mass spectrometry analysis, and identified 23 proteins interacting with GlyRS-C-Flag and 5 proteins interacting with Flag-N-Flag in the nucleus (Supporting Information Tables S1 and S2). Among the proteins binding to nuclear GlyRS, we selected NFκB1 for further experiments. NF-κB1 is a key transcription factor that binds to hundreds of gene promoters to orchestrate diverse gene expression programs that differ among cell types. We also found that NFκB1 binds to the gene promoters of mTOR for its expression and is required for activation of the mTOR signaling (Huang et al., 2017).
We next asked whether GlyRS mediates amino acids signaling to NFκB1 activation and downstream mTOR gene expression. We found that GlyRS inhibition completely abrogated the increase of the levels of NFκB1 and p-NFκB1 induced by Met (Figure 3a). To further reveal whether GlyRS affects NFκB1-mediated transcriptional activation of the mTOR signaling pathway, we performed ChIP-qPCR to determine whether GlyRS affects the binding of p-NFκB1 to its target genes involved in this pathway. We first predicted and verified that at least one consensus κB binding site (GGGRNNYYCC) exists in the gene promoters of mTOR, S6K1, 4EBP1, GlyRS, and NFκB1 itself by ChiP-PCR assays that used antibody against p-NFκB1. ChIP-qPCR assays further detected that the enrichments of these binding sequences in cells transfected with GlyRS siRNA were all dramatically lower compared with the control (Figure 3b) whereas were all significantly higher in cells overexpressing GlyRS (Figure 3c). We also observed that GlyRS also triggered its own and NFκB1 gene expressions (Figures 3b,c) implying a positive feedback loop in the regulation of GlyRS-NFκB1 signaling. We further found that NFκB1 inhibition largely reduced the phosphorylation of mTOR, S6K1, and 4EBP1 stimulated by GlyRS overexpression (Figure 3d). We also determined whether GlyRS phosphorylation and nuclear localization is needed for NFκB1 and mTOR activation. We found that only the WT but not Thr544/A or Ser704/A triggered NFκB1 and mTOR phosphorylation (Figure 3e), and only the WT but not R79A/K80A and K99A/R101A stimulated the phosphorylation of mTOR (Figure 3f). We observed that no effect was seen on mTOR protein levels in cells overexpressing GlyRS, through the phosphorylation of mTOR were significantly affected. Our data are consistent with previous reports that mRNA expression and phosphorylation of mTOR can be affected by amino acids with the protein levels of mTOR remained stable (Han et al., 2012; Zhang et al., 2018; Zhen et al., 2018; Zoncu et al., 2011).
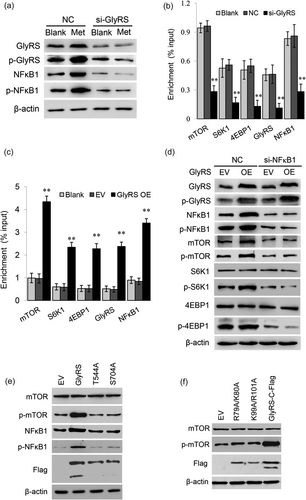
GlyRS regulates NFκB1 activation and its target gene expression. (a) Co-IP and mass spectrometry detected that NFκB1 might be a possible GlyRS-interacting protein (Supporting Information Tables S1 and S2), and the effects of GlyRS on NFκB1 activation were observed. The indicated protein levels were detected by western blot analysis in cells transfected with GlyRS siRNA and stimulated by Met for 24 hr. The antibody against p-GlyRS(Ser704) was used to detect the protein levels of phosphorylated GlyRS. (b,c) ChIP-qPCR analysis of the binding of p-NFκB1 to its target genes in cells transfected with a GlyRS siRNA (b) or an overexpression vector (c). Data represent the mean ± SE from three independent experiments, **p < 0.01, compared with control groups (blank, NC or EV). (d) Western blot analysis of the indicated protein levels in cells cotransfected with GlyRS overexpression vector and NFκB1 siRNA. The antibody against p-GlyRS(Ser704) was used to detect the protein levels of phosphorylated GlyRS. (e) Cells were transfected with an overexpression vector of GlyRS with mutated Thr544 and Ser704 to Ala. Indicated protein levels were detected by western blot analysis. The wild type of GlyRS showed two bands corresponding the the cytoplasmic form and nuclear form of GlyRS, whereas the two mutants only detected one protein band corresponding to the cytoplasmic form. (f) Cells were transfected with an overexpression vector of GlyRS with mutations in the NLSs. Indicated protein levels were detected by western blot analysis. The wild type of GlyRS showed two bands whereas the two mutants only detected one cytoplasmic form. ChIP-qPCR: Chromatin immunoprecipitation quantitative polymerase chain reaction; Co-IP: Co-Immunoprecipitation; GlyRS: glycyl tRNA synthetase; NC: negative control; EV: empty vector; NFκB1: nuclear factor kappa B1; SE: standard error; siRNA: small interfering RNAs
Taken together, these above results reveal that nuclear GlyRS is a positive regulator of NFκB1 activation and downstream mTOR gene expression.
3.5 GlyRS physically binds to NFκB1 and increase NFκB1 phosphorylation
We then detected the interaction of GlyRS with NFκB1 and explored the molecular mechanism via which GlyRS promotes NFκB1 activation. We analyzed the coprecipitates of NFκB1 and found that NFκB1 bound to p-GlyRS but not the nonphosphorylated GlyRS (Figure 4a). To observe where p-GlyRS interacts with NFκB1, we further transfected the GlyRS-C-Flag vector into cells and separated the cytoplasmic and nuclear fractions. Co-IP of Flag detected that NFκB1 bound to GlyRS in the nucleus, whereas p-NFκB1 did not bind to any form of GlyRS in BMECs (Figure 4b). We further validated that NFκB1 bound to the phosphorylated form of GlyRS in the nucleus (Figure 4c). Met enhanced the binding of GlyRS to NFκB1 by the observation of the colocalization of GlyRS-C-Flag with NFκB1 after Met stimulation (Figure 4d). We then validated the physical binding of GlyRS to NFκB1 by using the FRET approach. The mean FRET efficiency values of the interaction of these two proteins were dramatically higher compared with those of the controls (Figure 4e), demonstrating that the physical interaction between GlyRS and NFκB1. These above data reveal that p-GlyRS directly interacts with NFκB1 in the nucleus and amino acids enhance this interaction.
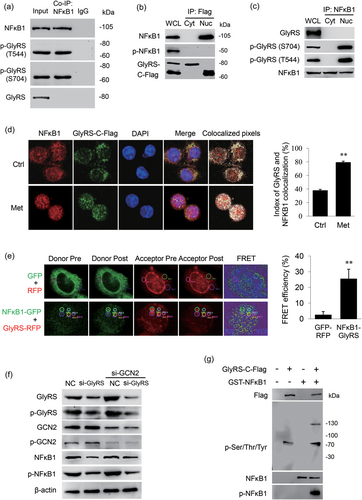
Nuclear p-GlyRS directly binds to NFκB1 and increases NFκB1 phosphorylation. (a) Western blot analysis of GlyRS and p-GlyRS coimmunoprecipitated with endogenous NFκB1. (b) BMECs were transfected with a C-Flag-tagged GlyRS vector. The cytoplasmic and nuclear fractions were extracted by using an extraction kit. NFκB1 and p-NFκB1 coimmunoprecipitated with cytoplasmic or nuclear GlyRS-C-Flag were detected by western blot analysis. (c) The cytoplasmic and nuclear fractions of cells were extracted by using an extraction kit. GlyRS and p-GlyRS coimmunoprecipitated with endogenous NFκB1 were detected by western blot analysis. (d) Cells were transfected with a C-Flag-tagged GlyRS vector and stimulated with Met (0.6 mM) for 24 hr. The colocalizations of GlyRS-C-Flag with NFκB1 in BMECs were detected by immunofluorescence observation. NFκB1 (red), GlyRS-C-Flag (green), nuclei were stained with DAPI (blue), and colocalization (white color) was visualized by using the ImageJ colocalization finder plugin. The index of colocalization was calculated by using ImageJ, more than 10 cells were imaged to obtain each colabeling. Data represent the mean ± SE of the overlap coefficient (R)*100, **p < 0.01. (e) FRET was measured by an acceptor photo-bleaching method. Cells were cotransfected with the pEGFP-C1-NFκB1 and pEX-7(pGCMV/RFP/MCS/Neo)-GlyRS plasmids. Cells cotransfected with the pEGFP-C1 and pEX-7 (pGCMV/RFP/MCS/Neo) plasmids were used as the negative control. Cells were imaged using a confocal microscope, NFκB1 (green), GlyRS (red), and FRET observation and FRET efficiencies between NFκB1 and GlyRS in BMECs were assessed. Over 15 randomly chosen regions of interest (ROI) were calculated. Data represent the mean ± SE, **p < 0.01, compared with the control group. (f) Western blot analysis of the indicated protein levels in BMECs cotransfected with GlyRS siRNA and GCN2 siRNA. Negative control RNA (NC) was transfected into cells as a control. The antibody against p-GlyRS(Ser704) was used to detect the protein levels of phosphorylated GlyRS. (g) The C-terminal Flag-tagged GlyRS vector was transfected into cells, then the nuclear fractions were separated, and GlyRS-C-Flag was purified by Flag affinity purification and subsequent phosphoprotein enrichment approaches. NFκB1 were expressed in Escherichia coli as glutathione S-transferase (GST) fusion proteins and purified on glutathione-sepharose beads. In vitro kinase assay was performed to detect the phosphorylation of GST-NFκB1 by purified nuclear GlyRS-C-Flag. BMECs: bovine mammary epithelial cells; DAPI: 4'-6-diamidino-2-phenylindole; FRET: fluorescence resonance energy transfer; GlyRS: glycyl tRNA synthetase; NFκB1: nuclear factor kappa B1; SE: standard error; siRNA: small interfering RNAs [Color figure can be viewed at wileyonlinelibrary.com]
We further asked the mechanism by which GlyRS triggers NFκB1 phosphorylation. General GCN2/eukaryotic translation initiation factor 2α (eIF2α) is a common pathway to regulate gene expression associated with amino acid supply (Chantranupong, Wolfson, & Sabatini, 2015). It is possible that p-GlyRS might stimulate aminoacylation of tRNAGly in the nucleus and thus inhibit the GCN2/eIF2α pathway to upregulate NFκB1 gene expression, thereby facilitating its phosphorylation. We observed that GlyRS knockdown did not significantly affect GCN2 phosphorylation, furthermore GCN2 knockdown almost restored the level of NFκB1 decreased by GlyRS inhibition, but the decreased p-NFκB1 level by GlyRS inhibition was not changed (Figure 4f), implying that GlyRS-triggered NFκB1 phosphorylation is independent of the GCN2 pathway and NFκB1 gene expression.
The facts that nuclear p-GlyRS physically bound to and triggered NFκB1 phosphorylation, and this activation was independent of NFκB1 gene expression, prompted us to suspect that nuclear p-GlyRS might directly increase NFκB1 phosphorylation. We next performed in vitro kinase assay to identify the activity of p-GlyRS on NFκB1 phosphorylation. We transfected GlyRS-C-Flag vector into BMEC, then separated the nuclear fractions and purified GlyRS-C-Flag by Flag affinity purification and subsequent phosphoprotein enrichment approaches. The purified p-GlyRS was capable of phosphorylating the recombinant protein GST-NFκB1 expressed and purified from E. coli (Figure 4g). These data reveal that nuclear GlyRS directly increases NFκB1 phosphorylation.
3.6 GlyRS is required for amino acids to inhibit autophagy
Since it is well established that amino acids activate mTOR leading to the inhibition of autophagy, and autophagy plays important role in milk synthesis and proliferation of BMECs (Gajewska, Sobolewska, Kozlowski, & Motyl, 2008; Xia et al., 2016), we further observed the effects of GlyRS on amino acid-inhibited autophagy in BMECs. By western blot analysis and immunofluorescence observation, we observed that autophagy was activated in GlyRS-knockdown or rapamycin-treated cells, as detected by the increase of LC3-II/LC3-I ratio (Figure 5a) and the number of GFP-LC3-II puncta compared with control cells (Figures 5b,c). We further observed that Met largely eliminated trehalose-induced cell autophagy, and GlyRS knockdown significantly inhibited this effect (Figures 5c−d). These above results reveal that GlyRS is required for the inhibition of autophagy by amino acids.
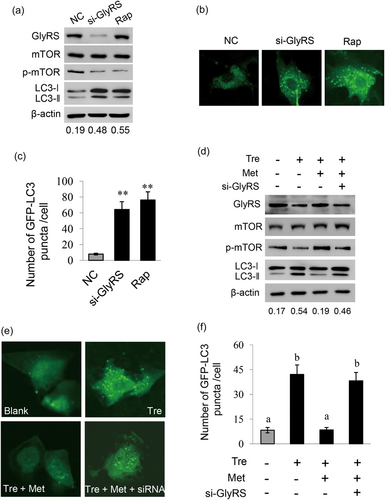
GlyRS is required for the inhibition of autophagy by Met. (a) Cells were transfected with GlyRS siRNA for 24 hr, cells transfected with NC were used as a negative control, and cells treated with rapamycin (8 mM) for 24 hr were used as a positive control. LC3-I/II were detected by western blot analysis with antibody against LC3B. The ratio of LC3-II/LC3-I shown in the bottom of the panel represents the level of autophagy. (b) Cells were transfected with EGFP-LC3B plasmid 1 hr before treatment as in (a). Accumulation of EGFP-LC3 in puncta was monitored using a confocal microscope. Ten cells were analyzed per sample. (c) Quantitative analysis of EGFP-LC3 puncta from (b) was performed by using ImageJ. Ten cells were analyzed per sample, and LC3 puncta per cell were calculated. (d) Cells were transfected with GlyRS siRNA and treated with trehalose (5 mM) plus Met (0.6 mM) for 24 hr. LC3-I/II were detected by western blot analysis and the ratio of LC3-II/LC3-I shown in the bottom of the panel represents the level of autophagy. (e) Cells were transfected with EGFP-LC3B plasmid 1 hr before treatment as in (d). Accumulation of EGFP-LC3 in puncta was monitored using a confocal microscope. Ten cells were analyzed per sample. (f) Quantitative analysis of EGFP-LC3 puncta from (e) was performed by using ImageJ. Ten cells were analyzed per sample, and LC3 puncta per cell were calculated. Data represent the mean ± SE from three independent experiments. Different superscripted lowercase letters indicate significant differences from each other (p < 0.05). * and ** indicate p < 0.05 and p < 0.01, respectively, compared with control group. GlyRS: glycyl tRNA synthetase; SE: standard error; siRNA: small interfering RNA [Color figure can be viewed at wileyonlinelibrary.com]
4 DISCUSSION
Our data reveal that amino acids trigger GlyRS nuclear localization leading to increased mRNA expression of mTOR in BMECs. GlyRS has a bipartite type of NLS in its WHEP domain, and amino acids (Met, Leu, and Lys) trigger GlyRS phosphorylation at Thr544 and Ser704 in the cytoplasm and subsequent nuclear translocation. We further show that nuclear p-GlyRS physically interacts with and stimulates NFκB1 phosphorylation, thereby stimulating the expression of its downstream gene mTOR and inhibiting autophagy.
We reveal that GlyRS translocates to nucleus by the direction of a bipartite type of NLS in its WHEP domain. The WHEP domain was appended to specific aaRSs with the tree of life ascended from lower to higher eukaryotes (X. L. Yang, 2013). GlyRS harbors a bipartite type of NLS in its WHEP domain, suggesting that this domain acquired by GlyRS during evolution aims for its nuclear localization. Other aaRSs also have NLS for nuclear localization. A unique motif appended to SerRS in species with closed circulatory systems harbors a robust NLS (Xu et al., 2012). Under stress conditions, TyrRS discloses its NLS and translocates to the nucleus to induce survival genes (Fu, Xu, Shi, Wei, & Yang, 2012). We further showed that the NLS in GlyRS is needed for its regulatory role on the activation of mTOR. Our results provide new evidence of aaRSs with NLSs to regulate gene expression.
We identify GlyRS phosphorylation at Thr544 and Ser704, and found that Met, Leu, and Lys all enhance GlyRS phosphorylation. Both Thr544A and Ser704A GlyRS mutants prevented GlyRS entering nucleus and abolished its regulatory function on NFκB1 and mTOR. These data reveal that GlyRS phosphorylation at Thr544 and Ser704 in the cytoplasm is a prerequisite for GlyRS to enter nucleus, revealing that both the NLS and the phosphorylation events are necessary for GlyRS nuclear translocation and regulatory roles. Other evidence have shown that phosphorylation of aaRSs are associated with their subcellular localization and noncanonical roles. LysRS phosphorylation creates steric clashes at the domain interface and disrupts its binding grooves for p38/AIMP2, releasing LysRS for nuclear translocation (Ofir-Birin et al., 2013). Two-site phosphorylation of Glu-ProRS dictates transcript-selective translational control of gene expression (Kaspy et al., 2013). These reports, together with our results, support that phosphorylation might be essential for performance of diverse signaling functions of aaRSs.
In our experiments, no amino acid starvation but only serum starvation is applied to cells. This is different from a number of studies looking at mTOR regulation, where cells are starved for amino acids before adding back of amino acids. Actually, we aim to study the regulatory roles of amino acids, that is, amino acids are not only substrates of protein translation, but also serves as signaling molecules. Thus, we build the in vitro cell culture model that additional amino acids (Met, Leu, or Lys) adding into the culture medium at the selected concentrations (0.6 mM) can significantly promote milk synthesis and activation of the mTOR signaling. The in vitro cell culture models in some literatures are similar to ours (Chen et al., 2018; Dong et al., 2018; Guo et al., 2018; Li et al., 2017).
Normally, NFκB1 protein is maintained in the cytoplasm by binding to IκBα. In our previous study, we found that methionine and estrogen trigger NFκB1 to dissociate from IκBα in the cytoplasm and enter nucleus via the PI3K signaling in BMECs (Huang et al., 2017). Our data further reveal that amino acids stimulate GlyRS phosphorylation and nuclear localization leading to nuclear NFκB1 activation. These data support the idea that amino acids trigger the nuclear localization of a number of signaling molecules and transcription factors and activate these transcription factors in the nucleus for gene transcription to stimulate cell anabolism.
We show that nuclear p-GlyRS directly interacts with and increases NFκB1 phosphorylation, leading to NFκB1-targeted mRNA expression of the mTOR-S6K1/4EBP1 signaling. It is intriguing that nuclear p-GlyRS might directly activate NFκB1, which suggests that GlyRS might be associated with kinase activity. To date, no aaRS has been identified as a protein kinase. One previous report suggests that an aaRS, bovine TrpRS, is associated with protein kinase activity (Paley, 1997). Furthermore, the coprecipitated proteins of mammalian threonyl-, tyrosyl- and arginyl-tRNA synthetases were found to be associated with protein kinase activity (Berg, 1993). A recent report also give further evidence that LeuRS in a Leu-dependent manner activates Vps34 kinase (Yoon et al., 2016). So far little is known whether some aaRSs has the structure like the well conserved structural aspects of kinases. Some studies have provided important hints on this. The aaRS class II sequence motifs in the protein kinase general control nonderepressible 2 (GCN2) regulate its phosphorylation of eukaryotic initiation factor 2α (eIF-2α; Lageix, Zhang, Rothenburg, & Hinnebusch, 2015; Zhu, Sobolev, & Wek, 1996). Sequence analysis revealed the presence in TrpRS of the major 11 conserved putative protein kinase motifs with unusual positions (Paley, 1997). Analysis of the crystal structures of GlyRS revealed that GlyRS has two ATP binding sites: one site binds to ATP and glycine, another site only binds to ATP, suggesting its role associated with ATP hydrolysis (Tan, Zhou, Zhang, Anderson, & Joachimiak, 2012). Further studies are needed to elucidate the structure basis of GlyRS associated with protein kinase activity.
In this study, we observe that amino acids trigger GlyRS nuclear localization to increase mTOR expression and GlyRS is needed for the inhibition of autophagy by amino acids. mTOR has been well established the main gateway to autophagy, it senses fluctuations in extracellular and intracellular amino acids and other stimuli to modulate cell growth and autophagy (Meijer, Lorin, Blommaart, & Codogno, 2015; Munson & Ganley, 2015; Rabanal-Ruiz et al., 2017). There are numerous reports on the signaling pathways sensing amino acids by mTOR, but it is still largely unknown how the gene expression of mTOR is stimulated by amino acids. Our data demonstrate that, in response to amino acid stimulation, GlyRS enters nucleus and binds to and activates NFκB1, leading to increased mTOR gene expression thereby facilitating its phosphorylation and inhibition of autophagy.
Our results point that amino acids activate the mTOR signaling, not only through direct activation of mTOR in the cytoplasm but also through stimulation of mTOR gene transcription in the nucleus. Our findings provide a new layer to explain the mechanism via which amino acid signals to mTOR and regulates autophagy, and help to explore the mechanism how amino acids regulate mTOR and autophagy for mammary gland development and milk synthesis.
FUNDING INFORMATION
This study was supported by grants from the National Natural Science Foundation of China (31472162 and 31671473).
CONFLICTS OF INTEREST
The authors declare no conflicts of interest




