Repression of TXNIP–NLRP3 axis restores intestinal barrier function via inhibition of myeloperoxidase activity and oxidative stress in nonalcoholic steatohepatitis
Abstract
Dysfunction of the intestinal barrier function occurs in hepatic injury, but the specific mechanisms responsible are largely unknown. Recently, NOD-like receptor 3 (NLRP3) inflammasome functions in impairing endothelial barrier function. In this study, we test the hypothesis that TXNIP–NLRP3 axis repression prevents against intestinal barrier function disruption in nonalcoholic steatohepatitis (NASH). First, lipopolysaccharide (LPS)-induced alterations in expression of ZO-1 and occludin, myeloperoxidase (MPO) activity, reactive oxygen species (ROS) level, and transepithelial electric resistance (TEER) in intestinal epithelial cells (IECs) isolated from C57BL/6 wild-type (WT) and TXNIP−/− mice were evaluated. The underlying regulatory mechanisms of TXNIP knockout in vivo were investigated with the detection of expressions of TXNIP, NLRP3 and ZO-1, and occludin, the interaction of TXNIP–NLRP3, MPO activity, ROS level, permeability of intestinal mucosa, levels of inflammatory factors in serum, and LPS concentration. We identified that TXNIP knockout promoted ZO-1 and occludin expression, yet reduced MPO activity, ROS level, and cell permeability in IECs, indicating restored the intestinal barrier function. However, LPS upregulated TXNIP and NLRP3 expression, as well as contributed to the interaction between TXNIP and NLRP3 in vitro. Furthermore, TXNIP was significantly upregulated in the intestinal mucosa of NASH mice and its knockout repaired the intestinal barrier disrupt, inhibited expression of inflammatory factors, and reduced LPS concentration as well as hepatic injury in vivo. Taken together, our findings demonstrated that inhibited the activation of the TXNIP–NLRP3 axis reduced MPO activity and oxidative stress and thus restoring the intestinal barrier function in NASH. TXNIP–NLRP3 axis may be a promising therapeutic strategy for the NASH treatment.
1 INTRODUCTION
Nonalcoholic steatohepatitis (NASH) concerns insulin resistance and disordered cholesterol homeostasis, with highly prevalent in all contemporary societies (Van Rooyen et al., 2011). NASH is associated with cirrhosis-related mortality, characterized by metabolic syndrome, hepatic steatosis, ballooning hepatocellular injury, liver inflammation, and fibrosis (Dunn et al., 2012). The disease can induce liver fibrosis and approximately 25% of patients with NASH can progress to cirrhosis, liver failure, and hepatocellular carcinoma (HCC; Takeuchi et al., 2015). NASH affects 20–40% of the general adult population, being predicted potentially the leading cause of advanced liver disorder in the coming decades (Vernon, Baranova, & Younossi, 2011). In addition, evidence confirms that NASH at early stage has a probability of 18–39% to progress to more advanced stages of hepatic fibrosis within 3.5–8.2 years (Chen et al., 2011). Genetic factors, high-fat and high-fructose diet, and an impaired intestinal barrier function play key roles in the development of NASH (Sellmann, Jin, Degen, De Bandt, & Bergheim, 2015). Unluckily, the diagnosis of NASH requires liver histology results, yet it was a difficulty due to the several disadvantages of liver biopsy, which is the only generally acceptable method for NASH diagnosis and assessment of its progression toward cirrhosis (Rinella et al., 2014, Sanyal et al., 2011). With this is mind, new approaches are needed to investigate related genes involved with the occurrence of NASH which may well aid in the development of future intervention and treatment strategies.
Lipopolysaccharide (LPS) has been implicated as an important cofactor in the development and progression of NASH in high-fat and high-fructose diet mice, where its superlow-dose sustains the low-grade activation of p38 MAPKs and neutrophil infiltration (Guo et al., 2016). Myeloperoxidase (MPO) is a highly oxidative enzyme secreted by leukocytes and has been implicated in NASH (Pulli et al., 2015). Also, oxidative stress resulted from reactive oxygen species (ROS) is of essential importance in the progression of NASH, in which it induces inflammatory cytokines, causing inflammation and a fibrogenic response, ultimately resulting in NASH worsening (Koek, Liedorp, & Bast, 2011, Rolo, Teodoro, & Palmeira, 2012). The increased oxidative stress was reported as a result triggered by thioredoxin-binding protein (TXNIP), which has been identified as a tumor suppressor gene in various solid tumors and hematological malignancies (Devi et al., 2012). In diabetic-related NASH, TXNIP is significantly increased whereas knockout of TXNIP by small interfering RNA (siRNA) prevents early abnormalities of NASH (Perrone, Devi, Hosoya, Terasaki, & Singh, 2010). TXNIP has been identified to exhibit a relation with the activation of NOD-like receptor 3 (NLRP3) inflammasome, inflammation, and lipid metabolism (Wang et al., 2013). Furthermore, NLRP3 is raising more attention as a crucial regulator of inflammatory responses against commensal microflora in the gut and inhibited NLRP3 signaling pathway has been proved to contribute to inflammatory bowel diseases via enhanced permeability across the epithelial barrier (Zaki, Lamkanfi, & Kanneganti, 2011). Previous study demonstrated that the activated TXNIP/NLRP3 pathway contributed to the inflammation in diabetic retinopathy (Chen et al., 2017). Repression of the TXNIP–NLRP3 inflammasome pathway can prevent liver inflammation and hepatocyte death (Cao et al., 2017). Hence, during this study, we set out to determine the interaction of TXNIP–NLRP3 in LPS-treated and high-fat and high-fructose diet-induced mice with nonalcoholic steatohepatitis and the effect on the intestinal barrier function.
2 MATERIALS AND METHODS
2.1 Ethics statement
The study was conducted with the approval of the Animal Ethics Committee of China-Japan Friendship Hospital. All animal experiments were performed in line with the approval of the Guide for the Care and Use of Laboratory Animal by International Committees.
2.2 Model establishment and high-fat and high-fructose diet inducement
Healthy 8–10-week-old male C57BL/6J mice (weight: 21 ± 3 g) in clean grade were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Specific TXNIP knockout mice were obtained through the following procedures. First, TXNIPflox/flox and Tie2-Cre/+ mice (purchased from Jackson Laboratory, Bar Harbor, Maine) were hybridized to obtain TXNIPflox/floxTie2-Cre/+ mice. TXNIPflox/floxTie2-Cre/+ mice were hybridized with TXNIPflox/flox mice and their generation was specific TXNIP knockout mice (TxNIP−/−) model. C57BL/6 wild-type (WT) mice and TXNIP−/− mice were fed with common forage with free access to food and water. After 3-day of adaptive feeding, the mice in the normal group (n = 6) were still fed with common forage, whereas mice in the model group were performed with intragastric administration of high-fat and high-fructose emulsion (10% cholesterol, 40% corn oil, 15% sucrose, 2% protein, 1% bile salt, 3.1% propylene glycol, and 3.6% Twain-80) with a dosage based on 1 ml/100 g every morning for 20 weeks (stopped for two days after every five consecutive days). Next, to clarify the roles of specific TXNIP knockout in the MPO activity and ROS level in the intestinal mucosa of mice, six WT mice and six TXNIP−/− mice were randomly selected and conducted with intraperitoneal injection of 2,2,6,6-tetramethyl piperidine-1-oxyl (TEMPO, 1 mg•kg•day) at the same time every day (Ding, Liu, Bi, & Zhang, 2017), ad libitum water for 4 weeks, as the TEMPO group. To study the effect of specific TXNIP knockout on the intestinal microflora in mice, six WT mice and six TXNIP−/− mice were randomly selected, treated with intraperitoneal injection of equal dose of phosphate buffer solution (PBS) and fed with water dissolved with compound antibiotics (ampicillin 1 g/ml, vancomycin 0.5 g/ml, neomycin 1 g/ml, and metronidazole 1 g/ml) for 4 weeks successively (Ochoa-Reparaz et al., 2009), as the antibiotic group. Subsequently, six WT mice and six TXNIP−/− mice were randomly selected and treated with intraperitoneal injection of equal dose of PBS and ad libitum to water, as the model mice. Following 24 weeks, mice were administrated with fluorescein isothiocyanate (FITC)-dextran (40 mg/100 g) through stomach tube when fasting, and injected with 200 μl of fluorescent staining ROStar intraperitoneally. Four hours later, mice were killed using CO2 and then the abdomen was exposed for liver tissues and intestinal mucosa collection, 1/3 of the tissues was fixed in 4% formaldehyde and the remaining was stored in a freezer at − 70°C for further usage.
2.3 Intestinal epithelial cell (IEC) treatment
Intestinal mucosa tissues from WT and TXNIP−/− mice were cut into pieces (3 mm2), washed with Hanks solution and incubated with Dispase I (2.4 U/ml) at 37°C for 30 min. After cell suspension was filtered via a 74-μm nylon mesh, cells were collected, washed, and centrifuged. Then cells were incubated with the addition of phycoerythrin (PE)-labeled-monoclonal antibody anti-mice CD326 (Ep-CAM, a specific antigen for epithelial cells) at 4°C for 30 min, rinsed with PBS and suspended. Then intestinal epithelial cells (IECs) were screened out by a flow cytometer (MOFLO S2500). The living cells were separated from particles and fragments by FSC, and then divided into CD326 negative and CD326 positive cells by the activity of PE. The positive group was small intestinal epithelial cells (Alenghat et al., 2013). Next, cells were adjusted to the density of 5 × 105, cultured with Dulbecco's modified Eagle's Medium (DMEM) containing 20% fetal bovine serum (FBS), added with 1% combination of penicillin and streptomycin, reacted with 0.25% trypsin containing ethylene diaminetetraacetic acid (EDTA), subcultured at the ratio of 1: 2 and cultured in an incubator containing 5% CO2 at 37°C. After 24-hr of subculture, the medium was replaced. In the following days, the medium was replaced once every day. The cells were subcultured once every 5–6 days. IECs in the logarithmic growth were given different stimulation and starved for 12 hr before experiment with serum-free medium. IECs from WT and TXNIP−/− mice were assigned into the control group (without any treatment) and the lipopolysaccharide (LPS) group (48-hr culture with the addition of LPS [10 μg/ml, Sigma, Deisenhofen, German] at the 5th day of culture; Evans, Flint, Somers, Eyden, & Potten, 1992). After that, the supernatant in each group was collected, concentrated to about 1/20 using a concentrated filter, and then the MPO activity was determined with a colorimetry in accordance with the instructions of the MPO kit. IECs in each group were added with dichlorofluorescin diacetate (DCFH-DA) at the ratio of 1:1,000, with the final concentration of 10 μmol/l, then incubated in an incubator at 37°C for 20 min, and washed three times with serum-free culture solution. Following, the intensity of fluorescence was detected at the extension wavelength of 488 nm and the emission wavelength of 525 nm using a fluorometer.
2.4 Cell transfection and grouping
IECs from TXNIP−/− mice in the logarithmic growth were seeded into 6-well plates and adjusted to a density of 2 × 105 cells/well. Two hours before the transfection, serum-free DMEM was prepared and then each well was incubated with the replacement of 2 ml of serum-free Eagle's minimum essential medium (MEM; Gibco, Carlsbad, California) without penicillin/streptomycin. After 2 hr, cells were preheated in a warm oven with 1.5 ml of opti-MEM. Empty vector and pcDNA-TxNIP (Shanghai Genechem Co., Ltd., Shanghai, China), Opti-MEM and Lipofectamine® 2000 transfection reagent (Invitrogen Inc., Carlsbad, California) were mixed well and allowed to stand for 5 min. Afterward, the mixture was added into the medium for IECs from TXNIP−/− mice and mixed evenly. After 24-hr culture, the medium was replaced by a fresh one. After the transfection of 48 hr, cells were treated with trypsin and then collected for the following experiments (Li et al., 2017). Next, cells were classified into the TXNIP−/− group (transfection with empty plasmids, as negative control) and the pcDNA-TxNIP group (transfection with TXNIP overexpression plasmids). IECs from WT mice in the logarithmic growth and transfected cells were fasted for 12 hr before experiment and the cells were divided into the WT group, the TXNIP−/− group and the pcDNA-TxNIP group. At the 5th day of culture, cells were added with 10 μg/ml LPS (Sigma, Deisenhofen, German) and incubated for 48 hr (Evans et al., 1992). Successively, the MPO activity and ROS level in IECs in each group were determined.
2.5 RNA isolation and quantitation
Total RNA was extracted using the Trizol extraction kit (16096020, Thermo Fisher Scientific Inc., Waltham, Massachusetts) and then 5 µg of RNA was prepared and reversely transcribed into complementary DNA (cDNA) in accordance with the instructions of the reverse-transcription quantitative polymerase chain reaction (RT-qPCR) kit (ABI Company, Oyster Bay, New York). The reaction system was 25 µl, including 300 ng of cDNA, 1 × PCR buffer, deoxyribonucleotide triphosphates (dNTPs; 200 μmol/l), forward primers (80 pmol/l) and reverse primers (80 pmol/l), and 0.5 U Taq enzyme (S10118, Shanghai Yuanye Biotechnology Co., Ltd., Shanghai, China). The reaction conditions consisted of predenaturation at 94°C for 5 min, followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 54.5°C for 30 s and extension at 72°C for 30 s, and a final elongation at 72°C for 10 min. The product was preserved at 4°C. The primer sequences of TXNIP, NLRP3, ZO-1, occludin, TNF-α, IL-1β, and β-actin are shown in Table 1. β-actin was used as the internal reference of TXNIP, NLRP3, ZO-1, occludin, TNF-α, IL-1β. The 2−ΔΔCt represented the ratio of the expression of the target gene in the experiment group and the control group. The formula was as follows: ΔΔCT = ΔCt experimental group − ΔCt control group, where ΔCt = Ct miRNA − Ct U6. Ct was the amplification cycles when the real-time fluorescence intensity of the reaction reached the set threshold, and the amplification was in the logarithmic growth. The experiment was independently repeated three times.
| Genes | Primer sequences |
|---|---|
| TXNIP | F: 5′-GCTCAATCATGGTGATGTTCAAG-3′ |
| R: 5′-CTTCACACACTTCCACTGTCAC-3′ | |
| NLRP3 | F: 5′-GGAGAGACCTTTATGAGAAAGCAA-3′ |
| R: 5′-GCTGTCTTCCTGGCATATCACA-3′ | |
| ZO-1 | F: 5′-AAAGTGCTGGCTTGGTCTGT-3′ |
| R: 5′-TAGGTGACGCTGGGTGATAG-3′ | |
| Occludin | F: 5′-GCTCAGGGAATATCCACCTATCA-3′ |
| R: 5′-CACAAAGTTTTAACTTCCCAGACG-3′ | |
| TNF-α | F: 5′-GTAGCAAACCACCAAGCAG-3′ |
| R: 5′-GGTATGAAGTGGCAAATCG-3′ | |
| IL-1β | F: 5′-TTGGGATCCACACTCTCCAG-3′ |
| R: 5′-AGAAGCTGTGGCAGCTACCT-3′ | |
| β-actin | F: 5′-GCACCGCAAATGCTTCTA-3′ |
| R: 5′-GGTCTTTACGGATGTCAACG-3′ |
- Note. F: forward; IL-1β: interleukin 1 beta; NLRP3: NLR family pyrin domain containing 3; RT-qPCR: reverse-transcription quantitative polymerase chain reaction; R: reverse; TNF-α: tumor necrosis factor alpha; TXNIP: thioredoxin-binding protein; ZO-1: zonula occludens protein-1.
2.6 Western blot analysis
Obtained protein samples were separated with sodium dodecyl sulfate-polyacrylamide gel (100 g/l), and then transferred onto polyvinylidene fluoride (PVDF) membranes. The membranes were blocked with 5% skim milk, and then incubated with the following rabbit antimouse antibodies at 4°C overnight and washed three times with PBS at room temperature, 5 min per time. The rabbit antimouse antibodies were NLRP3 (ab214185; 1:1,000), TXNIP (ab114981, 1:1,000) and β-actin (ab8227, 1:1,000). All antibodies were purchased from Abcam (Cambridge, Massachusetts). On the following day, the membranes were washed three times with Tris-buffered saline with Tween-20 (TBST; Wuhan Boster Company, Wuhan, Hubei, China). Horseradish peroxidase (HRP)-labeled goat antirabbit IgG (ab150077, 1:5,000, Abcam) was then incubated with the membranes at room temperature for 1 hr as the secondary antibody. The membranes were washed with TBST, then immersed in a mixture of A solution and B solution with equal dose obtained from a chemiluminescence (ECL) reaction solution (BB-3501, Amersham Pharmacia Biotech, Chicago, Illinois), and exposed using a gel imaging instrument. Finally, the membranes were imaged using a Bio-Rad imaging analyzer (Bio-Rad Laboratories, Hercules, California) and analyzed with the Quantity One v4.6.2. The ratio of the gray value of the target band to β-actin band was utilized as the relative expression of the protein. The experiment was independently repeated three times.
2.7 Coimmunoprecipitation
IECs were treated with lysate buffer (p0013, Beyotime Biotechnology Co., Ltd., Shanghai, China) on ice, centrifuged for 15 min at 40,256 × g and incubated at 4°C overnight with the mixture of 500–1,000 μg of proteins and 1 μg of TXNIP antibodies. Then cells were incubated at 4°C overnight with the addition of A/G gel beads (p2006, Beyotime Biotechnology Co., Ltd., Shanghai, China), centrifuged for 20 s at 25,764 × g, followed by the removal of the supernatant. The precipitation was washed three times with radioimmunoprecipitation assay (RIPA) buffer, and the sample buffer was added and boiled. Finally, NLRP3 protein content was determined via western bolt analysis.
2.8 Immunofluorescence assay
IECs were seeded into 24-well plates which were precoated with polylysine. Upon cell adhering to walls, the culture medium was absorbed. Then cells were washed gently three times with warmed 0.01 mol/l PBS, fixed in 4% polyoxymethylene at room temperature for 30 min, and washed three times with 0.01 mol/l PBS (10 min each time). Cells were added with blocking solution (Beyotime Biotechnology Co., Ltd., Shanghai, China) at 37°C for 60 min. After the blocking solution being discarded, cells were incubated at 4°C overnight with the addition of primary antibodies, including rabbit antimouse ZO-1 (ab96587, 1:400), rabbit antimouse occludin (ab31721, 1:400), both of which were purchased from Abcam. After three times washing with 0.01 mol/l PBS (10 min each time), cells were cultured for 1 hr under the environment void of light with the addition of secondary antibodies, including Alexa Fluor 594 donkey antirabbit (A21202, 1:400, Life Technologies, Gaithersburg, Maryland) and Alexa Fluor 488 donkey antimouse (A21207, 1:400, Life Technologies). Subsequently, cells were stained with 2,4-diamadino-6-phenylindole (Beyotime Biotechnology Co., Ltd.) for 5 min at the room temperature, added with appropriated fluorescence quenching (Beyotime Biotechnology Co., Ltd.), and imaged with a Molecular Devices. Molecular Devices MetaXpress Image Acquisition and Analysis Software was used for data analysis. The experiment was independently conducted three times.
2.9 Transepithelial electrical resistance (TEER)
Next, we established an IEC monolayer model. IECs in the logarithmic growth were extracted and incubated with trypsin, prepared into cell suspension and then inoculated into Transwell chambers at a density of 5 × 105 cell/well, followed by independent culture. The apical side of the chamber was 0.4 μm and the area was 1.12 cm2. Apical chamber was added with 0.5 ml of cell suspension and the basolateral chamber was added with 1.5 ml of culture solution, making the liquid level of internal and external ones basically flat. The culture solution was changed every other day at the first week, afterwards, the solution was replaced every day. Following 5-day inoculation, transepithelial electrical resistance (TEER) was assessed using an EVOM2 epithelial volt-ohm meter to identify the compactness and integrity of monolayer cells.
Electrical resistance was measured from the three angels of Transwell chambers and obtained the mean values: TEER (Ω·cm2) = (R1 − R0) × A, in which R1 and R0 represented the value of electrical resistance (Ω) with cell culture well and cell-free negative control well, respectively, and A represented polycarbonate membrane area (cm2). TEER reaching 500–1,500 Ω·cm2 was suggested that monolayer cells were close and complete. Three parallel controls were set in the experiment.
2.10 Reactive oxygen species (ROS) level detection
Intestinal mucosa tissues of mice were fixed in 4% polyoxymethylene for 3 days, dehydrated with 20% sucrose solution for 2 days, covered with a octenidine-gel (OCT) and then sectioned via a frozen slicer (CM1900, Leica, Germany) at the thickness of 30 μm. The sections were adherent onto the glass slide, washed three times with PBS (5 min each time), dried, sealed with glycerol and imaged under a fluorescence microscope at the extension wavelength of 490 nm and the emission wavelength of 530 nm. The Image ProPlus software was used to analyze the average optical density (OD), which referred to the relative ROS level in the intestinal mucosa.
2.11 MPO activity measurement
A total of 100 mg of intestinal mucosa tissues from mice were prepared into 5% homogenate with homogenate medium provided by the MPO measurement kit. The above procedure was processed in strict accordance with the instructions of the MPO measurement kit. MPO activity was expressed as unit of enzyme activities that per gram of intestinal mucosa contained, and recorded as U/g. The unit of enzyme activities was defined as 1 μmol H2O2 resolved at 25°C in one minute.
2.12 FITC-dextran permeability assay
Mice were treated with CO2, and the abdomen was open with celiac artery blood extracted, followed by anticoagulation with heparin. Intestinal mucosa tissues were centrifuged at 1,610 × g for 10 min and the plasma was obtained for later usage. Next, 25 μl of plasma was added with 100 μl of PBS. A fluorescence spectrophotometer was adopted to determine the intensity of fluorescence at the extension wavelength of 480 nm and the emission wavelength of 520 nm. A relative curve was drawn and the sample concentration was calculated. The experiment was independently repeated three times.
2.13 Detection of intestinal flora population
After feeding with compound antibiotic, the fresh feces of mice was obtained using an aseptic method, then placed into an aseptic tube and immediately stored in a freezer at − 80°C. Next, 0.2 g of feces was added with 200 μl of Tris-EDTA (TE) buffer containing lysozyme (20 mg/ml) and mixed with a pipette. Then the feces was incubated at room temperature for 40 min, added with 400 μl of digestion buffer provided by the kit and mixed evenly using a pipette. After 3 μl of protease K treatment and even mixture, samples were preserved at 55°C for 2–3 hr, followed by a centrifugation. Then the supernatant was added with 260 μl of absolute ethanol, mixed well and transcribed into the extraction cartridge of bacterial genomic DNA. The 3-times centrifugation was followed and the liquid collected into the centrifugation tube was the bacterial genomic DNA. The reaction volume of RT-qPCR contained 10 μl of 10 × buffer, 1 μl of 2 μmol/l forward and reverse sequences, respectively, 2 μl of DNA plate, 2.5 μl of MgCl2, 1 μl of dNTP, 0.4 μl of Taq enzyme, and brought up to 25 μl with double-distilled water. The reaction condition consisted of predenaturation at 95°C for 5 min, denaturation at 95°C for 20 s, and annealing at 50°C for prevotella and bifidobacterium, 55°C for Escherichia coli, Clostridium, and Lactobacillus for 45 s, extension at 72°C for 45 s, with a total of 40 cycles, and a final extension at 72°C for 5 min, preserving at 4°C. PCR products were processed with agarose electrophoresis, and then the target fragments were retrieved according to the instructions of the DNA product purification kit. Nucleic acid quantitation was used to detect the concentration of purified products. All retrieved products were treated with a quantitative one-step SYBR Green-based RT-qPCR system. The system software was used to automatically analyze Ct and generate standard curves based on the recoded fluorescence value. The absolute content of the bacteria was calculated by substituting the Ct of the sample into the standard curve. Full sequences of 16S rRNA of five bacteria were searched in the Genebank, which were compared with the selected primer sequences in the experiment using BLAST database (www.ncbi.nlm.nih.gov/BLAST). The standard curve is shown in Table 2.
| Primer | Sequence | Length/bp | Regression equation | R2 |
|---|---|---|---|---|
| Prevotella | F: 5′-GGTGTCGGCTTAAGTGCCAT-3′ | 151 | Y = 69.13–3.65× | 0.99485 |
| R: 5′-CGGAYGTAAGGGCCGTGC-3′ | ||||
| Bifidobacterium | F: 5′-TCGCGTCYGGTGTAAAG-3′ | 243 | Y = 62.77–3.32× | 0.99921 |
| R: 5′-CCACATCCAGCRTCCAC-3′ | ||||
| Escherichia coli | F: 5′-CATGCCGCGTGTATGAAGAA-3′ | 113 | Y = 62.06–2.89× | 0.99479 |
| R: 5′-CGGGTAACGTCAATGAGCAAA-3′ | ||||
| Clostridium | F: 5′-CGCAGAAGGTGAAAGTCCTGTAT-3′ | 100 | Y = 56.21–3.01× | 0.99627 |
| R: 5′-TGGTCCTCACTGATTCACACAGA-3′ | ||||
| Lactobacillus | F: 5′-CACCTTCCTCCGGTTTGTCA-3′ | 126 | Y = 57.53–2.58× | 0.99948 |
| R: 5′-CGAGCGCAACCCTTATTATCA-3′ |
- Note. F: forward; R: reverse.
2.14 Hematoxylin-eosin (HE) staining
Mice were treated with abdomen exposure and then the left lobe of liver was fixed with 4% paraformaldehyde, paraffin embedded routinely, sectioned (5 μm) and stained with hematoxylin-eosin (HE) according to the instructions of the HE kit (Beyotime Biotechnology Co., Ltd., Shanghai, China; Wang et al., 2017). The experiment was independently conducted three times. The remaining liver tissues were preserved in a freezer at − 70°C for the further experiments.
2.15 Biochemical parameter detection
We collected 100 μl of plasma from mice and utilized automatic biochemical analyzer (BS200, Mindray, Shenzhen, Guangdong, China) and the corollary reagent to detect the levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in serum. The experiment was independently repeated three times.
2.16 Enzyme-linked immunosorbent assay (ELISA)
The procedure was conducted in accord with the instructions of an enzyme-linked immunosorbent assay (ELISA) detection kit for LPS (Uscn Life Science, Inc., Wuhan, Hubei, China). The samples were diluted 10 times successively. Liver tissues (100 mg) were mixed with 2 ml of 0.5% human thyroid antibody (HTAB), and then the subcellular component was treated three times with ultrasonication (30 s per time). After centrifugation at 17,891 × g for 15 min at 0–4°C, the supernatant was diluted at the ratio of 1:5, and the corresponding reagents were diluted based on the instructions. Afterwards, the standardized well (addition with standard samples), the blank well (without any reagent), and the sample well to be detected (addition with samples to be detected) were set with parallel wells. Standard samples (100 μl) at different concentrations and diluted samples were added and incubated at 37°C for 2 hr. After the liquid being discarded, 100 μl of A detection solution was dropped and incubated together at 37°C for 1 hr. The liquid in the plate was discarded. The plate was rinsed with washing solution and dried using absorbent paper. After 3-times washing, 100 μl of B detection solution was dropped and incubated together for 30 min. Following 5-times washing, each well was added with 90 μl of substrate solution, developed at 37°C for 15–25 min, treated with 50 μl of termination solution and shaken gently to mix well until the color turned from blue to yellow. The OD value of each well was measured using a microplate reader (Thermo, Scientific, Milford, Massachusetts) at the wavelength of 450 nm. Cell growth curve was drawn with the concentration of standard sample as ordinate and the OD value of standard sample as abscissa. Standard curve method was performed to calculate the concentration of unknown samples, with three replications set for each sample.
2.17 Statistical analysis
All data were processed by SPSS 21.0 statistical software (IBM Corp. Armonk, New York). The measurement data were expressed as mean ± standard deviation. The t test was used to compare data between two groups and one-way analysis of variance (ANOVA) for comparisons among multiple groups. Enumeration data were expressed as the percentage (%) and the comparisons between groups were analyzed using Chi square test. A p value <0.05 was indicative of significant statistical difference.
3 RESULTS
3.1 LPS disrupts IEC barrier function by enhancing MPO activity and ROS level
Initially, to investigate the effect of LPS on the barrier function of IECs, CD326 positive cell subsets were separated by flow cytometry (Figure 1a), which was IECs of mice and treated with LPS. RT-qPCR (Figure 1b), western blot analysis (Figure 1c), immunofluorescence assay (Figure 1f) were used to measure the expression and location of ZO-1 and occludin in the control and LPS groups. MPO kit, ELISA, and resistance measuring instrument were used to measure the MPO activity (Figure 1d), ROS level (Figure 1e), TEER (Figure 1g) in the two groups. In the control group, ZO-1 and occludin mainly distributed in the cell membrane, showing strong fluorescence signals and close connections between cells without obvious fracture. The messenger RNA (mRNA) levels of ZO-1 and occludin in the control and LPS groups were 0.512 ± 0.051 and 0.385 ± 0.038, and the protein levels were 0.215 ± 0.021 and 0.235 ± 0.023, respectively. The MPO activity was 27.49 ± 2.71 U/L, ROS level was 398.35 ± 39.83 mol/L, and TEER was 156 ± 8 Ohm/cm2. In the LPS group, ZO-1 and occludin showed much lower fluorescence signal with fraction, unconnected cells, and exhibited a little fluorescence signal in the cytoplasm, the mRNA and protein levels as well as TEER were significantly decreased, whereas the MPO activity and ROS level were significantly increased (all p < 0.05). These findings demonstrated that the LPS treatment might promote the inhibition of ZO-1 and occludin expression, increase of the MPO activity and ROS level, as well as permeability of IECs, thus weakening the barrier function of IECs.
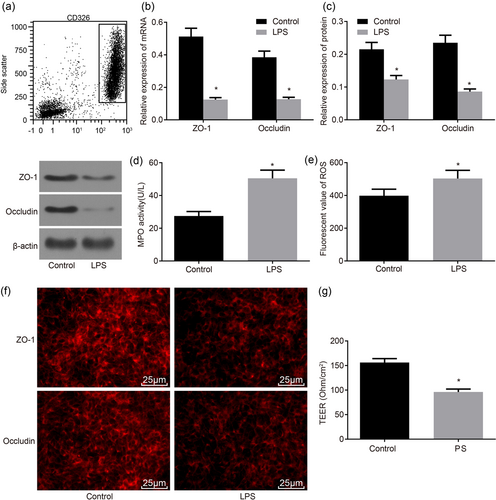
LPS treatment decreases ZO-1 and occludin expressions, the MPO activity, ROS level, and TEER, whereby reducing IEC barrier function. (a) CD326 positive cell subsets were sorted by flow cytometry; (b) RT-qPCR was utilized to determine the mRNA levels of ZO-1 and occludin in IECs; (c) western blot analysis was used to determine the protein levels of ZO-1 and occludin in IECs; (d) MPO kit was used to determine MPO activity; (e) ELISA was used to determine ROS level; (f) immunofluorescence assay of ZO-1 and occludin in IECs (×400); (g) resistance measuring instrument was used to measure TEER; *p < 0.05 versus the control group. Data are expressed by means ± standard error and analyzed by independent sample t test from three independent experiments. ELISA: enzyme-linked immunosorbent assay; IEC: intestinal epithelial cell; LPS: lipopolysaccharide; MPO: myeloperoxidase; mRNA: messenger RNA; ROS: reactive oxygen species; RT-qPCR: reverse-transcription quantitative polymerase chain reaction; TEER: transepithelial electric resistance [Color figure can be viewed at wileyonlinelibrary.com]
3.2 LPS disrupts IEC barrier function by upregulating expression of TXNIP and NLRP3 and the interaction of TXNIP–NLRP3
Subsequently, to further investigate the mechanism of LPS in IEC barrier function via the regulation to TXNIP–NLRP3 pathway, we used RT-qPCR to detect the expression of TXNIP and NLRP3, and coimmunoprecipitation to detect the interaction of TXNIP and NLRP3 protein in the control and LPS groups. As shown in Figure 2a,b, in IECs, the expression of TXNIP and NLRP3 were significantly increased and the content of TXNIP–NLRP3 protein was also significantly increased (p < 0.05), suggesting that the interaction of TXNIP–NLRP3 was significantly strengthened (Figure 2c,d). It can be concluded that LPS might upregulate the expression of TXNIP and NLRP3 and increase the interaction of TXNIP–NLRP3, disrupting IEC barrier function.
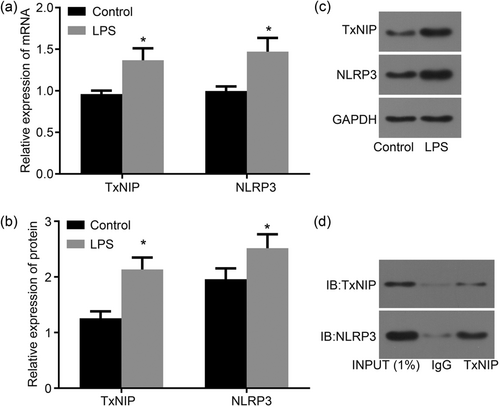
LPS elevates the expression of TXNIP and NLRP3 and strengthens the interaction of TXNIP–NLRP3, whereby reducing IEC barrier function. (a) RT-qPCR was used to determine the mRNA levels of TXNIP and NLRP3 in IECs; (b) the quantitative analysis of the protein levels of TXNIP and NLRP3 in IECs determined by western blot analysis; (c) the gray value of TXNIP and NLRP3 protein bands in IECs determined by western blot analysis; (d) the gray value of TXNIP–NLRP3 content evaluated by coimmunoprecipitation; *p < 0.05 versus the control group. Data are expressed by means ± standard error and analyzed by independent sample t test from three independent experiments. IECs: intestinal epithelial cells; LPS: lipopolysaccharide; mRNA, messenger RNA; RT-qPCR: reverse-transcription quantitative polymerase chain reaction
3.3 TXNIP knockout elevates ZO-1 and occludin expression yet lowers MPO activity and ROS level in IECs
To explore the effects of TXNIP on IEC barrier function, we conducted IEC separation from WT mice and TXNIP−/− mice, TXNIP overexpression in TXNIP−/− IECs, IECs in each group treated with LPS. Then immunofluorescence assay, RT-qPCR and western blot analysis were utilized to detect how ZO-1 and occludin expressed in the WT, TXNIP−/− and pcDNA-TXNIP groups. MPO kit, ELISA, and resistance measuring instrument were used to measure the MPO activity, ROS level, and TEER, respectively. As displayed in Figure 3e, in the WT group, ZO-1, and occludin mainly distributed in a mass in the cytoplasm, indicating weakened fluorescence signal and little connection between cells with obvious fracture. The mRNA levels of ZO-1, occludin and NLRP3 were 0.365 ± 0.044, 0.322 ± 0.053, and 0.861 ± 0.052 and the protein levels were 0.384 ± 0.051, 0.355 ± 0.031, and 0.921 ± 0.042, respectively (Figure 3a,b). The MPO activity was 29.82 ± 2.98 U/L, ROS level was 395.26 ± 39.52 mol/l, and TEER was 101 ± 7 Ohm/cm2 (Figure 3c,d,f). Although in the TXNIP−/− group, ZO-1, and occludin showed much stronger fluorescence signal without fraction and closely connected cells as compared with the WT group (Figure 3e), the expression of ZO-1, occludin, and NLRP3 was significantly increased (Figure 3a,b), whereas the MPO activity and ROS level were significantly decreased (Figure 3c,d) and TEER was significantly increased (Figure 3f; all p < 0.05). However, the above factors between the WT and pcDNA-TXNIP groups showed no significant differences (p > 0.05). Compared with the TXNIP−/− group, in the pcDNA-TXNIP group, ZO-1, and occludin showed much lower fluorescence signal and unconnected cells with remarkably increased fraction, the expression of ZO-1, occludin, and NLRP3 was decreased, whereas the MPO activity and ROS level were markedly increased and TEER was significantly decreased (all p < 0.05). These findings demonstrated that TXNIP knockout might elevate ZO-1 and occludin expression yet lower the MPO activity and ROS level and lessen permeability of IECs, thus restoring the barrier function of IECs.
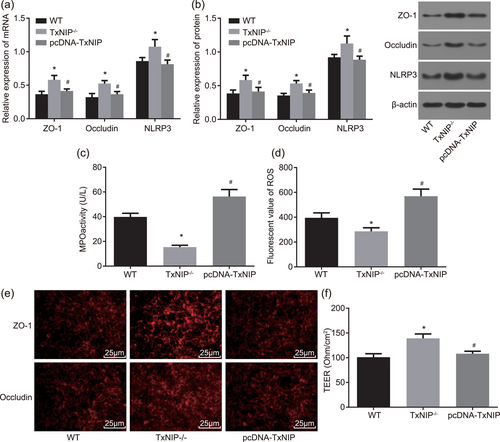
TXNIP knockout contributes to the elevation of ZO-1 and occludin expressions, the reduction of MPO activity and ROS level, whereby restoring IEC barrier function in IEC. (a) RT-qPCR was used to determine the mRNA level of ZO-1, occludin and NLRP3 in IECs; (b) the protein levels of ZO-1, occludin, and NLRP3 in IECs determined by western blot analysis; (c) MPO kit was utilized to determine the MPO activity in IECs from WT and TXNIP−/− mice,; (d) ELISA was applied to determine ROS level in IECs from WT and TXNIP−/− mice; (e) immunofluorescence assay was used to measure the protein levels of ZO-1 and occludin in IECs from WT and TXNIP−/− mice (×400); (f) resistance measuring instrument was used to test TEER; *p < 0.05 versus the WT group; #p < 0.05 versus the TXNIP−/− group. Data are expressed by means ± standard error and analyzed by one-way analysis of variance from three independent experiments. ELISA: enzyme-linked immunosorbent assay; IEC: intestinal epithelial cell; LPS: lipopolysaccharide; MPO: myeloperoxidase; mRNA: messenger RNA; ROS: reactive oxygen species; RT-qPCR: reverse-transcription quantitative polymerase chain reaction; TEER: transepithelial electric resistance; WT, wild-type [Color figure can be viewed at wileyonlinelibrary.com]
3.4 TXNIP knockout represses the MPO activity and oxidative stress in intestinal mucosa
It has been reported that TEMPO has mitochondria-targeted antioxidant activity and protects the kidney from aldosterone-induced damage (Ding et al., 2017), so we want to explore the protective effect of TEMPO on intestinal mucosa. Furthermore, to manifest the effects of TXNIP on the MPO activity and ROS level in the intestinal mucosa induced by high-fat and high-fructose diet in mice with steatohepatitis, WT, and TXNIP−/− mice models were established and then assigned into the normal, model, and TEMPO groups. As presented in Figure 4a,b, the MPO activity and ROS level in intestinal mucosa in the model and TEMPO groups were significantly elevated as compared with those in the normal group (p < 0.05). Compared with the model group, MPO activity, and ROS level in the intestinal mucosa in the TEMPO group were significantly descended (p < 0.05). In comparison to WT mice, MPO activity, and ROS level in the intestinal mucosa in the model and TEMPO groups from TXNIP−/− mice were significantly declined (all p < 0.05). After the TEMPO treatment, in WT mice, the clearance rate of ROS was 35.3%, and that of TXNIP−/− mice was 45.8%, indicating that the clearance rate of ROS was significantly increased in TXNIP−/− mice. The present findings suggested that high-fat and high-fructose diet can trigger elevated the MPO activity and oxidative stress, which can be inhibited by the knockout of TXNIP, and the TEMPO treatment can further repress oxidative stress.
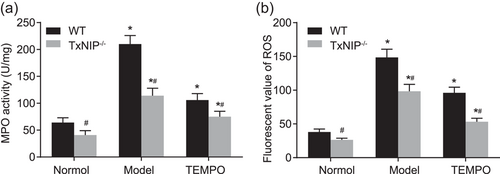
TXNIP knockout suppresses the MPO activity and ROS level, whereby restoring IEC barrier function in intestinal mucosa. (a) The MPO kit was applied to determine the MPO activity in intestinal mucosa from WT and TXNIP−/− mice; (b) ELISA was utilized to determine ROS level in intestinal mucosa from WT and TXNIP−/− mice; *p < 0.05 versus the normal group; #p < 0.05 versus the WT mice. Data are expressed by means ± standard error and analyzed by independent sample t test from three independent experiments. ELISA: enzyme-linked immunosorbent assay; MPO: myeloperoxidase; mRNA, messenger RNA; ROS: reactive oxygen species; TEMPO: 2,2,6,6-tetramethyl piperidine-1-oxyl; WT: wild-type
3.5 TXNIP knockout protects intestinal mucosal barrier function by reducing Escherichia coli
To demonstrate the effects of TXNIP on the intestinal mucosal barrier function of mice with steatohepatitis induced by high-fat and high-fructose diet, we used the immunofluorescence assay to detect how ZO-1 and occludin in intestinal epithelial tissue was located, RT-qPCR to detect the mRNA levels of TXNIP, ZO-1, and occludin, and FITC-Dextran to detect the permeability in the intestinal epithelial tissue from the normal and model groups. The results showed that compared with the normal group, ZO-1 and occludin in intestinal epithelial tissue exhibited mass distribution in cytoplasm and discontinuous gaps in fluorescent stained cells. Moreover, the connection between cells and cells was not close, whereas the expression of TXNIP, ZO-1, and occludin were significantly reduced and the FITC content in the intestinal epithelial tissue was significantly enhanced (all p < 0.05). In comparison to WT mice, TXNIP−/− mice showed no TXNIP expression whereas ZO-1 and occludin expressions were significantly increased (p < 0.05). ZO-1 and occludin displayed honeycomb linear fluorescence distributed around cell membrane and relatively complete outline; in addition, positive fluorescence staining intensity in cytoplasm decreased and the FITC content in intestinal epithelial tissue was noticeably ascended (all p < 0.05; Figure 5a–f).
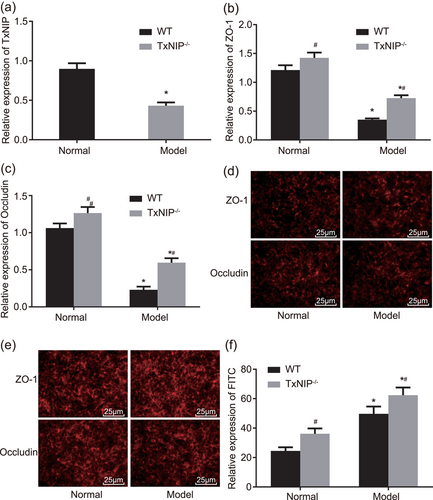
TXNIP knockout reduces expression of ZO-1 and occludin and increases the FITC content in intestinal mucosa. (a) RT-qPCR was utilized to determine the mRNA levels of TXNIP in the intestinal mucosa; (b) the relative expression of ZO-1 in intestinal mucosa of TXNIP−/− mice and WT mice; (c) the relative expression of occludin in intestinal mucosa of TXNIP−/− mice and WT mice; (d) the immunofluorescence assay was used to measure the protein levels of ZO-1 and occludin in WT mice (×400); (e) the immunofluorescence assay was used to measure the protein levels of ZO-1 and occludin in TXNIP−/− mice (×400); (f) FITC-Dextran was applied to test FITC content in TXNIP−/− mice and WT mice; *p < 0.05 versus the normal group; #p < 0.05 versus the WT mice. Data are expressed by means ± standard error and analyzed by independent sample t test from three independent experiments. FITC: fluorescein isothiocyanate; mRNA: messenger RNA; RT-qPCR: reverse-transcription quantitative polymerase chain reaction; WT: wild-type [Color figure can be viewed at wileyonlinelibrary.com]
To demonstrate the effects of TXNIP on the change of intestinal flora induced by high-fat and high-fructose diet, we classified mice into the normal, model, and antibiotic groups and utilized RT-qPCR to evaluate the content of intestinal flora in each group. As shown in Table 3, compared with the normal group, the number of Clostridium, Prevotella, Bifidobacterium, and Lactobacillus was obviously decreased whereas E. coli was obviously increased in the model and antibiotic groups (all p < 0.05). Compared with the model group, the number of Clostridium, Prevotella, Bifidobacterium, and Lactobacillus was obviously increased whereas E. coli was obviously decreased in the normal and antibiotic groups (all p < 0.05). Compared with WT mice, the number of Clostridium, Prevotella, Bifidobacterium, and Lactobacillus was obviously increased, whereas E. coli was obviously decreased in TXNIP−/− mice in the model and antibiotic groups (all p < 0.05). After the antibiotic treatment, in WT mice, the clearance rate of E. coli was 18.9%, and that in TXNIP−/− mice was 28.8%, suggesting that the clearance rate of E. coli in TXNIP−/− mice was much higher than that in WT mice. We demonstrated that TXNIP knockout can prevent the disorders in intestinal flora that resulted from high-fat and high-fructose diet and increase the clearance rate of E. coli. The above findings indicated that high-fat and high-fructose diet can accelerate the expression of TXNIP in intestinal mucosa, decrease the expression of ZO-1 and occludin and continuous distribution, increase the permeability of intestinal mucosa and intestinal flora disorder protects intestinal mucosal barrier function, thus making IEC barrier function disrupted. However, TXNIP knockout can restore intestinal mucosal barrier function triggered by high-fat and high-fructose diet.
| Type | Mice | Normal | Model | Antibiotic |
|---|---|---|---|---|
| Clostridium | WT | 12.582 ± 0.024 | 7.142 ± 0.012* | 11.524 ± 0.018* |
| TXNIP−/− | 13.628 ± 0.025# | 8.125 ± 0.017*# | 8.248 ± 0.016*# | |
| Prevotella | WT | 9.612 ± 0.015 | 5.427 ± 0.022* | 8.156 ± 0.032* |
| TXNIP−/− | 10.651 ± 0.017# | 6.253 ± 0.026*# | 9.125 ± 0.012*# | |
| Bifidobacterium | WT | 12.753 ± 0.036 | 7.018 ± 0.019* | 9.254 ± 0.041* |
| TXNIP−/− | 13.951 ± 0.038# | 9.536 ± 0.023*# | 11.254 ± 0.019*# | |
| Lactobacillus | WT | 14.874 ± 0.042 | 9.836 ± 0.026* | 10.305 ± 0.022* |
| TXNIP−/− | 15.847 ± 0.043# | 11.581 ± 0.024*# | 13.328 ± 0.017*# | |
| Escherichia coli | WT | 9.069 ± 0.011 | 14.019 ± 0.033* | 11.368 ± 0.019* |
| TXNIP−/− | 8.012 ± 0.010# | 13.027 ± 0.025*# | 9.281 ± 0.014*# |
- Note. TXNIP: thioredoxin-interacting protein; WT: wild-type; *p < 0.05 versus the normal group; #p < 0.05 versus the WT mice. Data are expressed as mean ± standard deviation and analyzed by unpaired t test. The comparison inside the group was analyzed by one-way analysis of variance. The experiment was independently conducted three times.
3.6 TXNIP knockout inhibits liver tissue injury
To explore whether TXNIP was involved in the process of steatohepatitis resulted from high-fat and high-fructose diet, we performed the RT-qPCR, automatic biochemical analyzer, ELISA, and HE staining. The results are shown in Figure 6a–e. Compared with the normal group, TNF-α and IL-1β expression, serum levels of ALT and AST, and LPS concentration were significantly elevated in the model group (all p < 0.05). Compared with WT mice, TNF-α and IL-1β expression, serum levels of ALT and AST, and LPS concentration were significantly descended in the model group from TXNIP−/− mice (all p < 0.05). In the normal group, liver tissue of mice showed no fatty degeneration and inflammatory cell infiltration. While in the model group from WT mice, liver tissue exhibited fat vacuoles with different size, more inflammatory cell infiltration and necrosis; in the model group from TXNIP−/− mice, liver tissue exhibited much less inflammatory cell infiltration and no noticeable decrease in fat vacuoles (Figure 6f). The results above manifested that TXNIP knockout can repress the expressions of inflammatory factors, lowers LPS concentration, and pathological injury of liver tissues, hence protecting against the development of steatohepatitis.
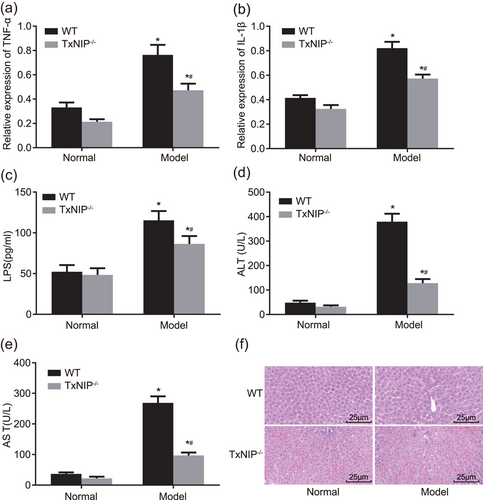
TXNIP knockout protects against liver tissue injury. (a) RT-qPCR was used to determine the mRNA levels of TNF-α in intestinal mucosa in TXNIP−/− mice and WT mice; (b) RT-qPCR was used to determine the mRNA levels of IL-1β in TXNIP−/− mice and WT mice; (c) ELISA was applied to determine the LPS concentration in TXNIP−/− mice and WT mice; (d and e) automatic biochemical analyzer was utilized to detect the serum levels of ALT and AST in TXNIP−/− mice and WT mice; (f) HE staining was conducted to observe the pathological changes of liver tissues (×400); *p < 0.05 versus the normal group; #p < 0.05 versus WT mice. Data are expressed by means ± standard error and analyzed by independent sample t test from three independent experiments. ALT: alanine aminotransferase; AST: aspartate aminotransferase; ELISA: enzyme-linked immunosorbent assay; HE: hematoxylin-eosin; LPS: lipopolysaccharide; mRNA: messenger RNA; RT-qPCR: reverse-transcription quantitative polymerase chain reaction; WT: wild-type [Color figure can be viewed at wileyonlinelibrary.com]
4 DISCUSSION
Epithelial mucins, tight junction proteins, and an impaired intestinal barrier function play key roles in the development of NASH (Matsumoto et al., 2017). A previous study on nonalcoholic fatty liver disease (NAFLD) revealed that oxidative stress and MPO activity both contribute to its development and progression (Pulli et al., 2015, Shih, Hwang, Yeh, & Yen, 2012). Hence, the process of the current study saw NASH models established to elucidate the mechanisms of TXNIP–NLRP3 in NASH. This study provides evidence that suppression of the TXNIP–NLRP3 axis restores intestinal barrier function by reducing MPO activity and oxidative stress in nonalcoholic steatohepatitis induced by high-fat and high-fructose diet (Figure 7).
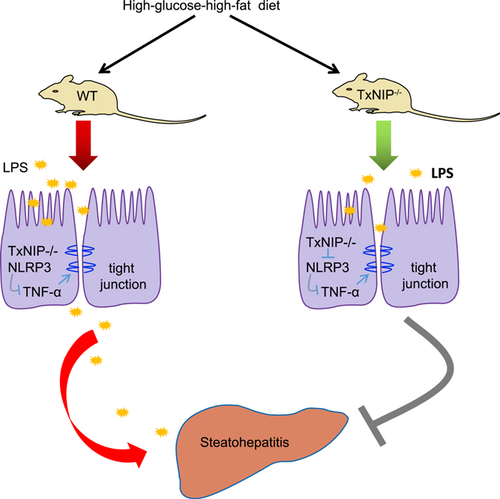
The mechanism map for the effect of TXNIP–NLRP3 axis on MPO activity and oxidative stress in nonalcoholic steatohepatitis induced by high-fat and high-fructose diet. High-fat and high-fructose diet induced disruption of ecological balance of intestinal flora and the production of LPS, thus the TXNIP–NLRP3 axis is activated and release of TNF-α is enhanced. Proinflammatory cytokines could induce the tight junction function injury of epithelial cells, leading to LPS penetrating through the epithelial barrier to the blood circulation and liver tissues. Consequently, the liver injury is aggravated. LPS: lipopolysaccharide; MPO: myeloperoxidase [Color figure can be viewed at wileyonlinelibrary.com]
Based on the obtained results, TXNIP presented to be highly expressed in IECs of NASH mice. TXNIP is considered as a pro-oxidative stress, proinflammatory, and proapoptotic protein under chronic hyperglycemia, diabetes, and cellular stress (Devi, Hosoya, Terasaki, & Singh, 2013). In hepatic gluconeogenesis, through G6pc upregulation via small heterodimer partner (SHP), TXNIP is overabundant in the hyperglycemic state and represses glucose uptake, resulting in imbalanced glucose homeostasis (Jo, Kim, Park, Kim, & Ahn, 2013). TXNIP is expressed at a high level in NAFLD where it mediates hepatic lipogenesis via protein arginine methyltransferase-1 (PRMT1) and proliferator-activated receptor gamma coactivator-1α (PGC-1α) regulation and inflammation (Park et al., 2014). Ablation of TXNIP suppresses the activated NLRP3 inflammasome, decreased oxidative stress, and worsened cell apoptosis (Xiao et al., 2014).
TXNIP links oxidative stress to NOD-like receptor family, NLRP3 inflammasome activation and this signaling axis may be involved in fructose-induced NAFLD, by which ROS-TXNIP pathway contributes to hepatocellular NLRP3 inflammasome activation, lipid accumulation, and inflammation (Zhang et al., 2015). Cadmium (Cd) is a persistent environmental and occupational contaminant that accumulates principally in the liver, functioning in the induction of oxidative stress, and inflammation (Hoet et al., 2012). Previous study reported that Cd-induced liver injury can be significantly alleviated by lowering serum levels of ALT and AST, repressing the production of proinflammatory cytokines, hindering the activation of NLRP3 inflammasome, ameliorating oxidative stress, and attenuating hepatocyte death (Cao et al., 2017). Another study demonstrated that the ischemic heart exhibited enhanced inflammasome activation as evidenced by increased NLRP3 expression, and also it exhibited enhanced interaction between TXNIP and NLRP3, which has been shown to be a mechanism for activating NLRP3 (Liu et al., 2014). In liver inflammation and hepatocyte death, the TXNIP–NLRP3 inflammasome pathway was found to act a promotion role (Wree et al., 2014). Moreover, TXNIP–NLRP3 inflammasome pathway mediated the attenuation of alcoholic cellular injury by Lycium barbarum polysaccharide (LBP; Xiao et al., 2014). Activation of NLRP3 affected epithelial barrier function in reactive cholangiocytes via synthesis of proinflammatory cytokines and impaction on epithelial integrity of cholangiocytes (Maroni et al., 2017). Also, the suppression of TXNIP–NLRP3 inflammasome activation ameliorated endothelial dysfunction, with AMP-activated protein kinase (AMPK) involved (Li et al., 2015). Hence, we further explore the possible mechanisms of the interaction between TXNIP and NLRP3 in intestinal barrier function in NASH.
Another founding in our study, the MPO activity and oxidative stress were manifested to be upregulated in IECs and exhibited a downregulation while silencing TXNIP. Oxidative stress from the generation of ROS is thought to be postulated to be a key component in the progression to NASH (Gornicka et al., 2011). Increased production of ROS leads to oxidative stress and cell death, involving in ATP, NAD, protein damage, and glutathione depletion (Ramírez, Vázquez-Sánchez, Carrión-Robalino, & Camacho, 2016). Oxidative stress also triggers production of inflammatory cytokines, causing inflammation and a fibrogenic response, which ultimately results in the development of NASH, and progresses to end-stage liver disease (Rolo et al., 2012). In addition, oxidative stress is reported to induce intestinal barrier function disruption via the activation of nuclear factor (erythroid-derived-2)-like 2 (Nrf2) and its downstream genes (Song et al., 2017). In human intestinal epithelial cells, the disruption of barrier function resulted from oxidative stress is mediated by H2O2 (Iraha et al., 2013). Emerging data showed that secreted MPO could potentiate oxidative stress and tissue damage by forming hypochlorous acid from hydrogen peroxide and reactive nitrogen species from nitrite or nitric oxide (Klebanoff, 2005). MPO participates in several key scenarios that promote progression to fibrosis (Pulli et al., 2015). It was reported that human livers with NASH contain higher amounts of MPO (Rensen et al., 2009). Moreover, MPO-derived oxidative stress activates transforming growth factor β (TGF-β) and hepatic stellate cells (HSCs), the most important source of collagen in the liver, and MPO-activated HSCs in turn secrete CXCL1 (Pulli et al., 2015). In mice with scald burn injury, MPO were found to be expressed at a high level in intestinal barrier function (Lutmer, Watkins, Chen, Velten, & Besner, 2013). These data demonstrated that TXNIP–NLRP3 axis might enhance MPO activity and oxidative stress leading to intestinal barrier disrupt in NASH.
Collectively, our results suggested that TXNIP knockout along with NLRP3 downregulation confers protection against LPS-induced liver inflammation and intestinal barrier function in NASH via inhibition of the MPO activity and ROS level. Therefore, TXNIP–NLRP3 axis could be a potential predictive biomarker for the treatment of NASH. However, due to the limited simple size and experimental conditions, further studies are needed to define the detailed mechanisms of TXNIP in IECs of NASH.
ACKNOWLEDGMENTS
This study was supported by the China-Japan Friendship Hospital Youth Science and Technology Excellence Project (No. 2015-QNYC-B-02) and the Research Fund of the China-Japan Friendship Hospital (No. 2017-1-MS-1). We would like to acknowledge the reviewers for their helpful comments on this article.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.




