Garcinol suppresses RANKL-induced osteoclastogenesis and its underlying mechanism
Abstract
Osteoclasts (OCs) are multinuclear giant cells responsible for bone resorption, and an excessive bone resorption by OCs plays an important role in osteoporosis. Commonly used drugs for the treatment of osteoporosis have severe side effects. As such, identification of alternative treatments is essential. Garcinol, a polyisoprenylated benzophenone extracted from the fruit of Garcinia indica, has shown a strong antitumor effect through the nuclear factor-κB (NF-κB) and mitogen-associated protein kinases (MAPK) signaling pathways. However, the role of garcinol in the osteoclastogenesis is still unclear. Here, we demonstrated that garcinol can inhibit the receptor activator of NF-κB ligand (RANKL)-induced osteoclastogenesis, osteoclastogenesis-related gene expression, the f-actin ring, and resorption pit formation. In addition, garcinol abrogated RANKL-induced osteoclastogenesis by attenuating the degradation of the MAPK, NF-κB, and PI3K-AKT signaling pathway as well as downstream factors c-jun, c-fos, and NFATC1. In vivo, suppression of osteoclastogenesis by garcinol was evidenced by marked inhibition of lipopolysaccharide-induced bone resorption. In conclusion, our data demonstrated that garcinol inhibited the RANKL-induced osteoclastogenesis by suppressing the MAPK, NF-κB, and PI3K-AKT signaling pathways and thus has potential as a novel therapeutic option for osteolytic bone diseases.
1 INTRODUCTION
Bone remodeling is maintained in a delicate balance by osteoblastic bone formation and osteoclastic bone resorption (Moverare-Skrtic et al., 2014). Osteoclasts (OCs) represent the only known cell type which are capable of resorbing bone matrix, and increasing OCs activity induces several lytic bone diseases such as osteoporosis (Bar-Shavit, 2007). Osteoporosis is associated with considerable disability and increased risk of mortality (Sattui & Saag, 2014). An epidemiological investigation demonstrated that the mean prevalence of osteoporosis in older adults was estimated at 15.7% in the China (Lin et al., 2015), and it is considered to be increasing gradually with the increasing age of the total population. Therefore, agents that modulate the formation and function of OCs are candidates for the treatment of lytic bone diseases, especially osteoporosis.
Osteoclastogenesis is modulated by two necessary factors including the tumor necrosis factor (TNF)-related cytokine receptor activator for the nuclear factor-κ B ligand (RANKL) and macrophage colony-stimulating factor (M-CSF; Boyle, Simonet, & Lacey, 2003), both of which are expressed by osteoblasts. The signaling pathways that regulate the OC differentiation mainly focus on RANKL-associated signaling pathways. The combined RANKL and RANK receptor recruit TNF receptor-associated factors (TRAFs). Only TRAF6 plays an important role in osteoclastogenesis (Bharti, Takada, Shishodia, & Aggarwal, 2004). TRAF6 activates the multiple downstream signaling pathways, mainly nuclear factor-κB (NF-κB), mitogen-associated protein kinases (MAPKs), and AKT, and thereby promotes the expression of oc-related genes.
Garcinol, a polyisoprenylated benzophenone extracted from the fruit of Garcinia indica (Balasubramanyam et al., 2004), has been extensively used to treat gastric ailments and skin irritation. Studies have demonstrated that garcinol exhibits antitumor and anti-inflammatory effects through the NF-κB, MAPK, and PI3K/AKT pathways (Liao, Sang, Ho, & Lin, 2005; Wang, Tsai, Chiou, Ho, & Pan, 2015; Yu et al., 2014), which are also essential for osteoclastogenesis. However, the effect of garcinol on OC formation and lipopolysaccharide (LPS)-induced osteolysis is not yet known.
In the current study, we investigated the effect of garcinol on RANKL-induced osteoclastogenesis and its underlying mechanism, and further assessed the use of garcinol to protect against LPS-induced osteolysis. Our study may contribute to the treatment and prevention of osteoporosis.
2 MATERIALS AND METHODS
Garcinol (Sigma-Aldrich, St. Louis, MO) was dissolved in dimethylsulfoxide (DMSO) and then diluted in cell culture medium so that DMSO comprised <0.1% of the total volume. Alpha modified minimal essential medium (α-MEM), fetal bovine serum (FBS), penicillin, and streptomycin were purchased from Gibco (Rockville, MD). Recombinant murine M-CSF and receptor activator of NF-κB ligand (RANKL) were purchased from Peprotech (Rocky Hill, NJ). The F-actin cytoskeleton staining kit was purchased from Millipore (Darmstadt, Germany). Anti-β-actin was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-c-Fos, anti-phospho-p44/42 MAPK (Erk1/2), anti-p38, anti-erk, anti-P-P65, anti-P65, anti-P-JNK, anti-JNK, anti IκBα, and anti-NFATC1 antibodies were purchased from Cell Signaling Technology. Anti-ERK1/2 (EP197Y) antibodies were purchased from Abcam Inc. (Cambridge, MA). Tartrate-resistant acid phosphatase (TRAP) enzymatic activity was detected using a Leukocyte Acid Phosphatase Staining kit (Sigma-Aldrich). All other reagents were purchased from Sigma-Aldrich unless otherwise stated.
2.1 Cell culture
Bone marrow monocytes (BMMs) were obtained from the femoral bone marrow of C57BL/6 mice at 6 weeks of age. The cells were isolated from the femoral and tibial bone marrow and cultured in α-MEM supplemented with 10% FBS, 1% penicillin/streptomycin, and 25 ng/ml M-CSF in an incubator with 5% CO2 at 37℃ until they reached 90% confluence. The BMMs were seeded in 96-well plates at a density of 8,000 cells/well in triplicate, in the presence of 25 ng/ml M-CSF, 100 ng/ml RANKL, and different concentrations of garcinol. Untreated cells were included as controls. The culture medium was replaced every other day, and multinucleated OCs were observed on differentiation Day 7.
2.2 Pit-formation assays
BMMs (5 × 103 cells/well) were seeded onto bovine cortical bone slices placed in 96-well culture plates in the presence of 100 ng/ml RANKL and 25 ng/ml M-CSF with or without varying concentrations of garcinol (1, 2, and 4 μM) and incubated. Medium was changed on Day 2. After 15 days, the slices were harvested and washed with phosphate-buffered saline (PBS) to remove the cells. Resorption pits were observed using a scanning electron microscope (FEI Quanta 250), and the area of bone resorption was quantified using the ImageJ software (National Institutes of Health, Bethesda, MD).
2.3 TRAP staining and activity assay
To perform TRAP staining, multinucleated OCs from BMMs were washed twice with PBS and then fixed with 4% paraformaldehyde. After a 20-min fixation, paraformaldehyde was washed out with PBS, and then the cells were incubated with TRAP staining solution for 30 min at 37°C. Images of multinucleated cells were captured by light microscopy, and the number of mature OCs was quantified by counting the number of multinucleated TRAP-positive cells (>3 nuclei) in a representative area in each of three replicate samples. The sizes of matured OCs were determined using the ImageJ software (National Institutes of Health).
2.4 Actin rings and nuclear staining
BMMs were incubated in 96-well plates (8 × 103 cells/well) and treated with garcinol at concentrations of 0, 1, 2, or 4 μM in medium supplemented with RANKL (100 ng/ml) and M-CSF (25 ng/mL) for 7 days. After formation of mature OCs, cells were fixed with 4% paraformaldehyde for 20 min and washed with PBS three times. Next, the cells were stained with Acti-stainTM 488 Fluorescent Phalloidin (Cytoskeleton Inc, Denver, CO) at room temperature in the dark for 30 min. After washing in PBS three times, the samples were treated with 4′,6-diamidino-2-phenylindole staining (1 μg/ml) for 5 min. Images were acquired using a fluorescence microscope (Eclipse TS100; Nikon).
2.5 Cytotoxicity assays
BMMs were seeded in 96-well plates at a density of 8,000 cells/well in triplicate in the presence of 25 ng/ml M-CSF. After culturing for 24 hr, the medium was replaced with fresh culture medium containing various concentrations of garcinol (0, 1, 2, 4, 8, 16, and 32 μM) for 24, 48, 72, and 96 hr. Each garcinol concentration was repeated in six wells. At each time point, 0.01 ml the 3-(4,5-dimethylthiazol-2-y1)-2,5-diphenyltetrazolium bromide (MTT) assay solution (5 mg/ml in PBS) was added to each well, and cells were incubated for 4 hr at 37°C. The medium was replaced with 200 µl DMSO for 10 min to solubilize crystals, and optical densities were measured at 570 nm (Multiskan GO; Thermo Fisher Scientific, Waltham, MA).
2.6 Quantitative real-time polymerase chain reaction assays
To detect the effect of garcinol on OC formation and function, BMMs were seeded at a density of 3 × 105 cells/well in 6-well plates with different methods of garcinol treatment. Total cellular RNA was isolated from the cells using Trizol reagent (Invitrogen, Tokyo, Japan). Total RNA (≤1,000 ng) was reverse-transcribed into complementary DNA (cDNA) at a 20 μl-reaction volume using a Double-Strand cDNA Synthesis Kit (Takara, Dalian, China). The polymerase chain reaction (PCR) reaction was performed with 1 μl of cDNA as template in triplicate using the Power SYBR® Green PCR Master Mix (Takara) on the ABI StepOnePlus System (Applied Biosystems, Warrington, UK). The total volume (20 μl) of each PCR reaction consisted of 10 μl of the SYBR Green QPCR Master Mix, 3 μl of double-distilled H2O, 1 μl of cDNA, and 10 μM each of forward and reverse primers. The cycling conditions were as follows: 95°C for 10 min (activation), followed by 40 cycles at 95℃ for 10 s, 60℃ for 20 s, and 72℃ for 90 s. β-Actin was utilized as the housekeeping gene, and independent experiments were repeated at least three times. The specific primers (based on the mouse sequences) used are shown in Table 1.
| Gene | Forward primers | Reverse primers |
|---|---|---|
| β-actin | 5′-AGC CAT GTA CGT AGC CAT CC-3′ | 5′-CTC TCA GCA GTG GTG GTG AA-3′ |
| TRAP | 5′-TCC TGG CTC AAA AAG CAG TT-3′ | 5′-ACA TAG CCC ACA CCG TTC TC-3′ |
| CTSK | 5′-CTT CCA ATA CGT GCA GCA GA-3′ | 5′-TCT TCA GGG CTT TCT CGT TC-3′ |
| NFATc1 | 5′-CAG CTG CCG TCG CAC TCT GGT C-3′ | 5′-CCC GGC TGC CTT CCG TCT CAT A-3′ |
| V-ATP d2 | 5′-AAG CCT TTG TTT GAC GCT GT-3′ | 5′-TTC GAT GCC TCT GTG AGA TG-3′ |
- Note. TRAP: tartrate-resistant acid phosphatase.
2.7 Western blot analysis
Cellular proteins were extracted after cell disintegration with the radioimmunoprecipitation assay lysis buffer and centrifuged at 16,000×g for 10 min at 4°C. The supernatant containing protein was collected, and the protein concentration was measured using a bicinchoninic acid protein assay kit. The protein was then mixed with sodium dodecyl sulfate-sampling buffer, followed by incubation at 95°C for 5 min. The protein samples were separated and transferred by electroblotting onto membranes, which were incubated with blocking buffer for 1 hr. The blocked membranes were then incubated with a targeted antibody overnight at 4°C, washed three times with 1× tris-buffered saline plus Tween for 5 min each time, and then incubated with a secondary antibody for 1 hr. Finally, the bands were detected via analysis of immunoreactivity using a Western Lighting Ultra Kit with a FujiFilm Las-4000 gel documentation system and quantified using a chemiluminescence imaging system (ChemiDoc XRS; Bio-Rad, Hercules, CA).
2.8 LPS-induced calvarial osteolysis mice model
To measure the osteolysis-suppressing effect of garcinol in vivo, a mouse calvarial osteolysis model was established. All experimental procedures were performed in accordance with the guidelines of the Animal Care Committee of Zhejiang University. C57BL/6 mice were purchased from Zhejiang University Institutional Animal Care and Use Committee (No. 11785). Briefly, twenty-four 6- to 8-weeks old C57 male mice were randomly assigned to four groups: control (injection with PBS), LPS (injection of 5 mg/kg LPS and PBS), low-dose garcinol (injection of 5 mg/kg LPS and 1 mg/kg garcinol), and high-dose garcinol (injection of 5 mg/kg LPS and of 5 mg/kg garcinol). Garcinol was dissolved in 5% DMSO in PBS. The mice received a subcutaneous injection over the sagittal midline suture of the calvarium under light inhalation anesthesia. PBS and garcinol were injected on alternating days over a 7-day period. At the end of the experiment, the mice were killed. The calvaria were separated and fixed with 4% paraformaldehyde for micro-CT and histological analysis. The calvaria samples were analyzed using a high-resolution micro-CT (Skyscan 1072; Skyscan, Aartselaar, Belgium). The bone volume/tissue volume (BV/TV) and the porosity of each sample were measured. The fixed calvaria were decalcified in 10% ethylenediaminetetraacetic acid (EDTA) (pH 7.4) for 2 weeks and then embedded in paraffin. Then, histological sections were prepared for TRAP and hematoxylin and eosin (H&E) staining, and the specimens were examined and imaged under a high-quality microscope. The number of TRAP-position OCs and OCs per bone surface (OCs/BS) were examined in each sample utilizing the ImageJ software.
2.9 Micro-computed tomography scanning
Three-dimensional (3D) reconstructions of whole calvaria were obtained from images acquired using a high-resolution micro-computed tomography scanner (µCT 100; Scanco, Switzerland). The image acquisition was carried out at a voltage of 80 kV and current of 100 mA. Cone Beam Reconstruction software (SkyScan) was then used to restrict a 3D image. A nummular region of interest (ROI; 3 × 3 × 1 mm) cantered around the midline suture was used to analyze the osteolysis-related index. The ratio of BV/TV % and the number and area of pores within the ROI were measured using the CT Analyzer software (SkyScan).
2.10 Histological analyses
After fixing in 10% formaldehyde for 3 days, calvarial bones were decalcified in 10% EDTA for 3 weeks and embedded in paraffin. Histological sections were prepared for TRAP staining and H&E staining, and the sections were analyzed using a high-quality microscope. The number of TRAP-positive multinucleated OCs normalized to the bone area and the OC TRAP (+) surface area normalized to the bone surface area was analyzed by the Image ProPlus 6.0 software for each sample.
2.11 Statistical analysis
All experimental data are presented as the mean ± standard deviation from at least three independent experiments. Statistical analyses were conducted using Student's t test, one-way analysis of variance, and SPSS 19.0 software (SPSS Inc.). Values of p < 0.05 and p < 0.01 were considered statistically significant.
3 RESULTS
3.1 Garcinol inhibits RANKL-induced OC formation in vitro
The chemical formula of garcinol is shown in Figure 1a. To assess cytotoxicity of garcinol, BMMs were exposed to varying doses of garcinol (0–32 μM) for 24, 48, 72, and 96 hr. The results showed that viability of BMMs was not affected by garcinol at doses up to 4 μM (Figure 1b).
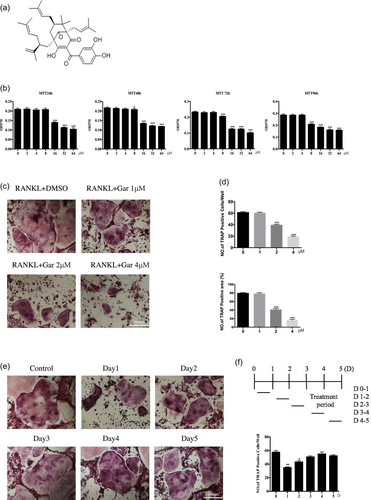
Garcinol inhibits RANKL-induced osteoclast (OC) formation in vitro. (a) Chemical structure of garcinol. (b) Effects of garcinol on viability of BMMs at 24, 48, 72, and 96 hr (n = 6). (c) BMMs were stimulated with RANKL at different concentrations of garcinol and histochemically stained for TRAP detection. TRAP-positive cells with ≥3 nuclei were considered OCs (magnification = 100×; scale bar = 200 μm). (d) Analysis of numbers and areas of TRAP-positive multinucleated (nuclei >3) cells formed in the presence of increasing concentrations of garcinol (n = 3). (e) BMMs were treated with garcinol (4 μM) at the indicated time, RANKL was stimulated in the BMMs, and samples were subjected to TRAP staining (magnification = 100×; scale bar = 200 μm). (f) Analysis of numbers and areas of TRAP-positive multinucleated (nuclei >3) cells by ImageJ. Values are the mean ± standard deviation (SD; n = 3; *p < 0.05, **p < 0.01, ***p < 0.001). BMMs: bone marrow monocytes; DMSO: dimethyl sulfoxide; RANKL: receptor activator for the nuclear factor-κ B ligand; TRAP: tartrate-resistant acid phosphatas [Color figure can be viewed at wileyonlinelibrary.com]
To examine the effects of garcinol on osteoclastogenesis, BMMs were treated with 100 ng/ml RANKL and 25 ng/ml M-CSF for 7 days with or without 1, 2, or 4 μM garcinol and then fixed and stained to visualize TRAP activity. The results demonstrated that garcinol significantly reduced the cell size and number of TRAP-positive multinucleated cells in a dose-dependent manner compared with the cells without garcinol treatment (Figure 1c). Analysis of the numbers and areas of TRAP-positive multinucleated (nuclei >3) cells are shown in Figure 1d.
Furthermore, to explore the inhibitory effect of garcinol on the process of osteoclastogenesis, garcinol was added to BMMs cultured with 100 ng/ml RANKL and 25 ng/ml M-CSF at five different time points (0–1, 1–2, 2–3, 3–4, and 4–5 days) (Figure 1e,f). The medium was replaced with new culture medium without garcinol after 24 hr culture. The results showed that garcinol strongly inhibited OC formation when BMMs were exposed to garcinol on Days 0–1 and 1–2; however, the inhibitory effect was not observed when garcinol was added on Days 2–3, 3–4, and 4–5. These results indicated that garcinol inhibits the OC differentiation in the early stage of OC differentiation, but does not affect the later stages of the process.
3.2 Garcinol attenuates the formation of the F-actin ring and bone resorption in vitro
After confirmation that garcinol suppresses OC differentiation in vitro, the effect of garcinol on RANKL-induced OC actin ring formation was evaluated. Actin ring formation is the most visible feature of mature OCs during osteoclastogenesis. The results showed that both size and number of actin ring structures were significantly reduced in a dose-dependent manner by treatment with garcinol (Figure 2a). To investigate the effect of garcinol on bone resorption, BMMs were cultured on bone slices following treatment with or without different concentrations of garcinol for 15 days. Bone-resorbed areas of garcinol-treated mature OCs were significantly reduced compared with those of the untreated controls (Figure 2c,d). These findings suggested that garcinol could be used as a medicine to inhibit osteoclastic bone resorption in vitro.
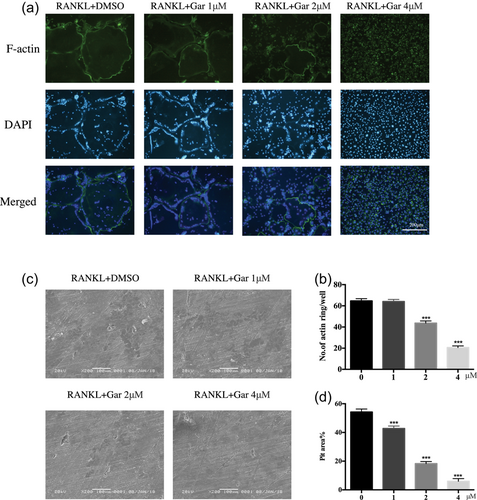
Garcinol attenuates the formation of F-actin ring and bone resorption in vitro. (a) BMMs were stimulated by RANKL with different concentrations of garcinol and stained with phalloidin-FITC and DAPI (magnification = 100×; scale bar = 200 μm). (b) The number and area of F-actin rings were measured using ImageJ (n = 3). (c) BMMs were cultured on bone slices and stimulated by M-CSF and RANKL in the presence of different concentrations of garcinol. After 15 days, bone resorption lacunae was observed by scanning electron microscopy (SEM) (magnification = 100×; scale bar = 200 μm). (d) Area of bone resorption was measured by ImageJ. Values are the mean ± standard deviation (SD; n = 3; *p < 0.05, **p < 0.01, ***p < 0.001). BMMs: bone marrow monocytes; DAPI: 4′,6-diamidino-2-phenylindole; DMSO: dimethyl sulfoxide; M-CSF: macrophage colony-stimulating factor; RANKL: receptor activator for the nuclear factor-κ B ligand [Color figure can be viewed at wileyonlinelibrary.com]
3.3 Garcinol inhibits OC-specific gene expression levels in vitro
To further clarify the role of garcinol on OC differentiation and function, we utilized qPCR to examine the expression of OC-specific genes. Genes evaluated were TRAP, CTSK, Nfatc1, and V-ATPase d2, which are generally upregulated during the process of osteoclastogenesis. Results revealed that the expression levels of these OC marker genes were markedly inhibited in a dose-dependent manner by garcinol after 3 days culture with RANKL (Figure 3a). As for the inhibitory effect of garcinol on the process of osteoclastogenesis, the results showed that garcinol suppressed RANKL-induced OC-specific gene expression through the process of osteoclastogenesis (Figure 3b). Therefore, the garcinol treatment strongly inhibited osteoclastogenesis by decreasing the expression of OC-specific genes. These data indicated that garcinol suppressed osteoclastogenesis by inhibiting RANKL-induced OC-related gene expression.
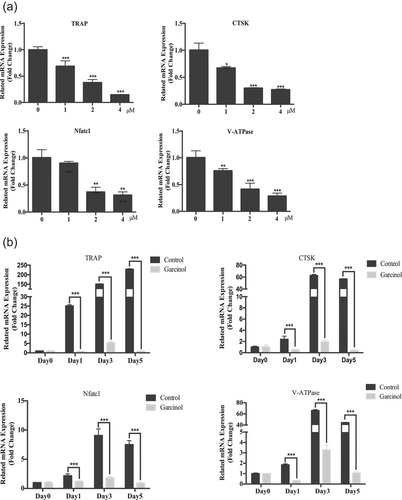
Garcinol inhibited osteoclast (OC)-specific gene expression levels in vitro. (a) qPCR was used to measure the relative levels of RANKL-induced OC-related gene expression in the presence of different concentrations of garcinol. The expression levels of these genes were normalized to the expression of β-actin (n = 3). (b) qPCR was used to measure the relative levels of RANKL-induced OC-related gene expression at the different times after treatment with garcinol. The expression levels of these genes were normalized to the expression of β-actin (n = 3). Values are the mean ± standard deviation (SD; n = 3; *p < 0.05, **p < 0.01, ***p < 0.001). mRNA: messenger RNA; qPCR: quantitative PCR; RANKL: receptor activator for the nuclear factor-κ B ligand
3.4 Garcinol inhibits RANKL-induced activation of the MAPK, NF-κB, and PI3K-AKT pathways
The MAPK, NF-κB, and PI3K-AKT signaling pathways are necessary for RANKL-induced OC differentiation and function. We investigated whether garcinol inhibited these signaling pathways during osteoclastogenesis. BMMs were incubated in serum-free medium and pretreated with or without garcinol (4 μM) for 1 hr. Then, cells were treated with RANKL, with or without garcinol for 0, 5, 10, 20, 30, and 60 min. Garcinol inhibited the MAPK signaling pathway (Figure 4a), as evidenced by reduction of RANKL-induced phosphorylation of ERK (p-ERK), p38 (p-p38), and JNK (p-JNK), the main signaling factors in the MAPK pathway. (Figure 4a).
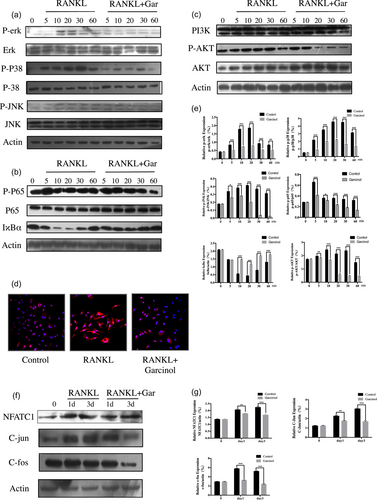
Garcinol decreased RANKL-stimulated MAPK, NF-κB, and PI3K-AKT signaling pathways. (a,b,c) BMMs were incubated in serum-free medium and pretreated with garcinol (4 μM) or vehicle for 1 hr and stimulated with RANKL for the indicated time. The cell lysates were probed for protein levels using western blot analysis (n = 3). (d) Nuclear translocation of p65 was visualized using immunofluorescence. (e) The expression of p-ERK related to total ERK, p-p38 related to total p38, p-JNK related to total JNK, p-p65 related to total p65, IκBα related actin and p-AKT related to total AKT were determined by ImageJ. (f) BMMs were cultured with RANKL (100 ng/ml) and M-CSF (25 ng/ml) with or without garcinol (4 μM) for 0, 1, or 3 days. The cell lysates were probed for NFATC1, C-fos, and C-jun protein levels using western blot analysis (n = 3). (g) The expression of NFATC1, C-fos, and C-jun relative to β-actin was determined using ImageJ. (SD; n = 3; *p < 0.05, **p < 0.01, ***p < 0.001). BMMs: bone marrow monocytes; MAPK: mitogen-associated protein kinases; NF-κB: nuclear factor-κB; p-ERK: phosphorylation of ERK; p-JNK: phosphorylation of JNK; p-p38: phosphorylation of p38; p-p65: phosphorylation of p65; RANKL: receptor activator for the nuclear factor-κ B ligand; SD: standard deviation [Color figure can be viewed at wileyonlinelibrary.com]
In addition, garcinol inhibited the NF-κB pathway (Figure 4b). Phosphorylation of p65 was significantly reduced by garcinol at each time point. IκBα is an upstream regulator of NF-κB that regulates nuclear translocation of NF-κB p65. We examined the cells for IκBα by western blot analysis and found that garcinol suppressed degradation of IκBα. (Figure 4b). The immunofluorescence staining of p65 also confirmed that garcinol abrogated the translocation of p65 from cytoplasm to nucleus (Figure 4d).
As RANKL-induced AKT activation is important in OC differentiation and function, we examined levels of AKT phosphorylation upon garcinol stimulation. As summarized in Figure 4c, garcinol inhibited AKT phosphorylation, but did not affect the expression level of PI3K.
NFATC1, C-jun, and C-fos, the downstream transcription factors involved in osteoclastogenesis, serve as the master regulators of osteoclastogenesis. In our study, we found that the protein expression levels of NFATC1, C-jun, and C-fos increased in a time-dependent manner at 0, 1, and 3 days as a result of stimulation with RANKL. However, garcinol treatment strongly inhibited the elevation of protein expression levels of NFATC1, C-jun, and C-fos (Figure 4e). Consequently, our data demonstrated that garcinol inhibited osteoclastogenesis through the MAPK, NF-κB, and PI3K-AKT signaling pathways.
3.5 Garcinol prevents LPS-induced osteolysis
After exploring the inhibitory effect of garcinol on osteoclastogenesis in vitro, the potential protective effect of garcinol in a mouse calvarial model of LPS-induced osteolysis was further explored. LPS (5 mg/kg) was injected into the sagittal suture of the calvarium with or without garcinol (1 mg/kg or 5 mg/kg garcinol) for 7 days. µCT and 3D reconstruction revealed that both the BV/TV and the porosity of the LPS-treated group were highly decreased compared with the control group (PBS injection). However, garcinol-treated groups showed a marked dose-dependent inhibitory effect on LPS-induced osteolysis (Figure 5a–d). In addition, histological assessment further confirmed that garcinol promoted the LPS-induced osteolysis (Figure 6a). The garcinol group showed increased BV/TV and decreased TRAP-positive OCs/BS in a dose-dependent manner (Figure 6b–d). Taken together, our data demonstrate that garcinol protects against LPS-induced osteolytic bone loss in vivo.
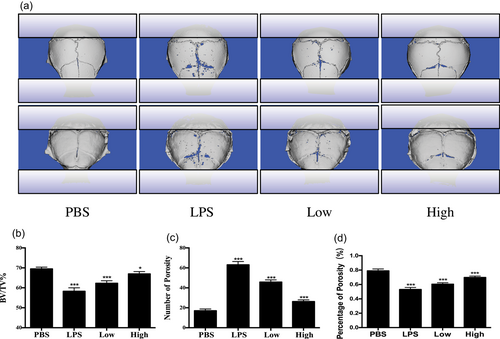
Garcinol protects against LPS-induced bone loss in vivo. (a) µCT scanning and 3D reconstruction of the entire calvaria of mice from the control group (PBS), LPS group (5 mg LPS/kg body weight), LPS with low-dose garcinol group (1 mg/kg), and LPS with high-dose garcinol group (5 mg/kg) (n = 6). (b) Bone volume to tissue volume (BV/TV, %), porosity, and the percentage of porosity were measured and analyzed by ImageJ (n = 6). (SD; n = 6; *p < 0.05, **p < 0.01, ***p < 0.001). LPS: lipopolysaccharide; µCT: micro-computed tomography; PBS: phosphate-buffered saline; SD:standard deviation; 3D: three-dimensional [Color figure can be viewed at wileyonlinelibrary.com]
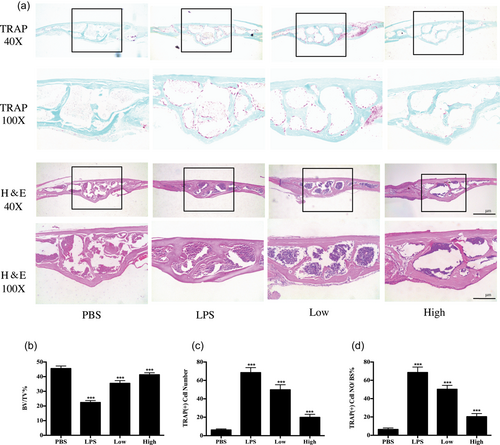
Histological and histomorphometric analysis of the effect of garcinol on LPS-induced bone loss in vivo. (a) Representative images of calvaria stained with H&E and TRAP from the control group (PBS), LPS group (5 mg LPS/kg body weight), LPS with low-dose garcinol group (1 mg/kg), and LPS with high-dose garcinol group (5 mg/kg). Black arrows indicate TRAP-positive cells. (b–d) Quantification of BV/TV, TRAP (+) cell number, and TRAP (+) cell number/bone surface (n = 6). (SD; n = 6; *p < 0.05, **p < 0.01, ***p < 0.001). BV/TV: bone volume/tissue volume; H&E: hematoxylin and eosin; LPS: lipopolysaccharide; PBS: phosphate-buffered saline; SD: standard deviation; TRAP: tartrate-resistant acid phosphatase [Color figure can be viewed at wileyonlinelibrary.com]
4 DISCUSSION
In this study, we demonstrated that garcinol markedly inhibits the OC formation and bone resorption by downregulating OC-related gene expression and attenuating the RANKL-induced MAPK, NF-κB, and PI3K-AKT signaling pathways in vitro. In addition, we demonstrated the dose-dependent protective effects of garcinol against LPS-induced osteolysis in vivo by µCT and histological analysis. Our study indicated that garcinol is a potential candidate for the treatment of osteolytic bone diseases, such as osteoporosis.
The underlying mechanism that modulates OC differentiation mainly focuses on the receptor activator for the NF-κB ligand (RANKL)-associated signaling pathways.
The MAPK signaling pathway, including the downstream transcription factors AP-1 (C-Fos and C-Jun), are essential for OC formation (Grigoriadis et al., 1994; Kashiwada et al., 1998). To date, many drug monomer compositions derived from natural plants have been confirmed to have inhibitory effects on OC differentiation and function by suppressing the MAPK signaling pathway. For example, artemether, a natural compound derived from Artemisia annua, impaired OC differentiation by inhibiting the RANKL-induced MAPK signaling pathways (Wu et al., 2018). Glycyrrhizin, a triterpenoid saponin glycoside, exhibits antiosteoclastogenesis effect through the MAPK signaling pathway (Ryu et al., 2018). In this study, we demonstrated for the first time that garcinol exerts antiosteoclastogenesis effects via suppression of RANKL-induced activation of the MAPK signaling pathway by downregulation of RANKL-induced p-ERK, p-p38, and p-JNK. In addition, p-ERK can translocate to the nucleus where it phosphorylates and activates OC-promoting AP-1 (c-Fos and c-Jun) (Qiao et al., 2016). We also showed that garcinol inhibited phosphorylated ERK-induced activation of AP-1 (c-Fos and c-Jun) in a time-dependent manner.
The binding of RANKL to the RANK receptor triggers activation of the NF-κB signaling pathway, which is an early signaling event leading to OC differentiation (J. M. Kim et al., 2017). In addition, two catalytic components IKKα and IKKβ, work together to degrade IκB after RANKL stimulation, resulting in NF-κB p65/p50 complex release and translocation from the cytoplasm to the nucleus (Soysa & Alles, 2009). Inactivation of IKK is sufficient for inhibition of osteoclastogenesis and for prevention of osteoporosis (Chaisson et al., 2004). In the current study, we observed that garcinol could abrogate the degradation of IKKα and suppress p65 phosphorylation. Moreover, immunofluorescence staining confirmed the inhibitory effects of garcinol on nuclear translocation of p65. NFATc1 is the key molecular target of the NF-κB signaling pathway in osteoclastogenesis (K. Kim, Kim, Youn, Jin, & Kim, 2010). We also demonstrated that garcinol inhibits RANKL-induced activation of NFATC1 in a time-dependent manner in this study.
Activation of the AKT pathway, a downstream mediator of RANK signaling plays an important role in regulating cell proliferation, differentiation, and apoptosis (Gingery, Bradley, Shaw, & Oursler, 2003). In this study, we found that garcinol dramatically suppressed the RANKL-induced phosphorylation of AKT in BMMs. These data imply that garcinol reduces RANKL-induced osteoclastogenesis through regulating the AKT signaling pathway.
LPS has been identified as a key pathogen in infective osteolytic diseases such as osteomyelitis and septic arthritis (Nason, Jung, & Chole, 2009). Inflammatory cytokines induced by LPS can cause bone loss by stimulating OC formation (Zhang et al., 2016). Therefore, we used LPS to induce bone osteolysis in a mouse calvarial model and explored whether administration of garcinol protects against LPS-induced bone loss. As expected, garcinol treatment significantly mitigated the severity of bone erosion in this model, illustrated by an increase in BV/TV and a decrease in porosity as shown by µCT scanning. Similarly, bone histomorphometry further confirmed that garcinol treatment could inhibit LPS-induced inflammatory bone loss in vivo as demonstrated by a decrease in TRAP-positive cells. Consistent with antiosteoclastogenic and antiresorptive effects in vitro, our in vivo results demonstrated that garcinol greatly reduced the bone resorption induced by LPS administration.
Despite our findings, we cannot exclude the possibility that garcinol might affect osteoblastic bone formation. In addition, compared with other studies that used an ovariectomy animal model to explore osteoporosis, we did not directly evaluate treatment of osteoporosis using garcinol. Thus, further investigations are needed.
In summary, we demonstrated that garcinol significantly inhibited the osteoclastogenesis and prevented bone loss in vitro and in vivo for the first time. Therefore, garcinol has potential therapeutic effects on OC-related osteolytic diseases.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (81572126), Natural Science Foundation of Zhejiang Province (LQ16H160013), and Zhejiang Basic Public Welfare Research Project (LGF18H060010).
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.




