Expression of myostatin in human hematopoietic cells unveils novel autocrine/paracrine actions for the hormone
Abstract
Myostatin is a member of the transforming growth factor β (TGFβ) superfamily that has a well-established role as a mediator of muscle growth and development. However, myostatin is now emerging as a pleiotropic hormone with multiple actions in the regulation of the metabolism as well as several aspects of both cardiac and smooth muscle cells physiology. In addition, myostatin is also expressed in several nonmuscular cells where its physiological role remains to be elucidated in most cases. In this report, we have shown that both myostatin and its receptor system are expressed in blood cells and in hematopoietic cell lines. Furthermore, myostatin treatment promotes differentiation of both HL60 and K562 cells through a mechanism that involves activation of extracellular signal-regulated kinases 1/2 and p38-mitogen-activated protein kinase, thus leading to the possibility that myostatin may be a paracrine/autocrine factor involved in the control of haematopoiesis. In addition, the presence of myostatin expression in immune cells could envisage a novel role for the hormone in the pathogenesis of inflammatory diseases.
1 INTRODUCTION
Myostatin, also termed growth/differentiation factor-8 (GDF-8), is a highly conserved member of the transforming growth factor β (TGFβ) superfamily that functions as an endogenous inhibitor of muscle growth. Loss-of-function of the Mstn gene leads to a dramatic and widespread increase in skeletal muscle mass (Grobet et al., 1997; McPherron, Lawler, & Lee, 1997; Schuelke et al., 2004; Wang et al., 2015), while overexpression or systemic administration of myostatin causes muscle atrophy (McPherron et al., 1997; Zimmers et al., 2002). All these actions are mainly exerted through the regulation of the proliferation and differentiation of myoblasts (Langley et al., 2002; Ríos, Carneiro, Arce & Devesa, 2001, 2002; Thomas et al., 2000).
The Mstn gene is predominantly expressed in cells of skeletal muscle lineage throughout embryonic development as well as in adult individuals (Lee, 2004). However, expression of the Mstn gene has been also reported in other tissues, although at a much lower level. These include both cardiac (Bish, Morine, Sleeper, & Sweeney, 2010; Sharma et al., 1999; Shyu et al., 2005) and smooth (Ciarmela, Wiater, Smith, & Vale, 2009; Kovanecz et al., 2017; Verzola et al., 2017) muscle cells, as well as white and brown adipose tissue (McPherron & Lee, 1997; Steculorum et al., 2016), mammary gland (Ji et al., 1998; Manickman et al., 2008), liver (Jiao et al., 2011; McFarlane et al., 2014), or placenta (Mitchell, Osepchook, Leung, McMahon, & Bass, 2006). In contrast with the skeletal muscle, the physiological role of myostatin in these tissues remains to be fully elucidated in most cases.
In the present report, we have investigated the presence of myostatin expression in cells of hematopoietic linage. Our findings demonstrate that myostatin is expressed in hematopoietic cells, where it may act as an autocrine/paracrine factor regulating cell differentiation and survival. On the view of these findings, an emerging role for myostatin as a regulator of hematopoietic function may be envisaged.
2 MATERIALS AND METHODS
2.1 Cell cultures and treatments
Experiments were carried out in HL60, Jurkat, and K562 cells, obtained from the European Collection of Animal Cell Cultures (ECACC, Wiltshire, UK). Cells were grown in Falcon polystyrene culture dishes (BD Biosciences, San Jose, CA) containing Roswell Park Memorial Institute 1640 medium (Sigma-Aldrich, St. Louis, MO) supplemented with 10% foetal calf serum (Life Technologies, Carlsbad, CA), 2 mM l-glutamine (Sigma-Aldrich), 100 UI/ml penicillin and 100 mg/ml streptomycin, at 37°C in 5% humidified CO2. Recombinant human myostatin and myostatin propeptide were purchased from R&D Systems (Minneapolis, MN). Dimethyl sulfoxide (DMSO) and 12-O-tetradecanoylphorbol-13-acetate (TPA) were purchased from Sigma-Aldrich.
2.2 Peripheral blood cells
Blood samples were obtained from normal human volunteers (five male and five female), aged 25–35 years. Informed consent was obtained in all cases, and the samples were destroyed at the end of the study. Samples were processed for both RNA extraction and immunocytochemistry as described below.
2.3 Reverse-transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from cell cultures with TRIzol reagent (Invitrogen, Carlsbad, CA). RNA from blood cells was extracted with a commercial kit, QIAamp® RNA Blood (Qiagen, Hilden, Germany), as described in the manufacturer’s protocol. In both cases, 1 μg of RNA was reverse-transcribed for 1 hr at 37°C with 200 U of Moloney Murine Leukemia Virus Reverse Transcriptase (Invitrogen), followed by 5 min at 95°C, in a 30 μl reaction mixture containing 50 mM Tris–HCl (pH 8.3), 5.5 mM MgCl2, 0.5 mM each deoxyribonucleotide triphosphate (dNTP), 40 U of RNaseOUT (Invitrogen) and 1.7 μg/μl of random primers (Invitrogen). Three microliters of the RT reaction were amplified by PCR with 1.25 U of Taq DNA polymerase (Invitrogen) in 50 μl of a reaction mixture containing 20 mM Tris–HCl (pH 8.4), 50 mM KCl, 2 mM MgCl2, 0.2 mM each dNTP and 0.4 μM each oligonucleotide primer. The housekeeping gene hypoxanthine guanine phosphoribosyl transferase (HPRT) was used as a control. The oligonucleotides sequences were as follows (products lengths are indicated in brackets): HPRT (138 basepair [bp]), upper: 5′-CAGCCCTGCCGTCGTGATTA-3′, lower: 5′-AGCAAGACGTTCAGTCCTGTC-3′; myostatin (345 bp), upper: 5′-GCACTGGTATTTGGCAGAGCA-3′, lower: 5′-CACACTCTCCAGAGCAGTAAT-3′; activin-like kinase 4 (ALK4; 267 bp), upper: 5′-CCCTCTTTGTCCAGCGCACAG-3′, lower: 5′-ATCAAACAGGGACCCTGCTCAT-3′; ActRIIA (388 bp) upper: 5′-TACT-GCTGCAGATGGACCTG-3′, lower: 5′-AGCTCCAGTTCAGAGTCCCA-3′; activin type IIB receptor (ActRIIB; 252 bp) upper: 5′-ACACGGGAGTGCATCTACTACAA-3′, lower: 5′-GGCAAATGAGTGAAGCGCTCGTT-3′. The PCR products were resolved in 2% agarose gel stained with ethidium bromide and visualised in a GelDoc 1000 (Bio-Rad Laboratories, Berkeley, CA).
2.4 Western blot analysis
Cells were collected by centrifugation, and the pellet was lysated by heating at 95°C for 5 min in radioimmunoprecipitation assay buffer (Sigma-Aldrich), containing aprotinin (30 μg/ml), Na3VO4 (100 mM) and phenyl methyl sulfonyl fluoride (PMSF; 10 mg/ml). Lysates were immediately cooled at 4°C and centrifuged (15,000g, 15 min, 4°C) to separate cellular debris. The lysates were resolved by sodium dodecyl sulphate-polyacrylamide gel electrophoresis, and electrotransferred onto a nitrocellulose paper (Whatman, Maidstone, UK). Membranes were then blocked overnight in tris-buffered saline (TBS) buffer with 0.1% Tween-20% and 0.2% casein, before incubation with the primary antibodies: Anti-myostatin (dilution 1:5000; kindly provided by Dr. Cadavid), anti-Smad2 (dilution 1:1000), anti-phospho-Smad2 (dilution 1:1000), anti-ERK-1/2 (dilution 1:1000), anti-phospho-ERK-1/2 (dilution 1:1000), anti-p38-mitogen-activated protein kinases (MAPKs; dilution 1:1000; all from Cell Signalling Technology, Danver, MA), and anti-tubulin (dilution 1:5000; Sigma-Aldrich). Immunoreactive bands were detected with a western-light chemiluminescence detection system (ECL; Amersham Pharmacy Biotech, Freiburg, Germany) and photographed (HyperfilmECL; GE Healthcare Bio-Sciences AB).
2.5 Immunocytochemistry
Cells were cytospined onto coated microscope slides (FLEX IHC microscope slides; Dako, Glostrup, Denmark) and fixed. To enhance antigen retrieval, cells were submerged in citrate buffer (10 mM, pH 6.0) and preheated in microwave (H2800 Microwave Processor; EBSciences, East Granby, CT). Slides were then rinsed twice in TBS and incubated with a polyclonal anti-myostatin antibody (dilution 1:200) overnight at 4°C. Myostatin immunoreactivity was detected using the EnVision detection kit (Dako) according with the manufacturer’s instructions. Endogenous peroxidase activity was quenched by incubating the slides in peroxidase-blocking solution (Dako) for 10 min. For negative controls, anti-myostatin antibodies were previously incubated with recombinant myostatin overnight at 4°C (data not shown).
2.6 Assessment of cell differentiation
HL60 cells were seeded in triplicate in 35 mm dishes (4 × 105 cells/dish) and treated with myostatin (125 ng/ml; R&D Systems), DMSO (1.3%), TPA (10 nM), myostatin + DMSO, myostatin + TPA, or vehicle. Cell differentiation was evaluated by determination of CD33 levels by flow cytometry, using an anti-CD33 monoclonal antibody coupled to fluorescein isothiocyanate (FITC; BD Pharmingen, Franklin Lakes, NJ). To investigate changes in cell morphology induced by myostatin treatment, cells were cytospined onto coated microscope slides, fixed, and stained with the Wright–Giemsa method using a Hema-tek automated haematologic slide stainer (Siemens Medical Solutions Diagnostics, Munich, Germany). Vehicle- or DMSO-treated cells were used as negative or positive controls, respectively. Differentiation of K562 cells was scored by benzidine staining that allows the determination of their haemoglobin content. To this end, cells were grown and treated as described above, collected, and washed twice with PBS. Cells were then incubated in a staining solution containing 1.5 mg/ml benzidine, 25 µl of 30% H2O2, and 50 µl acetic acid, for 30 min. Cells were scored in a Neubauer chamber, under a light microscope (Optiphot-2; Nikon Corporation, Tokyo, Japan). The brown–blue-stained cells were taken as the positive cells.
2.7 Cell growth assays
To evaluate the effect of myostatin on cell growth, 1 × 105 cells were seeded in triplicate in 15 mm dishes, and treated with recombinant myostatin (125 ng/ml) for 96 hr. Control cells were treated with isovolumetric amounts of vehicle. Cell number was determined every 24 hr with a Neubauer counting chamber. To evaluate cell cycle progression, cells were labelled with propidium iodide (PI) and analysed by flow cytometry using a FacScan (BD Pharmingen). Data analysis was performed with the CellQuest software (BD Pharmingen).
2.8 Assessment of cell survival
Apoptotic cell death was assessed in HL60 cells by both determination of annexin V binding and fluorescence microscopic analysis of cell DNA staining patterns with Hoechst 33258. Cells were seeded in triplicate in 15-mm dishes (3 × 105 cells/dish) and grown in serum-free media for 96 hr in the presence of myostatin (125 ng/ml) or vehicle. Annexin V binding was determined by flow cytometry using the annexin V-FITC apoptosis detection kit (BD Pharmingen), according with the instructions given by the manufacturer. PI was used as the viability marker. For determination of cell DNA staining patterns, cells were incubated with 50 nM Hoechst 33258 (Sigma-Aldrich) for 40 min at 37°C. After washing, cell morphology was examined under an Olympus fluorescence microscope (IX70; Olympus Optical Co, Tokyo, Japan) with the appropriate filter combination. Cells were scored for apoptosis by their nuclear morphology (shrinkage, condensation, and fragmentation) and the higher intensity of blue fluorescence. In K562 cells, cell viability was investigated by trypan blue staining (Sigma-Aldrich). Cells were cultivated and treated as indicated above and, at the end of the treatment period, cells were collected and resuspended Hank’s balanced salt solution (HBSS; Invitrogen). Finally, 0.2 ml of cell suspension were incubated with 0.5 ml trypan blue stain (0.4% wt/vol) and 0.3 ml HBSS for 5–15 min at room temperature. Positive cells were scored under a light microscope (Optiphot-2).
2.9 Statistical analysis
Statistical analysis was performed with the nonparametric Mann–Whitney test. Statistical significance was established at p < 0.05.
3 RESULTS
We used three different and complementary experimental qapproaches to investigate the existence of myostatin expression in both hematopoietic cell lines and human peripheral blood cells: Western blot analysis, immunocytochemistry, and RT-PCR. Western blot of protein extracts obtained from either HL60 or Jurkat cells revealed the presence of an immunoreactive band migrating at the size of about 25 kDa, while no immunoreactivity was detected in cell extracts obtained from K562 cells (Figure 1a). Similarly, the presence of myostatin immunoreactivity was detected by immunocytochemistry in both HL60 and Jurkat cells, but not in K562 cells (Figure 1b). Interestingly, myostatin immunoreactivity was mostly detected in association the plasma membrane regions neighbouring to other cells, thus suggesting that myostatin may be acting as a local signalling factor (Figure 1b). Evidence that the myostatin immunoreactivity detected in both HL60 and Jurkat cells arises from expression of the myostatin gene was obtained by RT-PCR. Amplification of messenger RNA (mRNA) obtained from these cells yielded a band migrating in keeping with the predicted size (345 bp) of the amplified fragment, that was not observed in K562 cells (Figure 1c). Finally, the existence of myostatin expression was also investigated in human peripheral blood cells. Immunocytochemical analysis of blood smears obtained from adult humans revealed the presence of myostatin immunoreactivity in both granulocytes and lymphocytes, but not in red blood cells (Figure 1e). As occurred with the cell lines, amplification of RNA from human peripheral blood cells also yielded a band migrating in keeping with the predicted size (345 bp) of the amplified fragment, thus suggesting that myostatin immunoreactivity arises from expression of the myostatin gene in peripheral blood cells (Figure 1f).
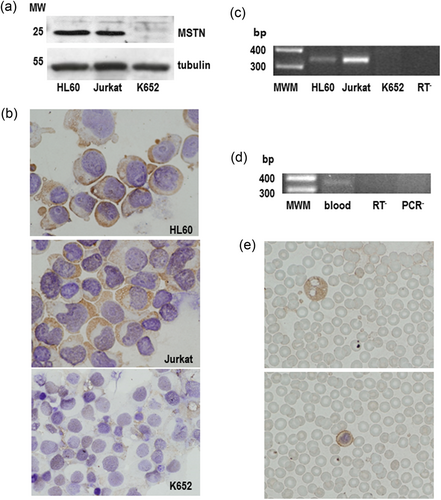
Myostatin is expressed in hematopoietic cell lines and human peripheral blood cells. (a) Western blot analysis of myostatin in HL60, Jurkat, and K562 cells. Whole cell lysates (500 mg) were resolved in 12% SDS-PAGE, electrotransferred onto a nitrocellulose paper and probed with a polyclonal anti-myostatin antibody (1:5000, overnight at 4°C). Tubulin was used as a loading control. (b) Immunocytochemistry of HL60, Jurkat, and K562 cells stained with a polyclonal anti-myostatin antibody (1:200, overnight at 4°C). Cells were cytospined onto coated slides and fixed, and myostatin immunoreactivity was detected with the EnVision system. Lens objective: ×40. (c) RT-PCR of RNA obtained from HL60, Jurkat, and K562 cells. One microgram of total RNA was reverse-transcribed and amplified (32 cycles) using specific primers. Amplimers were then resolved in 2% agarose gels and stained. MWM (100 bp ladder), RT−, negative control. (d) Immunocytochemistry of blood smears stained with a polyclonal anti-myostatin antibody. Myostatin immunoreactivity was detected in both granulocytes (top) and lymphocytes (bottom). Lens objective: ×40. (e) RT-PCR of RNA obtained from peripheral blood cells. One microgram of total RNA was reverse-transcribed and amplified as described above. RT− and PCR−, negative controls. Results shown in panels (d) and (e) are representative of samples obtained from 10 individuals. bp: basepair; MWM: molecular weight marker; RT-PCR: reverse-transcription polymerase chain reaction; SDS-PAGE: sodium dodecyl sulphate-polyacrylamide gel electrophoresis [Color figure can be viewed at wileyonlinelibrary.com]
Once demonstrated the expression of myostatin in haematic cells, we next investigated the presence of functional myostatin receptors. As occurs with other members of the TGFβ superfamily, myostatin transduces its signals through two related but distinct serine/threonine kinases known as the type I and type II receptors (Massague, 1998; Piek, Heldin, & ten Dijke, 1999). In the case of myostatin, there is compelling evidence indicating that the type II receptor used for intracellular signalling is the ActRIIB, whereas ALK4 is the type I receptor that is recruited and activated (Attisano & Wrana, 2002; Lee & McPherron, 2001; Rebbapragada, Benchabane, Wrana, Celeste, & Attisano, 2003; Ríos, Fernández-Nocelos, Carneiro, Arce & Devesa, 2004). In keeping with this, both ActRIIB and ALK4 transcripts were detected in the three cell lines investigated (Figure 2a). The demonstration that these receptors are functionally active was obtained by studying the myostatin-induced phosphorylation of Smad2, the main Smad involved in myostatin signalling (Ríos et al., 2004). As Figure 2b shows, administration of myostatin resulted in a clear-cut increase in the amount of phosphorylated Smad detected by western blot in both HL60 and K562 cells. Surprisingly myostatin treatment barely increased Smad2 phosphorylation in Jurkat cells, despite they express both ActRIIB and ALK4 (Figure 2b). Finally, and to ascertain the specificity of myostatin-induced Smad2 phosphorylation, we treated HL60 cells with myostatin in presence of the myostatin propeptide, which prevents myostatin binding with its receptor (Thies et al., 2001; Wolfman et al., 2003; Zimmers et al., 2002). As Figure 2c depicts, the ability of myostatin to promote Smad2 phosphorylation was prevented in a dose-dependent fashion in the presence of the myostatin propeptide.
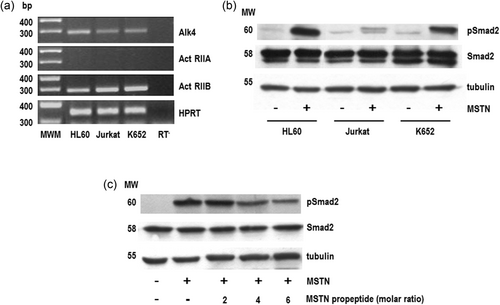
HL60 and K562 cells express functional myostatin receptors. (a) One microgram of total RNA obtained from HL60, Jurkat, or K562 cells was reverse-transcribed and amplified using specific primers for ALK4 (28 cycles), ActRIIA (26 cycles), and ActRIIB (30 cycles). The housekeeping HPRT gene was used as loading control. Amplimers were resolved in 2% agarose gels and stained. MWM (100 bp ladder); RT−, negative control. (b) Cells were serum-starved for 24 hr and treated with myostatin (125 mg/ml) or vehicle for 15 min. Cell lysates were resolved in 8% SDS-PAGE, electrotransferred onto a nitrocellulose paper and probed with anti-phospho-Smad2 (pSmad2) antibodies. Total Smad proteins were detected by anti-Smad2 antibodies. Tubulin was used as a loading control. (c) HL60 cells were serum-starved for 24 hr and treated with myostatin in the presence of increasing amounts (expressed as molar ratio) of the myostatin propeptide. Control cells were treated with vehicle. Cell lysates were resolved and probed as described above. ALK4: activin-like kinase 4; ActRIIB: activin type IIB receptor; HPRT, hypoxanthine guanine phosphoribosyl transferase; MSTN, myostatin; MWM, molecular weight marker
The major actions of myostatin in muscle cells include the regulation of cell differentiation, proliferation, and survival. Therefore, we next investigated the possibility that myostatin could exert similar effects in hematopoietic cells. Myostatin-induced differentiation of HL60 cells was first assessed by measuring the expression of CD33 a differentiation antigen of myeloid progenitor cells (Simmons & Seed, 1988). As Figure 3a depicts, myostatin treatment of HL60 cells resulted in a significant (p < 0.05) decrease in CD33 levels, and sharply potentiated the inhibitory effect of DMSO, a well-known inductor of HL60 differentiation, on CD33 expression (Figure 3a). In addition, myostatin treatment also induced morphologic changes in HL60 cells that are compatible with granulocytic maturation. These included increased cytoplasm, decreased nuclei versus cytoplasmic ratio, and presence of condensed, shrunk, twisted, and lobulated nuclei (Figure 3b). Overall, these morphological changes are like those observed after treating the cells with DMSO (Figure 3b). In contrast, myostatin treatment did not significantly affected TPA-induced CD33 inhibition (Figure 3a). Interestingly, TPA also failed to induce the phosphorylation of Smad2 (Figure 3c) and, at odds with DMSO and myostatin, induced differentiation of HL60 cells toward the monocyte/macrophage pathway (data not shown).
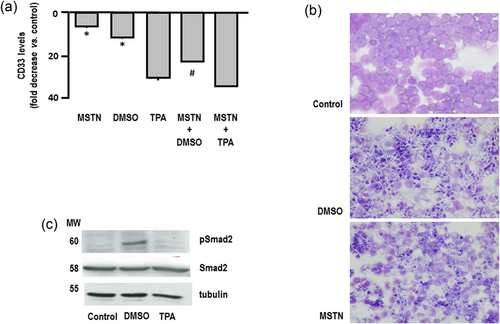
Myostatin promotes the differentiation of HL60 cells. HL60 cells were seeded in 15 mm plates (4 × 105 cells/plate) and treated every 24 hr for 96 hr with myostatin (125 ng/ml), DMSO (1.3%), TPA (10 nM), myostatin + DMSO or myostatin + TPA. Control cells were treated with vehicle. (a) CD33 levels were determined by flow cytometry, using an anti-CD33 monoclonal antibody coupled to FITC. Results (mean ± SEM of five different experiments in triplicate) are expressed as fold decrease versus control (vehicle-treated) cells. *p < 0.01 versus control; #p < 0.01 versus DMSO. (b) Cells were cytospined onto coated microscope slides, fixed, and stained with the Wright–Giemsa method. Differentiated cells were scored by the presence of condensed, shrunk, twisted and lobulated nuclei, and increased cytoplasm. Results are representative of three independent experiments. (c) Cells were serum-starved and treated with DMSO, TPA, or vehicle (control) for 24 hr. Cell lysates were resolved in 8% SDS-PAGE, electrotransferred onto a nitrocellulose paper and probed with anti-phospho-Smad2 (pSmad2) antibodies. Total Smad2 proteins were detected by anti-Smad2 antibodies. Tubulin was used as a loading control. Results are representative of three independent experiments. DMSO: dimethyl sulfoxide; FITC: fluorescein isothiocyanate; MSTN: myostatin; SDS-PAGE: sodium dodecyl sulphate-polyacrylamide gel electrophoresis; TPA: 12-O-tetradecanoylphorbol-13-acetate [Color figure can be viewed at wileyonlinelibrary.com]
Together with promoting cell differentiation, myostatin treatment also increased HL60 cell number (Figure 4a). However, this raise in cell number does not appear to be secondary to a boost in cell proliferation, as demonstrated by cell cycle analysis (Figure 4b). In contrast, myostatin treatment significantly reduced both annexin V expression and Hoechst 33258 staining as compared with control cells, thus indicating the existence of an increase in cell survival (Figures 4c,d).
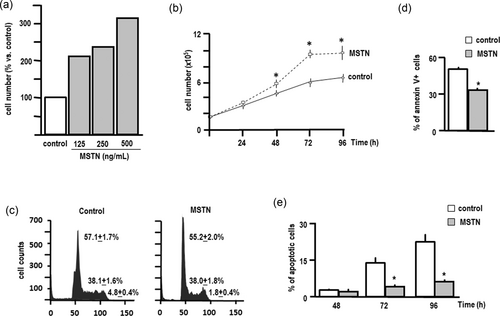
Myostatin promotes the survival of HL60 cells. (a) HL60 cells were seeded in 15 mm plates (105 cells/plate) and treated with different concentrations of myostatin or vehicle (control) every 24 hr for 72 hr, and cell number was determined in a Neubauer chamber. (b) Cells were treated with myostatin (125 ng/ml) or vehicle (control) every 24 hr for 96 hr, and cell number was determined every 24 hr in a Neubauer chamber. Each point represents the mean ± SEM of three independent experiments in triplicate (*p < 0.01 vs. control). (c) Cell cycle was analysed by flow cytometry of PI-stained cells. Numeric results are the mean ± SEM of three independent experiments in triplicate. Graphic results are representative. (d) HL60 cells were seeded in 15-mm plates (105 cells/plate) and treated with myostatin (125 ng/ml) or vehicle (control) every 24 hr for 96 hr. Apoptosis was determined by flow cytometry in cells stained with annexin V-FITC/PI. Results are the mean ± SEM of three independent experiments in triplicate (*p < 0.01 vs. control). (e) Apoptosis was determined by fluorescent microscopic analysis of cell DNA staining patterns with Hoechst 33258. Cells were scored for apoptosis by their nuclear morphology and the higher intensity of blue fluorescence. Each bar represents the mean ± SEM of three independent experiments in triplicate (*p < 0.01 vs. control). FITC: fluorescein isothiocyanate; MSTN: myostatin; PI: propidium iodide
At odds with the effects of myostatin administration, counteracting endogenous myostatin by means of myostatin propeptide treatment did not result in any significant effect on either cell proliferation or survival (Figures 5a,b). The ability of the myostatin propeptide to counteract myostatin actions was demonstrated by the fact that basal levels of phosphorylated Smad2 were clearly decreased (Figure 5c). Although we do not actually know the reasons for this lack of effect of thwarting endogenous myostatin, it is tempting to speculate that complex regulatory mechanisms may be activated to compensate the lack of myostatin action. In this regard, it is interesting to note that treatment with the myostatin propeptide resulted in a decreased expression of endogenous myostatin. Further studies are now in progress to investigate the mechanisms underlying these regulatory mechanisms.
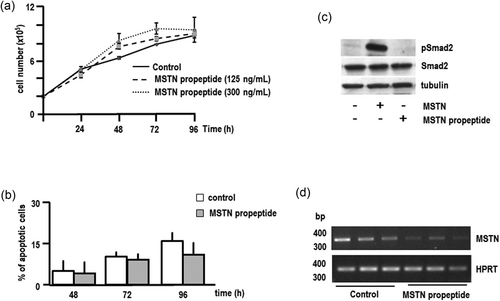
Effect of the myostatin propeptide on the proliferation and survival of HL60 cells. (a) HL60 cells were seeded in 15-mm plates (105 cells/plate) and treated with different concentrations of the myostatin propeptide or vehicle (control) every 24 hr for 96 hr, and cell number was determined in a Neubauer chamber. Each point represents the mean ± SEM of three independent experiments in triplicate. (b) HL60 cells were treated with the myostatin propeptide (300 ng/ml) or vehicle (control) every 24 hr for 96 hr. Apoptosis was determined by fluorescent microscopic analysis of cell DNA-staining patterns with Hoechst 33258. Cells were scored for apoptosis by their nuclear morphology and the higher intensity of blue fluorescence. Each bar represents the mean ± SEM of three independent experiments in triplicate. (c) HL60 cells were serum-starved for 24 hr and treated with myostatin (125 mg/ml) or the myostatin propeptide (300 ng/ml) for 15 min. Control cells were treated with vehicle. Cell lysates were resolved in 8% SDS-PAGE, electrotransferred onto a nitrocellulose paper and probed with anti-phospho-Smad2 (pSmad2) antibodies. Total Smad proteins were detected by anti-Smad2 antibodies. Tubulin was used as a loading control. (d) RT-PCR of RNA obtained from HL60 cells treated with the myostatin propeptide (300 ng/ml). One microgram of total RNA was reverse-transcribed and amplified (32 cycles) using specific primers. Amplimers were then resolved in 2% agarose gels and stained. MWM (100 bp ladder). The housekeeping HPRT gene was used as loading control. MSTN: myostatin; MWM: molecular weight marker; RT-PCR: reverse-transcription polymerase chain reaction; SDS-PAGE: sodium dodecyl sulphate-polyacrylamide gel electrophoresis
In keeping with the results obtained in HL60 cells, myostatin treatment also stimulated the differentiation of K562 cells. In this case, cell differentiation was evaluated by means of the determination of the haemoglobin accumulation by benzidine staining. As illustrated by Figure 6a, myostatin treatment induced a significant rise in the number of benzidine positive cells, thus indicating a positive effect on K562 differentiation. However, and in contrast with the results obtained in HL60 cells, induction of differentiation of K562 cells was associated with a significant decrease in cell number (Figure 6b) and cell viability (Figure 6c). Differentiation of K562 cells with different inducers, including TGFβ has been already shown to be accompanied by progressive cell death (Benito, Silva, Grillot, Nuñez, & Fernández-Luna, 1996).
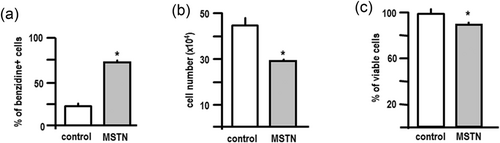
Myostatin promotes the differentiation but decreases the survival of K562 cells. K562 cells were seeded in 15-mm plates (103 cells/plate) and treated with myostatin (125 ng/ml) or vehicle every 24 hr for 96 hr. (a) Cells were stained with a benzidine solution that allows the determination of the haemoglobin content. The brown–blue-stained cells were taken as the positive cells. Each bar represents the mean ± SEM of three independent experiments in triplicate. (b) Cell number was determined in a Neubauer chamber. Each bar represents the mean ± SEM of three independent experiments in triplicate. (c) Cell viability was investigated by trypan blue staining. Positive cells were scored under a light microscope. Each bar represents the mean ± SEM of three independent experiments in triplicate. *p < 0.01 versus control. ERK: extracellular signal-regulated kinases; MSTN: myostatin; pERK: phospho ERK
In contrast with the results obtained in both HL60 and K562 cells, we were unable to find any effect of myostatin treatment on Jurkat cells, a human T-cell leukaemia line (data not shown), despite these cells express ActRIIB/ALK4 (Figure 2a). Since myostatin treatment also failed to effectively phosphorylate Smad2 in these cells (Figure 2b), it is tempting to speculate that lack of effect of myostatin on Jurkat cells may be secondary to defects in the ActRIIB/ALK4 signalling machinery. In this regard, it is noteworthy that activin A, which shares receptors with myostatin and regulates the haematopoiesis of various cellular lineages, does not exert any effect on T-cell haematopoiesis (Shav-Tal & Zipori, 2002).
Two of the major signalling pathways that are involved in the control of the proliferation, differentiation, and survival of hematopoietic cells depend on the activation of either ERK-1/2 or p38-MAPK, two members of the mitogen-activated kinases family (Huang, Chang, & Liu, 2004; Huang, Chiou, & Chang, 2006; Nagata & Todokoro, 1999; Nagata, Takahashi, Davis, & Todokoro, 1998). In keeping with this, western blot analysis of cell extracts obtained from HL60 cells revealed that myostatin treatment induced a sharp rise in both phospho p38-MAPK and phospho ERK-1/2 (Figure 7). In contrast, myostatin treatment induced p38-MAPK phosphorylation, without affecting phospho ERK-1/2 levels (Figure 7). Activation of p38-MAPK pathway is also one of the major mechanisms involved in the regulation of the differentiation of K562 cells, and p38-MAPK has been reported to participate in the erythroid differentiation of K562 cells induced by activin A (Huang et al., 2004; Huang, Li, & Chung, 2010). In contrast, the role of ERK-1/2 in the regulation of the differentiation of K562 cells has not been so clearly established, and it has been reported that ERK-1/2 activation can either promote or inhibit the differentiation of K562 cells depending on time and the inducing agent (Kang et al., 1999; Kawano et al., 2004; Woessmann, Zwanzger, & Borkhardt, 2004).
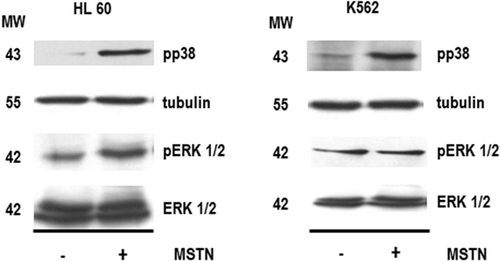
Effect of myostatin on the phosphorylation of p38-MAPK or ERK in HL60 or K562 cells. Cells were treated with myostatin (125 ng/ml) or vehicle every 24 hr for 96 hr. Cell lysates were resolved in 8% SDS-PAGE, electrotransferred onto a nitrocellulose paper and probed with specific antibodies raised against phospho-p38-MAPK (1:1000, overnight at 4°C), tubulin (1:1000, 1 hr at room temperature), phospho-ERK-1/2 (1:1000, overnight at 4°C) or ERK-1/2 (1:1000, 1 hr at room temperature). Results are representative of three independent experiments. ERK: extracellular signal-regulated kinases; MAPK: mitogen-activated protein kinases; MSTN: myostatin; MW: molecular weight; pERK: phospho-ERK
4 DISCUSSION
Accumulating evidence indicates that, apart from regulating skeletal muscle growth and development, myostatin exerts important effects on other systems, thus revealing itself as a pleiotropic hormone. Myostatin plays a major role in the regulation of the metabolism, secondary to its actions on both muscle and adipose tissue (Deng et al., 2017; Dong et al., 2016; Steculorum et al., 2016). In addition, myostatin might act as a regulator of cardiac growth and metabolism (Biesemann et al., 2014; Bish et al., 2010; George et al., 2010; Sharma et al., 1999) and exert an inhibitory effect of de growth of myometrial and vascular smooth muscle cells (Ciarmela et al., 2009; Kovanecz et al., 2017; Verzola et al., 2017; Wong et al., 2009). Finally, myostatin has also been proposed to act as a mediator of nutrient uptake and transport in the placenta (Mitchell et al., 2006; Peiris, Ponnampalam, Osepchook, Mitchell, & Green, 2010) and as a regulator of mammary epithelial cell differentiation (Ji et al., 1998; Manickam, Pena, & Whitelaw, 2008). In most of these cases myostatin actions are exerted through an autocrine/paracrine mechanism.
In this report, we demonstrate that both myostatin and its signalling system are expressed in in human blood cells. Furthermore, myostatin treatment stimulates the differentiation and survival of HL60 cells, while in the case of K562 cells, the induction of cell differentiation is associated with a significant decrease in cell number. Interestingly, some of these effects are like those induced by activin A, another member of the family of TGFβ. In fact, activin A induces the differentiation and suppress the growth of K562 cells (Frigon, Shao, Young, Maderazo, & Yu, 1992; Yu et al., 1987), and promotes the survival of HL60 cells (Saeki, Yuo, Kato, Miyazono, & Yazaki, 1997; Yamada et al., 1992). Since activin A and myostatin share a receptor system and activate similar signalling mechanisms that include Smad2, ERK-1/2, and p38-MAPK (Huang et al., 2004, 2006, 2010; Nagata et al., 1998; Nagata & Todokoro, 1999, and the current study), it seems conceivable that they may exert similar actions in hematopoietic cells. However, and at odds with our results, activin A inhibits the growth of HL60 cells (Saeki et al., 1997) and induces their differentiation toward the monocyte/macrophage lineage (Okabe-Kado, Honma, Hayashi, & Hozumi, 1991; Yamada et al., 1992), instead of the granulocyte lineage (the present work). Altogether, these results suggest that activin A and myostatin roles can also be distinguishable from one another.
Although we do not know the physiological relevance of these effects of myostatin on hematopoietic cells, the possibility exists that myostatin may play a role as an autocrine/paracrine factor involved in the regulation of haematopoiesis, as occurs with activin A (Naka & Hirao, 2017; Soderberg, Karlsson, & Karlsson, 2009). Since myostatin is mainly produced by skeletal muscle cells, the possibility exists that muscle myostatin can reach the bone marrow, thus making it difficult to distinguish between paracrine and endocrine effects. However, although muscle-derived myostatin has been detected in plasma, there is strong evidence indicating that this circulating myostatin is largely inactive because of the existence of different myostatin-interacting proteins that prevent the binding of myostatin to its receptor (Hill et al., 2002; Ríos et al., 2004). Therefore, it is unlikely that circulating myostatin may exert any effect on haematopoiesis, at least under physiological conditions. Nevertheless, it has been also reported that myostatin may override latency mechanisms under several pathological conditions (Zimmers et al., 2002) and therefore, the possibility exists that enough active myostatin may reach the bone marrow. Whether myostatin may be a factor modulating haematopoiesis under these circumstances is an intriguing possibility that remains to be elucidated.
In addition to its possible role on haematopoiesis, the fact that myostatin is being produced by immune cells, suggests the possibility that it may be also involved in pathogeny of some inflammatory diseases. This is the case of inflammatory myopathies, in which the infiltration of inflammatory cells is one of the hallmarks of the pathogenesis of the disease (Dalakas, 2015; Londhe & Guttridge, 2015). Although the role of myostatin in the inflammation-induced loss of skeletal muscle has been already established (Londhe & Guttridge, 2015), the possible involvement of myostatin produced by inflammatory cells has never been envisaged so far. In our model, muscle infiltration by inflammatory cells could result in locally-increased expression of myostatin, that would boost muscle loss (Figure 8). Interestingly, local production of myostatin has been also involved in the pathogenesis of inflammatory joint destruction in mice (Dankbar et al., 2015). More recently, myostatin expression has been reported in human monocytes, and it has been proposed, together with the myostatin produced by vascular smooth cells, to participate in the pathogenesis of atherosclerosis progression (Verzola et al., 2017). Overall, these findings support an emerging role for myostatin as a mediator of the progression of inflammatory diseases, other than myopathies.
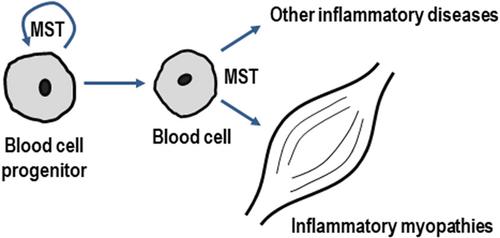
A proposed role for myostatin in haematopoiesis and inflammation. Myostatin produced by haematopoietic progenitors could act as an autocrine/paracrine factor regulating haematopoiesis. In addition, myostatin production by peripheral blood cells could be involved in the pathogenesis of both inflammatory myopathies and other inflammatory diseases. MST: myostatin [Color figure can be viewed at wileyonlinelibrary.com]
Acknowledgements
This study was supported by grants sponsors “Consellería de Cultura, Educación e Ordenación Universitaria, Ministerio de Ciencia e Innovación, Centro Singular de Investigación de Galicia and European Regional Development Fund (ERDF)” with grant numbers “GPC2010/402, GPC2014/030, SAF2008-00543, SAF2009–08629, ED431G/05.”
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.




