Exogenous nanoparticles and endogenous crystalline molecules as danger signals for the NLRP3 inflammasomes
Abstract
Inflammasome mechanisms are involved as some of the pathways of sterile inflammation. Inflammasomes are large multiprotein complexes in the cytosol and are a key system for the production of the pivotal inflammatory cytokines, interleukin (IL)-1β and IL-18, and inflammatory cell death called pyroptosis. Although a number of inflammasomes have been described, the nucleotide-binding oligomerization domain-, leucine-rich repeat-, and pyrin domain-containing 3 (NLRP3) is the most extensively investigated inflammasome. Exogenous pathogen-associated molecular patterns released during infection and endogenous crystalline danger/damage-associated molecular patterns (DAMPs) are well-known activators of NLRP3 inflammasomes. In addition, nanoparticle-associated molecular patterns (NAMPs), which are mediated by synthetic materials, including nanomaterials and nanoparticles, are proposed to be new danger signals of NLRP3 inflammasomes. Importantly, NAMP- and DAMP-triggered inflammation, a defining characteristic in inflammatory diseases, is termed as sterile inflammation because it occurs in the absence of foreign pathogens. This review focuses on the role of inflammasomes in exogenous NAMP- and endogenous crystalline DAMP-mediated sterile inflammation. Moreover, many regulatory mechanisms have been identified to attenuate NLRP3 inflammasomes. Therefore, we also summarize endogenous negative regulators of NLRP3 inflammasome activation, particularly induced by NAMPs or crystalline DAMPs.
1 INTRODUCTION
Inflammation is a complex protective immune response against harmful stimuli such as pathogens, damaged or dead cells, and irritants. This response is tightly regulated by the host, enabling survival after infection or injury and maintaining tissue homeostasis. Exposure to these stimuli causes various types of signal cascades, leading to the recruitment of inflammatory cells (including neutrophils, macrophages, and dendritic cells), which produce substantial amounts of cytokines and chemokines and induce the elimination of foreign matter or wound healing. However, insufficient inflammation can lead to persistent pathogenic infection or uncontrolled tissue functions, whereas excessive inflammation can cause chronic or systemic inflammatory diseases. Therefore, it is essential to regulate the inflammatory response appropriate for maintaining the homeostasis in the body.
Approximately 20 years ago, a new idea called “danger theory” was proposed (Matzinger, 1994). In this concept, “danger signals” of exogenous or endogenous origin have been posited to activate both the innate and adaptive immunity, resulting in an inflammation. A typical inflammatory response involves four components: (a) inflammatory inducers including “danger signals,” (b) sensors in immune cells (neutrophils, macrophages, and dendritic cells) that detect inflammatory inducers, (c) inflammatory mediators induced by the sensors, and (d) target tissues that are affected by the inflammatory mediators (Kopitar-Jerala, 2017; Medzhitov, 2008). It has been proposed that “classic” and “homeostatic” danger signals can be distinguished (Gallo & Gallucci, 2013). Classic danger signals are derived from pathogens and are recognized as exogenous pathogen-associated molecular patterns (PAMPs) released during infection (Akira, Uematsu, & Takeuchi, 2006; Kawai & Akira, 2009). Homeostatic danger signals are endogenous molecules released during cellular stress and recognized as endogenous danger/damage-associated molecular patterns (DAMPs; Akira et al., 2006). In addition, it has recently proposed new danger signals called “emerging” danger signals, which are caused by man-made materials, including nanomaterials and nanoparticles, which activate immune cells, resulting in tissue damage (Pallardy, Turbica, & Biola-Vidamment, 2017). It has been postulated that immune cells recognize nanomaterials and therefore nanoparticle-associated molecular patterns (NAMPs) would be an appropriate designation (Gallo & Gallucci, 2013; Pallardy et al., 2017). Importantly, NAMP- and DAMP-triggered inflammation, a defining characteristic in inflammatory diseases, is termed as sterile inflammation because it occurs in the absence of foreign pathogens.
Increasing evidence indicates that inflammasome mechanism has been found to be involved as one of the pathways of sterile inflammation (Guo, Callaway, & Ting, 2015; Rathinam, Vanaja, & Fitzgerald, 2012; Strowig, Henao-Mejia, Elinav, & Flavell, 2012). Inflammasomes are large multiprotein complexes in the cytosol and a key system for the production of the pivotal inflammatory cytokines, interleukin (IL)-1β and IL-18, and inflammatory cell death called pyroptosis. Although several types of inflammasome complexes have been described so far, nucleotide-binding oligomerization domain-, leucine-rich repeat-, and pyrin domain-containing 3 (NLRP3) inflammasome are known to be activated in response to NAMPs and DAMPs, indicating that NLRP3 inflammasome is a key mediator for the development of sterile inflammation. Indeed, we and other investigators have demonstrated that NLRP3 inflammasomes are involved in the wide variety of diseases, such as gout, silicosis, asbestosis, type 2 diabetes mellitus, atherosclerosis, obesity-induced insulin resistance, Alzheimer's disease, and cardiovascular and renal diseases (G. Y. Chen & Nunez, 2010; Davis, Wen, & Ting, 2011; Schroder, Zhou, & Tschopp, 2010; Takahashi, 2011; Vandanmagsar et al., 2011). This review focuses on the role of inflammasomes in exogenous NAMP- and endogenous crystalline DAMP-mediated sterile inflammation.
2 NANOPARTICLES AND CRYSTALLINE MOLECULES: LIVING AND DISEASES
Nanoparticles are particles whose size in one or more dimensions lies in the range 1–100 nm. Nanomaterials, defined as material with any external dimension in the nanoscale or having internal or surface structure in the nanoscale, are currently used in a wide variety of products including cosmetics, foods, electronics, print toner, paint, and drug delivery systems (Augustin & Sanguansri, 2009; Bowman, van Calster, & Friedrichs, 2010; Petros & DeSimone, 2010). The interaction of nanomaterials with human body may be beneficial, for example, in therapies such as vaccination and drug delivery. In contrast, some interactions are harmful, such as those involving accidental exposure to environmental and industrial nanoparticles. Among environmental and industrial nanoparticles, crystalline silica is one of the most abundant minerals on the Earth, being a major component not only of sand and rocks but also of environmental particulate matter of <2.5 µm in diameter (Pozzi, De Berardis, Paoletti, & Guastadisegni, 2003). Although the ingestion of amorphous silica is harmless and nontoxic, the inhalation of crystalline silica can lead to acute lung inflammation, resulting in silicosis characterized by shortness of breath, fever, and bluish skin (Hornung et al., 2008; Nakayama, 2018; Pallardy et al., 2017; Strowig et al., 2012). In addition, the inhalation of crystalline asbestos in occupational exposures can result in pulmonary fibrosis (asbestosis) and lung cancer (Dostert et al., 2008; Nakayama, 2018; Pallardy et al., 2017; Strowig et al., 2012).
Due to lifestyle changing, aging, and the development of diseases, various molecules are increased and function as endogenous danger signals in vivo, inducing the inflammatory response (Goto, 2008). Crystal deposition diseases are a group of disorders in which inflammatory damage is elicited by exposure to endogenous crystalline molecules (Strowig et al., 2012). For instance, medically relevant endogenous crystals, such as monosodium urate (MSU) crystals, have long been noted during gout, a common crystal-induced arthritis, because MSU crystals are deposited within joints and soft tissues and elicit an inflammatory response (Dalbeth, Merriman, & Stamp, 2016; Mitroulis, Kambas, & Ritis, 2013). Calcium pyrophosphate dehydrate and hydroxyapatite crystals are frequently found in the region of atherosclerosis and osteoarthritis synovial fluid (Jin et al., 2011; Karasawa & Takahashi, 2017a). In contrast, cholesterol crystals in the early atherosclerotic lesions activate inflammatory responses and promote inflammatory cell infiltration, resulting in the progression of atherosclerosis and the development of cardiovascular disease (Duewell et al., 2010; Karasawa & Takahashi, 2017b). In addition, endogenous fatty acid crystals trigger inflammation and may be involved in the development of atherosclerosis and the enhancement of insulin resistance (Freigang et al., 2013; Karasawa et al., 2018; Strowig et al., 2012; Wen et al., 2011).
Thus, it is evident that exogenous and endogenous nanoparticles and crystalline molecules cause human diseases. These nanoparticles and crystalline molecules are recognized by innate immune cells, such as macrophages and neutrophils (Nakayama, 2018; Pallardy et al., 2017). These NAMPs and endogenous crystalline DAMPs have been found to cause inflammation via the NLRP3 inflammasomes (Guo et al., 2015; Rathinam et al., 2012; Strowig et al., 2012).
3 NLRP3 INFLAMMASOMES
Inflammasomes are large multiprotein complexes in the cytosol that recognize various inflammation-inducing stimuli such as PAMPs, DAMPs, and NAMPs. These are a part of the system that tightly regulates the production of potent proinflammatory cytokines such as IL-1β and IL-18 (Davis et al., 2011; Rathinam et al., 2012; Takahashi, 2011). Inflammasomes have also been found to regulate another important aspect of inflammation and tissue repair such as pyroptosis, an inflammatory form of cell death (Wen, Miao, & Ting, 2013). They are typically composed of an NLR, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), and caspase-1 as an IL-1β-converting enzyme (Gu et al., 1997). While the leucine-rich repeat domain of NLRs is involved in direct or indirect sensing of the activation signal, the nucleotide-binding domain is involved in the regulation of homo-oligomerization, which is essential for inflammasome assembly. In response to PAMPs, DAMPs, and NAMPs, components of inflammasomes assemble through the interaction of pyrin domain (PYD) and caspase recruitment domain (CARD). Although many types of the inflammasomes have been reported, NLRP3 inflammasomes are most widely studied and activated in response to a wide array of stimuli, including exogenous and host ligands (bacterial RNA, cytosolic DNA, and microbial toxins) as well as endogenous danger signals, such as DAMPs and NAMPs (Figure 1; Guo et al., 2015; Rathinam et al., 2012). When NLRP3 is activated, it nucleates ASC into prion-like filaments through PYD–PYD interactions (A. Lu et al., 2014). Then, ASC must be linearly ubiquitinated for the NLRP3 inflammasome assembly, after which pro-caspase-1 interacts with ASC using CARD–CARD interactions and forms its own prion-like filaments (Cai et al., 2014). A landmark study revealed that the formation of the inflammasome complex induces the activation of caspase-1 (Martinon, Burns, & Tschopp, 2002; Martinon, Petrilli, Mayor, Tardivel, & Tschopp, 2006). Since caspase-1 is a cysteine protease, its activation cleaves the precursor cytokines pro-IL-1β and pro-IL-18, generating the biologically active cytokines IL-1β and IL-18, respectively (Guo et al., 2015; Rathinam et al., 2012; Strowig et al., 2012). Further, active caspase-1 is able to induce an inflammatory form of cell death known as pyroptosis. In this regard, recent investigations have revealed that caspase-1 can cleave gasdermin D to induce pyroptosis (Kayagaki et al., 2015; J. Shi et al., 2015). In addition with caspase-1-containing inflammasomes, a noncanonical inflammasome has been recently demonstrated (Kayagaki et al., 2015; Knodler et al., 2014). Murine caspase-11 (human caspase-4 and -5) is cytosolic lipopolysaccharide (LPS) sensor and mediates a noncanonical inflammasome pathway (Kayagaki et al., 2011). Activation of caspase-11 by intracellular LPS cleaves and activates gasdermin D, and its N-terminus induces pyroptosis. Additionally, activated caspase-11 also cleaves pannexin-1, a membrane channel for adenosine triphosphate (ATP), which induces potassium efflux and subsequent activation of NLRP3 inflammasomes (Kayagaki et al., 2015; D. Yang, He, Munoz-Planillo, Liu, & Nunez, 2015). Noncanonical inflammasome is activated by Gram-negative bacteria-derived LPS, and it has a critical role in antimicrobial defense at the intestinal mucosal surface, and protective action for fungal infection and alcoholic hepatitis (Crowley, Vallance, & Knodler, 2017; Khanova et al., 2018; Knodler et al., 2014).
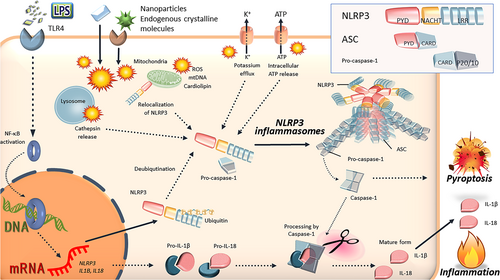
Basic mechanisms of the main NLRP3 inflammasome activation. NLRP3 is activated by various cellular events: potassium efflux, intracellular ATP release, deubiquitination of NLRP3, relocalization, the generation of ROS, mitochondrial dysfunction, lysosome rapture, and cathepsin release. Then, inflammasome components, including NLRP3, ASC, and pro-caspase-1, are formed the NLRP3 inflammasome complexes. Finally, activated caspase-1 induces the inflammatory form of cell death known as pyroptosis and cleaves the precursor cytokines pro-IL-1β and pro-IL-18, generating the biologically active cytokines IL-1β and IL-18. ASC: apoptosis-associated speck-like protein containing a caspase recruitment domain; ATP: adenosine triphosphate; IL: interleukin; LRR: leucine-rich repeat; NF-κB: nuclear factor κB; mtDNA: mitochondrial DNA; NLRP3: nucleotide-binding oligomerization domain-, leucine-rich repeat-, and pyrin domain-containing 3; PYD: pyrin domain; ROS: reactive oxygen species; TLR4: toll-like receptor 4 [Color figure can be viewed at wileyonlinelibrary.com]
The production and secretion of IL-1β mature form are regulated through two steps, including the transcription of pro-IL-1β (signal 1) and proteolytic processing into a mature form IL-1β by inflammasomes (signal 2; Figure 1; Guo et al., 2015; Rathinam et al., 2012; Strowig et al., 2012). Before activation of NLRP3 inflammasomes, NLRP3 must be primed by distinct steps in most cell types. A nuclear factor-κB (NF-κB)-activating stimuli, such as LPS, upregulate mRNA expression of NLRP3 and IL-1β, resulting in the elevated expression of NLRP3 and pro-IL-1β protein (Guo et al., 2015; Rathinam et al., 2012; Strowig et al., 2012). In contrast, another priming step facilitates the rapid induction of NLRP3 inflammasomes by the deubiquitination of NLRP3, independent of de novo protein synthesis (Figure 1; Juliana et al., 2012; Py, Kim, Vakifahmetoglu-Norberg, & Yuan, 2013). Having received the activation signal, NLRP3 can respond to the stimuli and assemble the NLRP3 inflammasomes.
The upstream mechanisms of NLRP3 activation have been elucidated by many studies, including the release of cathepsins into the cytosol after lysosomal destabilization, potassium efflux, the generation of mitochondrial reactive oxygen species (ROS), and the release of mitochondrial DNA or cardiolipin (Figure 1; Lamkanfi & Dixit, 2014; Vanaja, Rathinam, & Fitzgerald, 2015). Lysosomal rupture is essential for inflammasome activation, especially by NAMPs and endogenous crystalline DAMPs. Particularly, the cytosolic leakage of cathepsin B after lysosomal damages activates NLRP3 inflammasomes (Martinon et al., 2006). This leakage of cathepsin B also leads to potassium efflux and mitochondrial damage. It has been suggested that plasma membrane is damaged by particle contact with the cellular surface and that this phenomenon causes potassium leakage and reduced potassium concentration within cells, resulting in inflammasome activation (Strowig et al., 2012). In contrast, the increased cellular production of ROS has been observed in response to most inflammasome activators (Cassel et al., 2008; Hornung et al., 2008). It is proposed that proteins modified by oxidative stress directly bind and activate NLRP3. Indeed, the complex formed by ROS-detoxifying protein thioredoxin (TRX) and thioredoxin-interacting protein (TXNIP) has been proposed to link ROS and NLRP3 (Zhou, Tardivel, Thorens, Choi, & Tschopp, 2010). Although TXNIP is associated with TRX under normal conditions, the presence of free radicals oxidizes TRX that cannot bind TXNIP; thus, TXNIP interacts with and activates NLRP3 (Rabolli, Lison, & Huaux, 2016). Furthermore, recent investigations have demonstrated that NLRP3 inflammasomes are tightly regulated by multiple mechanisms, including ubiquitination, phosphorylation, nitrosylation, microRNAs, and endogenous regulators (e.g., pyrin-only proteins and CARD-only proteins; S. Chen & Sun, 2013; Karasawa et al., 2015; Kawashima et al., 2017; Rathinam et al., 2012).
Following NLRP3 activation through above described regulatory mechanisms, the activity regulation of NLRP3 with localization change occurs. Indeed, although NLRP3 localizes to endoplasmic reticulum (ER) under normal conditions, NLRP3 translocates from ER to the mitochondria and complexes with ASC after the activation of NLRP3 (Misawa et al., 2013). Then, IL-1β and IL-18 secretion is regulated by caspase-1 activation by many NLRP3 inflammasome activators, including MSU crystals, silica crystals, asbestos, and cholesterol crystals (G. Y. Chen & Nunez, 2010; Davis et al., 2011; Schroder et al., 2010; Takahashi, 2011). For details of the structure, activation mechanism, and physiological and pathophysiological role of the NLRP3 inflammasomes, refer to reviews of Lamkanfi and Dixit (2014), Strowig et al. (2012), Vanaja et al. (2015), and Wen et al. (2013).
4 NLRP3 INFLAMMASOME ACTIVATION BY EXOGENOUS NANOPARTICLES
The NLRP3 inflammasomes are activated by various PAMPs (e.g., fungal, bacterial, and viral pathogens) and DAMPs (e.g., ATP, pore-forming toxins, and nucleic acids). Furthermore, NAMPs, such as exogenous nanoparticles, also activate NLRP3 inflammasomes and cause sterile inflammation, leading to pathogenesis. The effects of major NAMPs on the activation of NLRP3 inflammasomes are summarized in Table 1.
| Molecules | Target types | Effect on NLRP3 inflammasomes | Identified mechanisms | Representative references |
|---|---|---|---|---|
| Silica | Macrophages | Caspase-1 activation | Phagocytosis | Cassel et al. (2008) |
| IL-1β and IL-18 secretion | ROS production | |||
| ASC multimerization, Caspase-1 activation | SR-B1 receptor | Tsugita, Morimoto, and Nakayama (2017) | ||
| IL-1β production and secretion | ||||
| NLRP3 and caspase-1 protein expression | Macrophage accumulation | M. Yang et al. (2016) | ||
| IL-1β secretion | NF-κB pathway | |||
| Dendritic cells | IL-1β and IL-18 secretion | Extracellular ATP release | Nakanishi et al. (2016) | |
| ROS production | ||||
| IL-1β production and secretion | MyD88, a downstream of TLR pathways | Winkler et al. (2017) | ||
| Placental cells | Caspase-1 activation | Phagocytosis | Shirasuna et al. (2015) | |
| Neutrophils | ||||
| IL-1β secretion | ROS production and lysosome rupture | |||
| Silica and TiO2 | Macrophages | Caspase-1 activation | Extracellular ATP release | Baron et al. (2015) |
| IL-1β secretion | ATP/ADP/adenosine signaling | |||
| Silica and TiO2 | Macrophages | IL-1β secretion | Lysosome rupture and ROS production | Tsugita, Morimoto, Tashiro, Kinoshita, and Nakayama (2017) |
| Silica and alum | Macrophages | Caspase-1 activation | Phagocytosis | Hornung et al. (2008) |
| IL-1β secretion | Lysosome acidification and rupture | |||
| Silica and asbestos | Macrophages | Caspase-1 activation | ROS production | Dostert et al. (2008) |
| IL-1β production and secretion | ||||
| TiO2 | Intestinal epithelial cells | Caspase-1 activation | Phagocytosis | Ruiz et al. (2017) |
| Macrophages | ||||
| IL-1β and IL-18 secretion | ROS production | |||
| Macrophages | Caspase-1 activation | Neutrophil accumulation | Yazdi et al. (2010) | |
| IL-1β production and secretion | ROS production | |||
| AgNP | Macrophages | ASC speck formation | Phagocytosis | Simard et al. (2015) |
| Caspase-1 activation, ILA-1β secretion | ER stress | |||
| Hepatoma cells | Caspase-1 activation | ER stress | Mishra et al. (2016) | |
| IL-1β secretion | Autophagy-lysosomal pathway | |||
- Note. ADP: adenosine diphosphate; AgNP: silver nanoparticles; alum: aluminum salts; ATP: adenosine triphosphate; ER: endoplasmic reticulum; IL: interleukin; NF-κB: nuclear factor-κB; NLRP3: nucleotide-binding oligomerization domain-, leucine-rich repeat-, and pyrin domain-containing 3; ROS: reactive oxygen species
4.1 Crystalline silica and nanosilica
Crystalline silica, also known as silicon dioxide (SiO2), is found in nature as sand or quartz (Pozzi et al., 2003). It found not only in the lungs of silica-exposed workers, such as miners and foundry workers, but also in the lungs of urban residents who have never worked with silica dust (Brauer et al., 2001). In particular, nanosilica particles (≤100 nm in diameter) are also frequently used as additives in cosmetics, such as foundation creams and sunscreens, due to their hydrophilic nature along with a range of other advantageous properties (Bowman et al., 2010). Many nanoparticles, such as nanosilica, carbon nanotubes, titanium dioxide (TiO2), and quantum dots, have been demonstrated to have toxic effects in the tissue of the lungs, liver, spleen, and kidneys in mice (De Jong et al., 2008; Isoda et al., 2012; R. S. H. Yang et al., 2007). Furthermore, we and other researchers have recently reported that pregnant mice treated with nanosilica develop pregnancy complications, such as smaller uteruses, smaller fetuses, and placental dysfunction with the activation of NLRP3 inflammasomes (Shirasuna et al., 2015; Yamashita et al., 2011). These nanoparticles (<100 nm) have recently been reported to cause acute inflammation, suggesting that a reevaluation of their safety is necessary.
In 2008, results published from three research groups demonstrated that crystalline silica activates NLRP3 inflammasomes (Cassel et al., 2008; Dostert et al., 2008; Hornung et al., 2008). In mouse macrophages and human peripheral blood mononuclear cells, crystalline silica activates NLRP3 inflammasomes and causes caspase-1 activation, resulting in maturation and secretion of IL-1β. In contrast, cells lacking NLRP3, ASC, and caspase-1 failed to release cleaved IL-1β in response to silica, indicating a requirement for NLRP3 inflammasome complexes in the processing of IL-1β after exposure to silica (Cassel et al., 2008; Hornung et al., 2008; M. Yang et al., 2016). Interestingly, silica-induced macrophage cell death is independent of NLRP3; the extent of cell death in wild-type and NLRP3-deficient murine macrophages is similar (Cassel et al., 2008).
It is important to understand the “upstream” mechanism of NLRP3 inflammasome activation by silica. Clearly, silica binding and uptake by phagocytosis are prerequisites for the activation of the NLRP3 inflammasomes in humans and mice (Cassel et al., 2008; Dostert et al., 2008; Hornung et al., 2008). In in vitro experiments, macrophages rapidly engulf silica into intracellular compartments by phagocytosis, whereas phagocytosis of silica is suppressed when cells are treated with an inhibitor (cytochalasin D, which impairs actin filament assembly), resulting in reduced NLRP3 inflammasome activation and IL-1β secretion (Cassel et al., 2008; Dostert et al., 2008; Hornung et al., 2008). Class A scavenger receptors such as SR-A1 (also known as MSR1) and SR-A6 (also known as MARCO) have been considered to be involved in the internalization of particles such as silica and TiO2, in addition to the phagocytosis of silica (Prabhudas et al., 2014; Thakur, Hamilton, & Holian, 2008). Tsugita, Morimoto, Tashiro, Kinoshita, and Nakayama (2017) demonstrated that the SR-B1 receptor on the macrophage membrane specifically recognizes silica (but not TiO2 or MSU crystals), after which the silica is ingested by phagocytosis, resulting in NLPR3 inflammasome activation.
After being internalized, crystalline silica localizes lysosomes within the cells. Interestingly, although both silica and TiO2 nanoparticles activate NLRP3 inflammasomes and induce IL-1β secretion in human and mouse macrophages, silica localizes in lysosomes, while TiO2 does not, indicating a distinct cellular mechanism depending on nanoparticles (Tsugita, Morimoto, & Nakayama, 2017). Lysosomes contain a plethora of proteolytic enzymes, many of which are activated by the acidification of lysosomal pathways. Bafilomycin A, an inhibitor of the vacuolar H+ ATPase system, required for the acidification of lysosomal compartments, completely suppresses silica-induced IL-1β release, indicating a pivotal role for lysosomes in silica-induced NLRP3 inflammasome activation (Hornung et al., 2008). It is well known that lysosomal destabilization is necessary for the activation of the NLRP3 inflammasomes. In particular, lysosomal destabilization and subsequent cathepsin B release are a common pathway for NLRP3 inflammasome activation by crystals and particulate matter. Indeed, silica stimulates the extracellular release of cathepsin B independently of NLRP3, and treatment with cathepsin B-specific inhibitor (CA-074-Me) suppress NLRP3 inflammasome activation and IL-1β secretion (Hornung et al., 2008). Therefore, NLRP3 inflammasomes sense lysosomal contents released into the cytosol after silica-induced lysosomal damage.
The increased cellular production of ROS has been observed in response to inflammasome activators. Indeed, silica stimulates ROS production from macrophages and dendritic cells in humans and mice (Cassel et al., 2008; Nakanishi, Tsukimoto, Tanuma, Takeda, & Kojima, 2016; Shirasuna et al., 2015; Winkler et al., 2017). ROS inhibitors such as NAC (an antioxidant), DPI (an NADPH oxidase inhibitor), and MitoTEMPO (a mitochondria-targeted antioxidant) significantly block silica-induced NLRP3 inflammasome activation and IL-1β secretion (Cassel et al., 2008; Shirasuna et al., 2015). Cassel et al. (2008) demonstrated that NLRP3-deficient murine macrophages were capable of producing ROS, but not of inducing IL-1β secretion by silica, indicating that ROS generation is essential as an upstream factor for NLRP3 inflammasome-dependent IL-1β secretion.
4.2 Titanium dioxide
Similar to silica, TiO2 is a frequently used nanoparticles in product manufacturing, and an increased incidence of respiratory diseases in workers exposed to TiO2 is reported (Mitrano, Motellier, Clavaguera, & Nowack, 2015). TiO2 nanoparticles increase the expression of NLRP3, caspase-1, and IL-1β, leading to the production of active caspase-1 and IL-1β secretion in mice (B. G. Kim, Lee, Lee, Park, & Jang, 2017; Ruiz et al., 2017). Mature IL-1β secretion by stimulation with TiO2 nanoparticles is completely dependent on NLRP3 inflammasome activation (Yazdi et al., 2010). In addition, TiO2 nanoparticles clearly induce the secretion of mature IL-1α form another IL-1 family mediating innate immunity. Interestingly, TiO2 nanoparticles injection induces IL-1α release and neutrophil accumulation induced by TiO2 nanoparticles is clearly decreased in IL-1α-deficient but not in NLRP3-deficient mice, indicating that the inflammation caused by TiO2 nanoparticles in vivo is largely caused by a biological effect of IL-1α but not of IL-1β (Yazdi et al., 2010). Intriguingly, activators of the NLRP3 inflammasome induce the secretion of IL-1α as well as IL-1β. Although most activators induce IL-1α secretion in an inflammasome-dependent manner, particulate or crystalline activators, such as MSU and silica, induce the processing and secretion of IL-1α in an inflammasome-independent, calpain-like protease-dependent manner (Gross et al., 2012).
4.3 Asbestos
Asbestos is also well known to be dangerous to humans through inhalation, causing illnesses such as mesothelioma and lung cancer (Mossman & Gee, 1989). It has been reported that the scavenger receptor MARCO may associate with asbestos-induced lung fibrosis in mice (Murthy et al., 2015). Asbestos is internalized by macrophages, thus stimulating ROS production, and inducing IL-1β secretion, a process dependent on NLRP3 inflammasomes (Dostert et al., 2008). In the above mechanisms, actin polymerization is essential for asbestos-induced inflammasome activation because cytochalasin D treatment clearly reduces phagocytosis of asbestos and assembly of NLRP3 and ASC components, resulting in the decrease of IL-1β and IL-18 secretion (MacPherson, Westbom, Kogan, & Shukla, 2017). In addition, Thompson et al. (2014) reported that asbestos fibers oxidize TRX, resulting in the release of TNXIP and subsequent activation of NLRP3 inflammasomes.
4.4 Aluminum
Aluminum salts (alum) are the most commonly used vaccine adjuvants, and all alum preparations contain crystals (Lindblad, 2004). Alum via phagocytosis also plays a role in triggering NLRP3 inflammasomes and inducing the cleavage of IL-1β (Hornung et al., 2008). Lysosome rupture and cathepsin B release are the important inducers of NLRP3 inflammasome activation by alum (Hornung et al., 2008). These findings indicate that NLRP3 sense lysosomal damage by alum as an endogenous danger signal. In contrast, alum regulate the induction of prostaglandin E2, which is important in various types of inflammation, in macrophages via NLRP3 inflammasome-independent manner (Kuroda et al., 2011).
4.5 Silver nanoparticles
Silver nanoparticles (AgNPs) are among the most commonly used nanoparticles in nanomedicine; they possess potent antimicrobial properties, and there is increased interest their use in drug delivery (J. S. Kim et al., 2007). AgNPs stimulate ASC speck assembly formation, caspase-1 activation, and mature IL-1β secretion, indicating the activation of NLRP3 inflammasomes by AgNPs in human THP-1 monocytes and hepatic cells (Mishra, Zheng, Tang, & Goering, 2016; Simard, Vallieres, de Liz, Lavastre, & Girard, 2015). In the ER, stress results in an unfolded protein response, a hallmark of cytotoxicity; Simard et al. (2015) showed that AgNPs induce ER stress by degrading ER stress sensor activating transcription factor 6 (ATF-6). In addition, inhibition of AgNP-induced ATF-6 degradation completely blocks the effect of AgNPs on pyroptosis and IL-1β secretion. They demonstrated that AgNPs induce ER stress sensed by the degradation of ATF-6, leading to NLRP3 inflammasome activation in human monocytes.
5 NLRP3 INFLAMMASOME ACTIVATION BY ENDOGENOUS CRYSTALLINE MOLECULES
Similar to NAMPs, endogenous crystalline DAMPs also activate NLRP3 inflammasomes and cause sterile inflammation, including joint inflammation, atherosclerosis, and cardiovascular disease. The effects of crystalline DAMPs on the activation of NLRP3 inflammasomes are summarized in Table 2.
| Molecules | Target types | Effect on NLRP3 inflammasomes | Identified mechanisms | Representative references |
|---|---|---|---|---|
| MSU | Macrophages | Caspase-1 activation, IL-1β secretion | Neutrophil accumulation | Martinon et al. (2006) |
| Caspase-1 activation, IL-1β secretion | Decrease of AMP-activated protein kinase | Y. Wang et al. (2016) | ||
| IL-1β secretion | Increase of C5a and immune cell accumulation | Khameneh et al. (2017) | ||
| ROS production | ||||
| Transport of ASC in mitochondria to NLRP3 on ER | Acetylation of α-tubulin | Misawa et al. (2013) | ||
| IL-1β secretion | Microtubule system | |||
| Caspase-1 activation | Mitochondrial ROS, potassium efflux | Nomura et al. (2015) | ||
| IL-1β secretion | Decrease intracellular ATP | |||
| Dendritic cells | Caspase-1 activation | Potassium efflux, phagocytosis | Gross et al. (2012) | |
| IL-1β and IL-18 secretion | ROS production, lysosome rupture | |||
| DNA damage, ROS production | Licandro et al. (2013) | |||
| Knee joints of mice | Caspase-1 activation | Increase of ROS production and LTB4 secretion | Amaral et al. (2012) | |
| IL-1β secretion | Neutrophil accumulation | |||
| Cholesterol crystal | Macrophages | IL-1β production and secretion | Neutrophil accumulation and atherosclerosis | Duewell et al. (2010) |
| Transcription factor NF-E2-related 2 (Nrf2) | Freigang et al. (2011) | |||
| ASC speck formation, IL-1β secretion | Autophagy dysfunction | Razani et al. (2012) | ||
| Endothelial cells | Colocalization of NLRP3, ASC, and caspase-1 | ROS production | Koka et al. (2017) | |
| Caspase-1 activation, IL-1β secretion | TXNIP | |||
| Oxidized LDL | Macrophages | Caspase-1 activation | Potassium efflux, ROS production | Sheedy et al. (2013) |
| IL-1β production and secretion | Lysosome rupture, CD36 receptor | Chen et al. | ||
| Endothelial cells | NLRP3 expression and caspase-1 activation | NF-κB pathway | S. Wang et al. (2017) | |
| Calcium phosphate | Macrophages | Caspase-1 activation, IL-1β secretion | Potassium efflux, phagocytosis | Pazar et al. (2011) |
| ROS production | ||||
| Caspase-1 activation, IL-1β secretion | Lysosome acidification and rupture | Usui et al. (2012) | ||
| Vascular smooth muscle cells | Caspase-1 activation, IL-1β secretion | Spleen tyrosin kinase, exosome release | Dautova et al. (2018) | |
| CPPD | Macrophages | Caspase-1 activation, IL-1β secretion | Neutrophil accumulation | Martinon et al. (2006) |
| Hydroxyapatite | Macrophages | Caspase-1 activation | Potassium efflux, phagocytosis | Jin et al. (2011) |
| IL-1β and IL-18 secretion | ROS production, lysosome rupture | |||
| Saturated fatty acids (palmitic and stearic acid) | Macrophages | Caspase-1 activation, IL-1β secretion | Intracellular crystallization | Karasawa et al. (2018) |
| Mitochondrial ROS, dysregulation of autophagy | ||||
| lysosomal dysfunction | ||||
- Note. AMP: adenosine monophosphate; ASC: apoptosis-associated speck-like protein containing a caspase recruitment domain; ATP: adenosine triphosphate; CPPD: calcium phosphate dihydrate; ER: endoplasmic reticulum; IL: interleukin; LDL: low-density lipoprotein; MSU: monosodium urate; NF-κB: nuclear factor κB; NLRP3: nucleotide-binding oligomerization domain-, leucine-rich repeat-, and pyrin domain-containing 3; ROS: reactive oxygen species
5.1 MSU crystals
The saturation of uric acid in body fluids results in the formation of MSU crystals. They are identified as danger signals from dying cells, resulting in an acute and chronic inflammatory response known as gout associated with the deposition of MSU crystals (Y. Shi, Evans, & Rock., 2003). In 2006, Martinon et al. (2006) demonstrated that MSU crystals activate NLRP3 inflammasomes, resulting in the production of active IL-1β and neutrophil accumulation in mice, suggesting a pivotal role for the inflammasomes in inflammatory diseases.
Similar to the mechanisms of NLRP3 inflammasome activation induced by nanoparticles, MSU crystals are also taken up by phagocytosis and lysosomal damage is induced, resulting in the release of cathepsin B and stimulation of ROS production from mitochondria (Gross et al., 2012). These complex signals result in mature IL-1β secretion and inflammation. In addition, MSU crystal exposure induces oxidative DNA damage mediated by ROS in an NLRP3 inflammasome-dependent manner because MSU-induced DNA fragmentation is markedly ameliorated in NLRP3- and caspase-1-deficient mouse macrophages (Licandro et al., 2013).
Colchicine, a tubulin polymerization inhibitor, is used to treat gout; this suggests a relationship between NLRP3 inflammasome activation and microtubule function (Misawa et al., 2013; Y. Wang, Viollet, Terkeltaub, & Liu-Bryan, 2016). Recently, Misawa et al. (2013) reported essential findings on NLRP3 inflammasome activation mechanisms induced by MSU and crystalline silica in human macrophages. Under resting conditions, ASC is localized in the mitochondria, cytosol, and nucleus, whereas the NLRP3 is localized in the ER. NLRP3 inflammasome activators, such as MSU, induce mitochondrial damage, followed by the accumulation of acetylated α-tubulin. Then, using microtubules, acetylated α-tubulin mediates dynein-dependent transportation of ASC-containing mitochondria to an area with ER, resulting in an increased colocalization of ASC and NLRP3, and hence activation of NLRP3 inflammasomes. Interestingly, using colchicine to inhibit the transport of ASC to NLRP3 in ER and IL-1β production, Misawa et al. (2013) demonstrated that microtubules mediate the assembly and activation of NLRP3 inflammasomes in response to MSU and silica.
Extracellular ATP is a well-known danger signal for NLRP3 inflammasome activation (Luna-Gomes, Santana, & Coutinho-Silva, 2015), whereas intracellular ATP released after cellular stress or activation and purinergic signaling has been shown to modulate inflammation (Baron et al., 2015; Eltzschig, Sitkovsky, & Robson, 2012; Nomura, So, Tamura, & Busso, 2015). Regarding MSU stimulation in human macrophages, potassium efflux and calcium mobilization occur first, followed by depletion of intracellular ATP via the inhibition of glycolysis (Nomura et al., 2015). As a result, extracellular ATP levels increase. This extracellular ATP can then be recognized by purinergic P2 receptors, specifically either by ATP-gated ion channel P2X or G-protein coupled P2Y receptors, and activates P2X7 receptor to amplify ATP release (Baron et al., 2015). Thereby, in the extracellular space, ATP is rapidly hydrolyzed in a stepwise manner to adenosine diphosphate (ADP), adenosine monophosphate, and adenosine by ectoenzymes. Baron et al. (2015) reported that metabolite ADP and adenosine from extracellular ATP through their receptors activate the NLRP3 inflammasomes, resulting in IL-1β secretion. In addition, Nomura et al. (2015) have suggested that the reduction of intracellular ATP may represent a danger signal for NLRP3 activation and assembly because MSU crystals induce the depolarization of mitochondrial membrane potential as well as the reduction of intracellular ATP, resulting in the induction of IL-1β secretion through NLRP3 and caspase-1 activation.
In addition to NLRP3 inflammasome activation, gout is characterized by neutrophil infiltration into intra- and periarticular spaces (Mitroulis et al., 2013). Indeed, MSU and calcium phosphate dihydrate (CPPD) crystals, as danger signals for pseudogout, elicit an increase in the recruitment of neutrophils with IL-1β secretion in mice. However, in NLRP3-, ASC-, caspase-1-, and IL-1β-deficient mice, this neutrophil infiltration is impaired, indicating the importance of NLRP3 inflammasomes for neutrophil accumulation by MSU and CPPD (Martinon et al., 2006). Moreover, MSU crystals also activate the complement system including C5a, which regulates the inflammatory response, and C5a receptor antagonists suppress MSU-induced neutrophil infiltration (Khameneh et al., 2017). C5a induced by MSU crystals triggers ROS production, contributing to the activation of NLRP3 inflammasomes in mouse macrophages, and in turn releasing IL-1β that acts as a recruiter of neutrophils (Khameneh et al., 2017). Furthermore, Amaral et al. (2012) reported that leukotriene B4 drives NLRP3 inflammasome activation and neutrophil recruitment in response to MSU crystals, resulting in joint inflammation and dysfunction in mice. Further information on the importance of the association between neutrophils and NLRP3 inflammasomes in gout can be found in the following excellent reviews (Dalbeth et al., 2016; Mitroulis et al., 2013).
5.2 Cholesterol crystals and oxidized low-density lipoprotein (LDL)
Atherosclerosis is an inflammatory disease characterized by the deposition of cholesterol-rich lipids and macrophage infiltration into the vascular walls (Tall & Yvan-Charvet, 2015). In contrast to the normal forms of cholesterol and LDL, crystalline cholesterol, and oxidized LDL (oxLDL) extensively participate in forming atherosclerotic plaques, which are known endogenous danger signals that activate NLRP3 inflammasomes (Duewell et al., 2010; Karasawa & Takahashi, 2017a, 2017b). In mouse macrophages, oxLDLs are recognized by the CD36 receptor and phagocytosed, thereby provoking intracellular crystallization of cholesterol (Sheedy et al., 2013). In human peripheral mononuclear cells, oxLDL induces IL-1β secretion by activating NLRP3 inflammasomes dependent on ROS generation (Duewell et al., 2010; Freigang et al., 2011; Liu, Yin, Zhou, He, & Dai, 2014). Cholesterol crystals also lead to lysosome rupture, resulting in the release of cathepsin B in cytosol and are a strong candidate activator for NLRP3 inflammasomes (Duewell et al., 2010; Freigang et al., 2011). Duewell et al. (2010) showed that deficiency of inflammasome-related molecules, such as NLRP3, ASC, or IL-1β, attenuated the development of atherosclerotic lesions in mice, indicating that NLRP3 inflammasomes, induced by cholesterol crystals, play an important role in the progression of atherosclerosis. In addition to macrophages, cholesterol crystals also markedly increase the formation and activation of NLRP3 inflammasomes in carotid arterial endothelial cells, as shown by increased colocalization of NLRP3 with ASC or caspase-1, enhanced caspase-1 activity, and elevated IL-1β secretion in mice (Koka et al., 2017), suggesting an essential role for endothelial inflammasome activation in arterial dysfunction and atherosclerosis (S. Wang et al., 2017).
5.3 Calcium phosphate crystals
Calcium phosphate, also a particulate material, can accumulate in atherosclerotic lesions and is associated with vascular calcification. We and other research groups showed that when calcium phosphate crystals, such as hydroxyapatite and tricalcium phosphate, are phagocytosed, and they induce NLRP3 inflammasome activation through potassium efflux, ROS generation, lysosomal rupture, and cathepsin B in human and mouse macrophages and vascular smooth muscle cells (Dautova et al., 2018; Jin et al., 2011; Pazar et al., 2011; Usui et al., 2012). Jin et al. (2011) demonstrated that mice lacking NLRP3 inflammasome components are protected against hydroxyapatite-induced neutrophilic inflammation in the air pouch model of synovitis.
5.4 Fatty acid crystals
Obesity is considered a low-grade chronic systemic inflammation (Prieto, Contreras, & Sanchez, 2014). Free fatty acids levels are elevated in the plasma of obese humans (Boden, 2002), and it has been proposed that free fatty acids promote inflammatory responses by directly engaging toll-like receptors and inducing the NF-κB-dependent production of inflammatory cytokines (Shi et al., 2006; Wen et al., 2011). In particular, one of the major saturated fatty acids, palmitic acid (PA), induces NLRP3 inflammasome activation by ROS generation and autophagy dysfunction, resulting in mature IL-1β secretion (L'Homme et al., 2013; Shirasuna et al., 2016; Wen et al., 2011). Additionally, we have recently demonstrated that saturated fatty acids, such as PA and stearic acid, cause intracellular crystallization in macrophages and induce NLRP3 inflammasome activation via lysosomal destabilization (Karasawa et al., 2018). Similar to other crystalline molecules, intraperitoneal administration of PA crystal induces neutrophil recruitment in an IL-1β-dependent manner (Karasawa et al., 2018).
6 NEGATIVE REGULATION FOR CRYSTAL-INDUCED NLRP3 INFLAMMASOMES
Although the NLRP3 inflammasomes are essential for maintaining homeostasis in the presence of DAMPs, PAMPs, and NAMPs, the overactivation of the NLRP3 inflammasomes leads to various immune-related diseases, including autoinflammatory diseases, metabolic syndrome, and atherosclerosis. Therefore, it is important to maintain an appropriate NLRP3 inflammasome activation level. At present, several IL-1 blockades, such as the recombinant IL-1 receptor antagonist anakinra, the neutralizing IL-1β antibody canakinumab, and the soluble decoy IL-1 receptor rilonacept, have been used to treat the patients with autoinflammatory diseases (Guo et al., 2015). Although specific inhibitors of NLRP3 inflammasome activation are currently unavailable as a clinical medicine, a number of factors and/or compounds have been reported to suppress NLRP3 inflammasome activation in experimental models. In the latter part of this review, we summarize endogenous negative regulators of NLRP3 inflammasome activation, particularly induced by NAMPs or crystalline DAMPs (Figure 2).
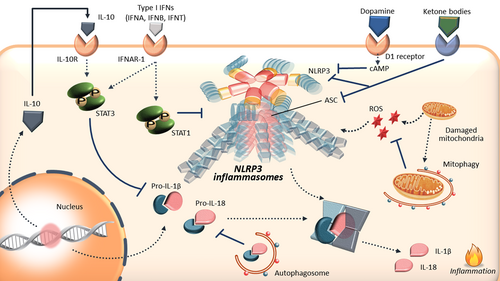
Negative regulation of NLRP3 inflammasomes. Type I IFN directly and indirectly suppresses NLRP3 inflammasome activation, ROS production, and IL-1β secretion via increased IL-10. Autophagy associates with NLRP3 inflammasomes via the removal of damaged mitochondria and eliminates the precursor cytokines pro-IL-1β and pro-IL-18. Ketone bodies and dopamine via their receptors also blocks NLRP3 inflammasome activation. ASC: apoptosis-associated speck-like protein containing a caspase recruitment domain; cAMP: cyclic adenosine monophosphate; IFN: interferons; IFNA: IFN-α; IFNB: IFN-β; IFNT: IFNτ; IL: interleukin; NLRP3: nucleotide-binding oligomerization domain-, leucine-rich repeat-, and pyrin domain-containing 3; ROS: reactive oxygen species; ROS: reactive oxygen species; STAT: signal transducers and activator of transcription [Color figure can be viewed at wileyonlinelibrary.com]
6.1 Type I interferons (IFNs)
Type I IFNs, including IFN-α (IFNA) and IFN-β (IFNB), play crucial roles in the first line of defense against viral infections, activation of the innate and adaptive immune systems, and pathogenesis of various diseases (Gough, Messina, Clarke, Johnstone, & Levy, 2012; Stark, Kerr, Williams, Silverman, & Schreiber, 1998). Anti-inflammatory properties are a prominent feature of type I IFNs, and several studies have shown that IFNB inhibits IL-1β secretion and NLRP3 inflammasome activation (Guarda et al., 2011; Inoue et al., 2012; Shao, Xu, Han, Su, & Liu, 2015). Inoue et al. (2012) have reported that IFNB therapy ameliorates the development of murine experimental autoimmune encephalomyelitis (a model for multiple sclerosis) by inhibiting NLRP3 inflammasomes. Type I IFN signal via signal transducers and activator of transcription (STAT)1/STAT3 increases anti-inflammatory cytokine IL-10 synthesis, IL-10-mediated STAT3 activation, and suppression of IL-1β precursor synthesis (Kopitar-Jerala, 2017). We and other research group reported that in human macrophages, type I IFNs, such as IFNB in humans or specific IFN in ruminants (IFNτ), stimulate IL-10 secretion via STAT3 activation, and these IFNs suppress nanosilica-induced NLRP3 inflammasome activation, ROS production, and IL-1β secretion (Guarda et al., 2011; Hara et al., 2014).
6.2 Autophagy
Autophagy is a genetically regulated metabolic process responsible for the turnover of cellular proteins and damaged or superfluous organelles (Mizushima & Komatsu, 2011). The cellular lysosomal degradation pathway of autophagy has been demonstrated to be the major component of the cellular stress response (White & Lowe, 2009). Autophagy is considered to be an endogenous inhibitor of NLRP3 inflammasomes (S. Chen & Sun, 2013; Rathinam et al., 2012; Razani et al., 2012). In autophagocytosis-deficient mice (autophagy-related gene 16-like 1 deficient), TLR signals (signal 1 of the NLRP3 inflammasome activation) are exaggerated, resulting in the increased production of IL-1β and IL-18 (Levine, Mizushima, & Virgin, 2011; Saitoh et al., 2008). In the absence of autophagy machinery, damaged mitochondria accumulate and generate excessive ROS; these events induce the activation of NLRP3 inflammasomes (Zhou, Yazdi, Menu, & Tschopp, 2011). In addition, autophagosomes isolate pro-IL-1β and target it for degradation, thereby suppressing the substrate available for caspase-1 to process the mature form of IL-1β (Rathinam et al., 2012). Regarding the association among nanoparticles, autophagy, and NLRP3 inflammasomes, nanoparticles (silica, AgNPs, and MSU crystals) stimulate autophagic and lysosomal activity with the activation of NLRP3 inflammasomes in macrophages and osteoblasts (Allaeys, Marceau, & Poubelle, 2013; Jessop, Hamilton, Rhoderick, Shaw, & Holian, 2016; Li et al., 2014; Mishra et al., 2016). Importantly, autophagy deficiency in mice enhances NLRP3 inflammasome activity (caspase-1 activation and IL-1β production), cytotoxicity, and lung inflammation in vivo (Jessop et al., 2016), suggesting the essential of basal autophagy in maintaining NLRP3 inflammasome homeostasis. Autophagy is also related to the development of atherosclerosis with NLRP3 inflammasomes. Razani et al. (2012) showed that classic inflammasome markers were robustly stimulated in autophagy-deficient macrophages, when coincubated with cholesterol crystals. Additionally, in macrophage-specific autophagy-deficient mice, cholesterol crystals appeared to be increased in plaque development with proatherogenic inflammasome activation, suggesting an essential role for basal levels of autophagy in atheroprotection (Razani et al., 2012).
6.3 Ketone bodies
Ketone bodies, including β-hydroxybutyrate (BHB), are produced in the liver and serve as alternative energy sources to ATP for various tissues such as the brain, heart, and skeletal muscles during nutrient deprivation (Newman & Verdin, 2014). Intriguingly, metabolic interventions (such as caloric restriction) induce fatty acid oxidation and ketone body production, and caloric restriction and BHB reduce inflammation and extend lifespan in animals (Dessein, Shipton, Stanwix, Joffe, & Ramokgadi, 2000; Edwards et al., 2014; Mitchell et al., 2016). In 2015, Youm et al. (2015) demonstrated that BHB inhibited MSU crystal-, silica-, and PA-induced caspase-1 activation and NLRP3 inflammasome-mediated IL-1β and IL-18 production by preventing potassium efflux, ASC oligomerization, and speck formation. In addition, elevation of BHB via ketogenic diet blocks NLRP3 inflammasome activation and IL-1β secretion both in murine macrophages and neutrophils, resulting in protection against acute gout induced by MSU injection (Goldberg et al., 2017). These data highlight the specificity with which BHB targets the NLRP3 inflammasomes, making it a potential candidate for preventing NLRP3-driven inflammation, for therapeutic target in gout patients.
6.4 Dopamine
Yan et al. (2015) showed that dopamine, a neurotransmitter, is also an endogenous regulator of NLRP3 inflammasome activation and demonstrated that dopamine inhibits NLRP3 inflammasome activation and IL-1β secretion in murine macrophages induced by various stimuli (MSU, alum, Nigericin, and ATP). Via dopamine D1 receptor signaling, dopamine reduces NLRP3 protein level by promoting NLRP3 ubiquitination. In this process, an E3 ubiquitin ligase MARCH7 is required for the dopamine-induced ubiquitylation and degradation of NLRP3. In addition, NLRP3 degradation is dependent on the second messenger cyclic AMP. Importantly, dopamine mitigates MSU-induced peritoneal inflammation by inhibiting NLRP3 inflammasomes in vivo.
6.5 MCC950
Coll et al. (2015) discovered that MCC950, a diarylsulfonylurea-containing compound known to inhibit caspase-1-dependent processing of IL-1β, also specifically inhibits NLRP3 inflammasome activation, but not other inflammasomes. They showed that MCC950 inhibits the secretion of IL-1β and NLRP3-induced ASC oligomerization, but is not associated with NLRP3 priming, potassium efflux, or NLRP3-ASC interactions in macrophages (Coll et al., 2015). In murine macrophages, combined LPS and cholesterol crystal stimulation results in a robust release of IL-1β, which is completely inhibited by MCC950 treatment. In addition, treatment with MCC950 reduces the development of atherosclerotic lesions in vivo. On the other hand, in adenine-rich diet-induced nephropathy, induction of intrarenal crystal formation activates NLRP3 inflammasomes and IL-1β production, resulting in kidney fibrosis in mice, and MCC950 treatment strongly attenuates kidney fibrosis by inhibiting NLRP3 inflammasome in vivo (Sakai, Furuichi, & Wada, 2016). Recently, many other studies have also reported that the beneficial effects of MCC950 in various types of inflammatory diseases involving the NLRP3 inflammasomes (Huang et al., 2018; Jiang et al., 2017).
7 CONCLUSION
NLRP3 inflammasomes are clearly activated by exogenous nanoparticles and endogenous crystalline molecules. These nanoparticles and crystalline molecules induce three critical events: Lysosome destabilization, ROS production, and mitochondrial damage. Once activated, NLRP3 inflammasomes drive a robust release of mature IL-1β, initiating a positive-feedback loop resulting in the accumulation of other immune cells (neutrophils and macrophages) and an increase in the “danger” cytokines and chemokines. Excessive or uncontrolled activation of NLRP3 inflammasomes by nanoparticles or crystalline molecules, therefore, triggers the onset and progression of various types of inflammatory diseases, such as silicosis, asbestos, gout, pseudogout, atherosclerosis, and metabolic diseases. Considering the potential for excessive NLRP3 inflammasomes and IL-1β production, it is not unexpected that multiple negative regulatory mechanisms exist in nature to control inflammasome function. Understanding these negative regulatory processes is essential for the identification of new targets for the treatment of crystal-related diseases.
AUTHOR CONTRIBUTIONS
K. S. and T. K. wrote the manuscript. M. T. critically revised the manuscript. All authors read and approved the final manuscript.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.




