PURB is a positive regulator of amino acid-induced milk synthesis in bovine mammary epithelial cells
Abstract
Previous studies have implicated that purine-rich element binding protein B (PURB) is a key regulator of gene transcription and cell physiology. Whether PURB plays a regulatory role in milk synthesis in bovine mammary epithelial cells (BMECs) is not known. We observed that Met and Leu increased PURB expression and nuclear localization. Overexpression of PURB led to increased milk protein and fat synthesis as well as mTOR and SREBP-1c expression whereas PURB knockdown had the opposite effects. The PI3K inhibitor wortmannin totally abolished the stimulation of Met and Leu on PURB expression, and we further confirmed that PURB was required for Met and Leu to stimulate mTOR phosphorylation and SREBP-1c expression. We also demonstrated that PURB binds to the promoters of mTOR and SREBP-1c, and these bindings were increased by Met and Leu stimulation. In summary, our data reveal that PURB is required for amino acids to stimulate mTOR and SREBP-1c gene expression, and PURB is a positive regulator of amino acid-induced PI3K-regulated milk protein and fat synthesis in BMECs.
1 INTRODUCTION
The purine-rich element binding protein B (PURB) is a member of the evolutionarily-conserved Pur family of single-stranded nucleic acid sequence-binding proteins known as PURA, PURB, and two forms of PURG (Daniel & Johnson, 2018; Kelm, Lamba, Levis, & Holmes, 2018). The Pur family proteins can form homo- and heteromeric complexes and bind specifically and with high affinity to the purine-rich ssDNA (sense or antisense) or RNA. They prefer to bind the GGN (N is not G) repeat-containing sequence. These proteins are structurally related and have repeated nucleic acid binding domains that are highly conserved. These domains form the central region of the protein whereas the amino and carboxyl termini are more divergent and are associated with the interaction of Pur proteins with certain protein or nucleic acid-binding partners (Kelm, Cogan, Elder, Strauch, & Getz, 1999; Kelm, Wang, Polikandriotis, & Strauch, 2003). For example, mouse PURB is a 324 amino acid protein with five repeated nucleic acid binding domains. It requires all five repeats (37-263aa) to bind to purine-rich ssDNA and RNA, whereas the N-termini (1-36aa) and C-termini (264-324aa) serve as regulatory sequences for this binding (Wortman, Johnson, & Bergemann, 2005). Among the Pur family members, in higher eukaryotes, PURA and PURB are ubiquitously expressed whereas PURG is at relatively low levels in most tissue or cells (Liu, & Johnson, 2002).
The Pur family proteins play pivotal roles in modulating DNA replication and gene expression, controlling cell growth and differentiation in a cell type-dependent specific manner. Both PURA and PURB have been linked with tumor development, cell senescence, postnatal brain development (Johnson, Daniel, & Gordon, 2013; Khalili et al., 2003; Mulnix et al., 2013). Mutations of Pur proteins have recently been described to cause Pur syndrome, a neurodevelopmental disorder (Reijnders et al., 2018; Rezkalla, Von Wald, & Hansen, 2017). In humans, PURA can function as a transcriptional activator for certain gene, regulating cell cycle transition (Daniel & Johnson, 2018; Johnson et al., 2013). PURB is the dominant repressor among the Pur family proteins of smooth muscle α-actin (SMA) gene expression in fibroblasts and vascular smooth muscle cells (Hariharan, Kelm, & Strauch, 2014; Knapp et al., 2006). The physiological roles and molecular mechanism of Pur proteins still need for further study.
Milk protein and fat are synthesized in mammary epithelial cells (MECs), and the mechanistic target of rapamycin (mTOR) and sterol regulatory element-binding protein 1c (SREBP-1c) are two main signaling molecules controlling these courses (Herve, Quesnel, Lollivier, & Boutinaud, 2016; Martignani, Cravero, Miretti, Accornero, & Baratta, 2014; Zhang et al., 2018). mTOR is a central signaling kinase, which responds to diverse extra- and intra-cellular stimuli and phosphorylates its substrates ribosomal protein S6 kinase beta-1 (S6K1) and eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) leading to increased protein synthesis (Saxton & Sabatini, 2017; Wolfson & Sabatini, 2017). SREBP-1c has been established as the main transcription factor controlling gene expression of fat synthesis enzymes and the main regulator of milk fat synthesis (Han, Gao, Yang, & Loor, 2018; N. Li et al., 2014, P. Li et al., 2018). mTOR can also regulate SREBP-1c expression and activity to regulate milk synthesis (Bakan & Laplante, 2012; Matsuzaka & Shimano, 2016).
Amino acids are not only substrates for protein synthesis but also signaling molecules regulating diverse cellular physiology including protein and lipid synthesis, cell growth, and differentiation (Abu-Remaileh et al., 2017; Wolfson & Sabatini, 2017). Amino acids such as methionine (Met) and leucine (Leu) can upregulate the expression of mTOR and SREBP-1c and therefore enhance these signaling leading to increased milk protein and fat synthesis (P. Li et al., 2018; Zhang et al., 2018). The detailed mechanism underlying how amino acids regulate the expression of mTOR and SREBP-1c is still largely unknown.
In our previous promeotics study, we found that PURB expression is increased in bovine MECs (BMECs) stimulated by Met. We hypothesized that PURB might be a regulator of the expression of mTOR and SREBP-1c and milk protein and fat synthesis. In this study, we sought to determine whether PURB is a key regulator of amino acid-induced milk synthesis in BMECs.
2 MATERIALS AND METHODS
2.1 Cell culture and treatments
BMECs was isolated according to previous reports (Cui et al., 2015; Wang et al., 2014). Briefly, BMECs were isolated from the mammary gland tissues of mid-lactation dairy cows and cultured in DMEM/F-12 medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Gibco10091-148, Grand island, NY) and 50 U/ml penicillin/ml streptomycin. Cell purity was tested by immunofluorescence observation of the expression of the epithelial marker cytokeratin 18 (CK18). BMECs purified in 5–10 passages were stored in liquid nitrogen, and for experimental assays, cells were thawed and cultured in six-well plates at 3 × 105 cells/well. To observe the effects of amino acids, Met and Leu (0.6 mM) were added to the culture medium, and the cells were cultured for 24 hr. To inhibit phosphotidylinositol 3-kinase (PI3K) activity, wortmannin (Wm; 400 nmol/l) was added to cell culture medium 1 hr before Met or Leu (0.6 mM) treatment.
2.2 Immunofluorescence assay
Immunofluorescence assay was performed according to a previous report (Zhang et al., 2018). The subcellular localization of CK18 and PURB were detected with corresponding primary antibodies. Primary antibodies: CK 18 (sc-51582, Santa Cruz, Santa Cruz, CA) and PURB (18128-1-AP, Proteintech, Chicago, IL). These antibodies were detected using the appropriate Alexa Fluor 647 or Alexa Fluor 488 secondary antibody (ZSGB-BIO, Beijing, China). Next nucleus were stained with 4′,6-diamidino-2-phenylindole (DAPI). Cells were visualized with a confocal microscope (TCS-SP2 AOBS, Leica, Heidelberg, Germany).
2.3 Western blot analysis
Western blot analysis was performed using standard techniques to detect the expression of PURB and other specific proteins. Briefly, cells were lysed in cold RIPA lysis buffer (Beyotime, Beijing, China). Protein concentrations were determined with a bicinchoninic acid assay kit (Beyotime). Sample buffer was added, and the samples were boiled. Equal amounts of protein were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and blotted to polyvinylidene fluoride membranes and probed with the corresponding primary and secondary antibodies. Primary antibodies: PURB (18128-1-AP, Proteintech); α-casein (bs-8245R), β-casein (bs-0813R), Bioss, Beijing, China; mTOR (#2983), p-mTOR (Ser2448) (#5536), Cell Signaling Technology, Danvers, MA; SREBP-1c (sc-365513), AKT1 (sc-1618), p-AKT1 (Thr308) (sc-135650), lamin B1 (sc-20682), β-tubulin (sc-9935), and β-actin (sc-47778), Santa Cruz. Densitometry was performed using Image J software and normalized to β-actin.
2.4 Detection of triglyceride secretion
Triglyceride (TG) amounts in the culture medium were determined using the TG GPO-POD Assay kit (ApplygenTech Inc., Beijing, China) according to the manufacturer's protocol.
2.5 Detection of lipid droplet
Lipid droplet in BMECs was detected by a fluorescence-based method (N. Li et al., 2014; Liang et al., 2014). Briefly, cells were grown on glass coverslips in six-well plates to 30%–50% confluence. Then cells were fixed in 4.0% (w/v) paraformaldehyde for 10 min. The hydrophobic fluorescent probe Bodipy 493/503 (Invitrogen; 1 μg/ml) were added to culture medium and cells were incubated for 15 min. Then, cells were incubated for 10 min in a dark room with DAPI. Finally, the integral optical density of lipid droplets was detected using a confocal microscope (TCS-SP2 AOBS, Leica).
2.6 PURB overexpression
Bovine PURB CDS sequence (NM_001206632) was amplified from BMEC cDNA and subcloned into pGCMV/IRES/EGFP expression vector (GenePharma, Shanghai, China) and completely sequenced for verification. Cells were transfected with the recombinant plasmid using Lipofectamine 2000 (11668027, Invitrogen) according to the manufacturer's instruction, and cells transfected with the empty vector were used as control. Cells were harvested 24 hr after transfection.
2.7 PURB knockdown
PURB knockdown was performed according to a previous report (Zhang et al., 2018). A mixture of three small interfering RNAs (siRNAs) targeting different regions of PURB mRNA and the negative control siRNA (NC) were purchased from GenePharma. The three PURB siRNA sequences were as follows: PURB-siRNA-393: 5′-GCGAGAACCGCAAGUACUATT-3′, PURB-siRNA-773: 5′-GCCAUCACGGUCCCUUUCATT-3′, and PURB-siRNA-859: 5′-GCAGAGGGAUAAGCUUUAUTT-3′. The mixture of siRNAs was transfected into cells using Lipofectamine 2000 (Invitrogen), and cells transfected with NC were used as control. Cells were harvested 24 hr post-transfection.
2.8 Nuclear and cytoplasmic fractionation
The nuclear and cytoplasmic fractions were separated by using NE-PER Nuclear and Cytoplasmic Extraction Kit (P0028, Beyotime).
2.9 Quantitative real-time PCR assays
Total RNA was extracted from BMECs using Trizol reagent (Invitrogen), and RNA quality was quantified by gel analysis. Total RNA (~2 μg) was reverse-transcribed into cDNA using a thermoscript reverse transcriptase (TaKaRa, Dalian, China). The qRT-PCR Kit SensiMix™ SYBR & Fluorescein was used for quantitative real-time PCR (qRT-PCR) reactions. The analysis was carried out on ABI PRISM 7500 RT-PCR System (Applied Biosystems, Foster City, CA). qRT-PCR primers to analyze the mRNA expression of mTOR and SREBP-1c were synthesized according to the previous reports (Huang et al., 2017; Wang et al., 2014), qRT-PCR primers for PURB: F : 5′-AAACGGTTACCAGTCCCTGT-3′, R : 5′-CGGTTTATTCTAGGGAGATTA-3′. All target mRNAs were normalized to β-actin mRNA level. The relative expression of the target genes was quantified using the 2−ΔΔCt method.
2.10 Chromatin immunoprecipitation assays
Chromatin immunoprecipitation (ChIP) was performed using a ChIP assay kit (P-2078, Beyotime) with antibodies against PURB (18128-1-AP, Proteintech). An antibody against IgG served as the negative control and antibody against RNA polymerase as the positive control. To select the binding site of PURB in the promoters of mTOR and SREBP-1c, eight primers to amplify the different regions of the gene promoter (−1 to −2000 nt) of mTOR and 7 primers to amplify the different regions of the gene promoter of SREBP-1c (−1 to −2000 nt) were designed and sequenced, then the primers amplifying predicted sequence by ChIP-qPCR were used for next ChIP-qPCR analysis. Only one sequence was amplified in each promoter (−1480 to −1266 nt in the promoter of mTOR and −217 to −27 nt in the promoter of SREBP-1c), and the corresponding primers were used for next ChIP-qPCR. ChIP-qPCR primers: mTOR, F: 5′-CTTAGGAAGATAAAAATCCTATA -3′, R: 5′-GTTGAAGCTGAAACTCCAATACT-3′; SREBP-1c, F: 5′- GCGCGCGTCCCCTCCGCCTCT CTCT-3′, R: 5′-TGGAGACCCGCAGCCAGCTCAAGTG -3′. For ChIP-qPCR, the data represent the percentage of input that is immunoprecipitated.
2.11 Statistical analysis
Analysis of statistics was calculated by SPSS 21 software (IBM, Armonk, New York, NY). Data are presented as means ± SE (standard error) of three independent experiments. Differences between means were determined using one-way analysis of variance or Student's t-test, and statistical significance was detected when p < 0.05 or p < 0.01.
3 RESULTS
3.1 PURB is associated with amino acid-induced milk synthesis in BMECs
To determine whether PURB is a regulator of milk synthesis in BMECs, we first observed the effects of amino acids on PURB expression. The purity of cultured primary BMECs was identified by the immunofluorescence observation of the expression of CK18 (Figure 1a). Met and Leu (0.6 mM) addition in the culture medium significantly increased α-casein (Figure 1b,c) and β-casein (Figure 1b,d) synthesis in BMECs by Western blot analysis analysis. Met and Leu treatments also significantly increased the secretion of TG by cells in the culture medium (Figure 1e) and promoted lipid droplet formation in the cytoplasm of BMECs (Figure 1f). Met and Leu treatments also led to increased mTOR phosphorylation (Figure 1b,g) and SREBP-1c expression (Figure 1b,h).
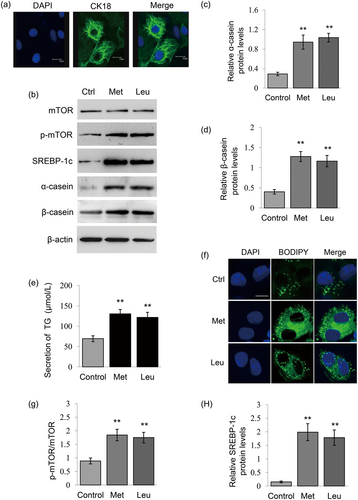
Met and Leu stimulate milk protein and fat synthesis in BMECs. (a) The purity of cultured primary BMECs were identified by the immunofluorescence observation of the expression of CK18. CK18 (green), nuclei were stained with DAPI (blue), Scale bar = 15 μm. (b) Western blot analysis of indicated protein levels in BMECs stimulated by Met or Leu (0.6 mM). Normal cultured cells were used as controls. β-actin served as a loading control. Figure shows one representative result from three independent experiments. (c,d) Relative folds of α-casein (c) and β-casein (d) protein levels (protein/β-actin) from the western blots in (b) were quantified by gray scale scan. (e) TG amounts secreted by cells into the culture medium were detected using a TG assay kit. (f) Fluorescence staining of lipid droplets in cells. Lipid droplet (green), DAPI (blue). Scale bar represents 15 μm. (g) The ratio of p-mTOR to total mTOR from the western blots in (b) were quantified by gray scale scan. (h) Relative fold of SREBP-1c protein levels (SREBP-1c/β-actin) from the western blots were quantified by gray scale scan. Data were the mean ± standard error (SE) from three independent experiments. *p < 0.05; **p < 0.01. BMECs: bovine mammary epithelial cells; DAPI: 4′,6-diamidino-2-phenylindole; TG: triglyceride [Color figure can be viewed at wileyonlinelibrary.com]
By Western blot analysis and qRT-PCR analysis, we also observed that Met and Leu treatments upregulated the protein (Figure 2a,b) and mRNA (Figure 2c) levels of PURB. Immunofluorescence assay detected that PURB was located in the nucleus of BMECs, and Met and Leu treatments led to an increased nuclear localization of PURB (Figure 2d). These data suggest that PURB might be associated with the stimulation of amino acids on milk synthesis in BMECs.
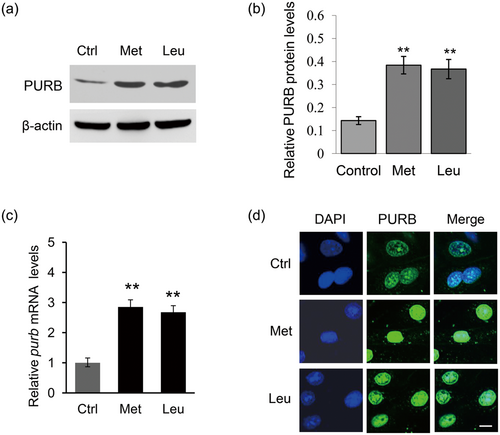
Met and Leu stimulate PURB expression and nuclear localization. (a) Western blot analysis of PURB protein levels in BMECs stimulated by Met or Leu. Normal cultured cells were used as controls. β-actin served as a loading control. Figure shows one representative result from three independent experiments. (b) Relative folds of PURB protein levels (PURB/β-actin) from the western blots in (a) were quantified by gray scale scan. (c) qRT-PCR analysis of purb mRNA levels in cells treated as in (a). (d) The subcellular localization of PURB was observed by imunofluorescence staining using an antibody against PURB. PURB (green), DAPI (blue). Scale bar = 15 μm. Data were the mean ± standard error (SE) from three independent experiments. *p < 0.05; **p < 0.01. BMECs: bovine mammary epithelial cells; DAPI: 4′,6-diamidino-2-phenylindole; PURB: purine-rich element binding protein B; qRT-PCR: quantitative real-time PCR [Color figure can be viewed at wileyonlinelibrary.com]
3.2 The effects of PURB on milk protein and fat synthesis
To investigate the regulatory role of PURB on milk synthesis, we overexpressed PURB in BMECs by transfecting an overexpression vector into cells and knocked down PURB in cells by transfection of a PURB siRNA mixture into cells. Western blot analysis showed that PURB is overexpressed or knocked down in cells after this tranfection (Figure 3a,b). Overexpression of PURB led to increased α-casein (Figure 3a,c) and β-casein (Figure 3a,d) synthesis in BMECs, whereas PURB knockdown significantly decreased α-casein (Figure 3a,c) and β-casein (Figure 3a,d) synthesis. PURB overexpression also significantly increased the secretion of TG by cells in the culture medium (Figure 3e) and promoted lipid droplet formation in the cytoplasm of BMECs (Figure 3f), whereas PURB knockdown had the opposite effects (Figure 3e,f). These data reveal that PURB is a positive regulator of milk protein and fat synthesis in BMECs.
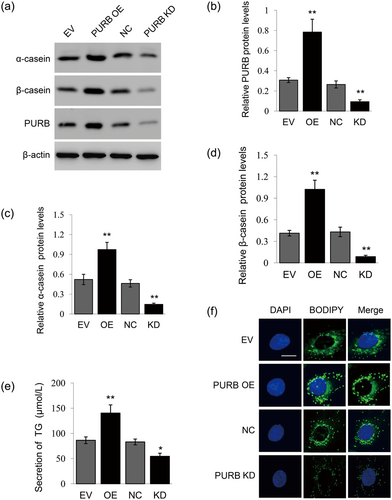
The effects of PURB overexpression and knockdown on milk synthesis. (a) Western blot analysis of indicated protein levels in BMECs transfected with a PURB overexpression vector or PURB siRNA mixture. Cells transfected with empty vector (EV) or NC were used as controls. Figure shows one representative result from three independent experiments. (b–d) Relative folds of PURB (b), α-casein (c), and β-casein (d) protein levels (protein/β-actin) from the western blots in (a) were quantified by gray scale scan. (e) TG amounts secreted into the culture medium by cells treated as in (a) were detected using a TG assay kit. (f) Fluorescence staining of lipid droplets in cells treated as in (a). Lipid droplet (green), DAPI (blue). Scale bar represents 15 μm. Data were the mean ± standard error (SE) from three independent experiments. *p < 0.05; **p < 0.01. BMECs: bovine mammary epithelial cells; DAPI: 4′,6-diamidino-2-phenylindole; NC: negative control; PURB: purine-rich element binding protein B; siRNAs: small interfering RNAs; TG: triglyceride [Color figure can be viewed at wileyonlinelibrary.com]
We next determined the effects of PURB overexpression and knockdown on the signaling pathways leading to milk protein and fat synthesis. Western blot analysis showed that PURB overexpression led to increased mTOR phosphorylation (Figure 4a,b) and SREBP-1c protein level (Figure 4a,c), whereas PURB knockdown had the opposite effects (Figures 4a–c). qRT-PCR analysis further detected that PURB overexpression led to increased mTOR and SREBP-1c mRNA expression, whereas PURB knockdown had the opposite effects (Figure 4d). These data suggest that PURB positively regulates milk protein and fat synthesis via stimulating mTOR and SREBP-1c signaling.
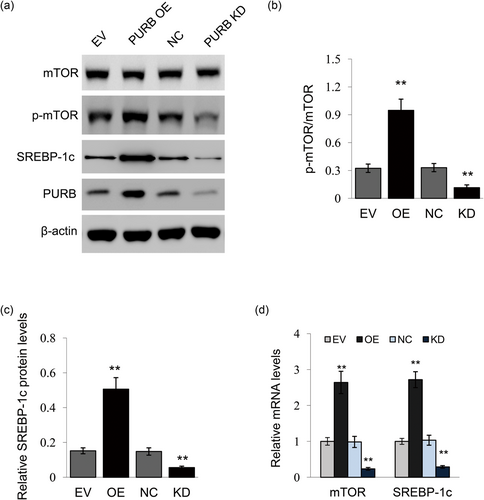
The effects of PURB on signaling molecules associated with milk synthesis. (a) Cells were treated as in Figure 3. Indicated protein levels were measured by Western blot analysis analysis, β-actin served as a loading control. Figure shows one representative result from three independent experiments. (b) The ratio of p-mTOR to total mTOR from the western blots in (a) were quantified by gray scale scan. (c) Relative fold of SREBP-1c protein levels (SREBP-1c/β-actin) from the western blots were quantified by gray scale scan. (d) qRT-PCR analysis of mTOR and SREBP-1c mRNA levels in cells. Data were the mean ± standard error (SE) from three independent experiments. *p < 0.05; **p < 0.01. qRT-PCR: quantitative real-time PCR [Color figure can be viewed at wileyonlinelibrary.com]
3.3 Amino acids trigger PURB expression through the PI3K signaling
We further investigated how amino acids regulate PURB expression and subcellular localization. Western blot analysis detected that PURB is located in the nucleus and Met and Leu markedly increased PURB nuclear localization (Figure 5a). We previously found that Met regulates mTOR and SREBP-1c mRNA expression through the PI3K signaling in BMECs (Huang et al., 2017); thus, we speculated that PI3K activity might be required for amino acids to regulate PURB expression. The PI3K inhibitor wortmannin (Wm, 400 nM) totally abolished AKT phosphorylation, showing its inhibitory effect on PI3K (Figure 5b,c). We further observed that PI3K inhibition totally abolished the stimulation of Met and Leu on PURB expression (Figure 5b,d). These data reveal that amino acids regulate PURB expression via the PI3K signaling.
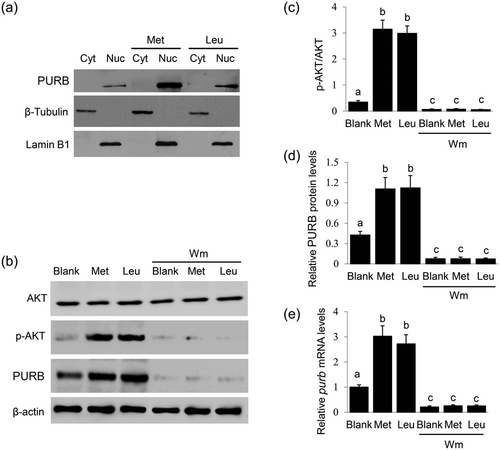
Effects of PI3K inhibition on PURB expression. (a) BMECs were stimulated by Met or Leu (0.6 mM) for 24 hr. PURB in the cytoplasm and nucleus were analyzed by Western blot analysis. Lamin B1 and β-tubulin were used to show the purities of nucleus and cytoplasm. Figure shows one representative result from three independent experiments. (b) Cells were treated with Met or Leu (0.6 mM) together with Wm (400 nM) for 24 hr. Western blot analysis of the indicated protein levels, β-actin served as a loading control. Figure shows one representative result from three independent experiments. (c) The ratio of p-AKT to total AKT in cells treated as in (b) were quantified by gray scale scan. (d) Relative folds of PURB protein levels (PURB/β-actin) from the western blots in (b) were quantified by gray scale scan. (e) qRT-PCR analysis of purb mRNA levels in cells treated as in (b). Data were the mean ± standard error (SE) from three independent experiments. Value with different superscripted lowercase letter indicates significant difference (p < 0.05). BMECs: bovine mammary epithelial cells; PURB: purine-rich element binding protein B; qRT-PCR: quantitative real-time PCR
3.4 PURB is a key mediator for amino acid to stimulate the mTOR and SREBP-1c signaling pathways
We further investigated whether PURB mediates amino acid stimulation on the mTOR and SREBP-1c signaling pathways. BMECs were stimulated with Met or Leu and transfected with PURB siRNAs, and the effects of PURB knockdown were verified by Western blot analysis (Figure 6a,b). PURB knockdown dramatically suppressed the stimulation of Met or Leu on the phosphorylation of mTOR (Figure 6a,c). PURB knockdown also abolished the stimulation of Met or Leu on the protein levels of SREBP-1c (Figure 6a,d). These data reveal that PURB is required for amino acids to regulate the mTOR and SREBP-1c signaling pathways.
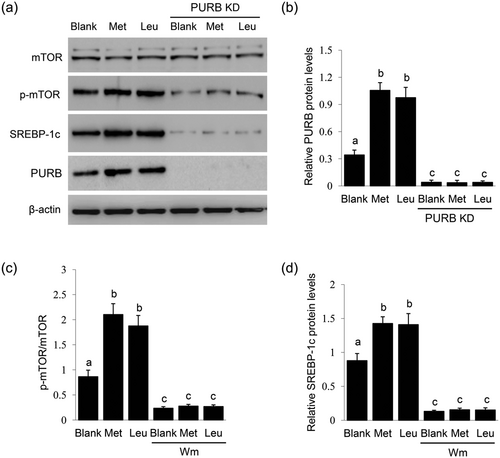
Effects of PURB knockdown on the regulatory roles of Met and Leu. (a) BMECs were transfected with PURB siRNAs and treated with Met or Leu (0.6 mM) for 24 hr. Western blot analysis analysis of the indicated protein levels, β-actin served as a loading control. Figure shows one representative result from three independent experiments. (b) Quantification of PURB protein levels (PURB/β-actin) from the western blots by gray scale scan. (c) The ratio of p-mTOR to total mTOR from the western blots were quantified by gray scale scan. (d) Relative fold of SREBP-1c protein levels (SREBP-1c/β-actin) from the western blots were quantified by gray scale scan. Data were the mean ± standard error (SE) from three independent experiments. Value with different superscripted lowercase letter indicates significant difference (p < 0.05). BMECs: bovine mammary epithelial cells; PURB: purine-rich element binding protein B; siRNAs: small interfering RNAs
3.5 Amino acids trigger PURB to bind to the gene promoters of mTOR and SREBP-1c
We next investigated whether PURB binds to the gene promoters of mTOR and SREBP-1c, thus regulating their gene transcription. ChIP-qPCR with PURB antibody detected that only one sequence (−1480 to −1266 nt) was amplified in the promoter of mTOR (Figure 7a), suggesting there might be only one binding site of PURB in the promoter of mTOR. Both Met and Leu treatments significantly increased the binding of PURB to this region by ChIP-qPCR analysis (Figure 7b). ChIP-qPCR also detected that only one sequence (−217 to −27 nt) was amplified in the promoter of SREBP-1c (Figure 7c), and Met and Leu treatments increased this binding (Figure 7d). These data, combined with the data in Figures 2 and 4d, reveal that amino acids stimulate PURB expression and binding to the gene promoters of mTOR and SREBP-1c for their gene transcription.

ChIP-qPCR analysis of the binding of PURB to the gene promoters of mTOR and SREBP-1c. (a) ChIP-qPCR analysis of the binding of PURB to the gene promoters of mTOR. Eight primers to amplify the different regions of the gene promoter (−1 to −2000 nt) of mTOR were used for ChIP-qPCR. Only one sequence (−1480 to −1266 nt) was amplified in the promoter of mTOR, and the corresponding primers were used for next ChIP-qPCR. Figure depicts the binding region of PURB in the gene promoters of mTOR. (b) ChIP-qPCR analysis of the binding of PURB to the gene promoters of mTOR in cells stimulated by Met and Leu. (c) ChIP-qPCR analysis of the binding of PURB to the gene promoters of SREBP-1c. Seven primers to amplify the different regions of the gene promoter (−1 to −2000 nt) of SREBP-1c were used for ChIP-qPCR. Only one sequence (−217 to −27 nt) was amplified in the promoter of SREBP-1c, and the corresponding primers were used for next ChIP-qPCR. Figure depicts the binding region of PURB in the gene promoters of SREBP-1c. (d) ChIP-qPCR analysis of the binding of PURB to the gene promoters of SREBP-1c in cells stimulated by Met and Leu. ChIP: chromatin immunoprecipitation; PURB: purine-rich element binding protein B
4 DISCUSSION
Some evidence indicates that PURB plays a critical role in gene transcription, but the reports are relatively fewer compared to many other transcriptional factors (Daniel & Johnson, 2018; Johnson et al., 2013). In the current study, we show that PURB is a positive regulator of milk protein and fat synthesis in BMECs. PURB is located in the nucleus, and amino acids (Met and Leu) stimulates PURB expression via the PI3K signaling. PURB can bind to the gene promoters of mTOR and SREBP-1c, and Met and Leu stimulate this binding. We also show that PURB mediates the stimulation of Met and Leu on the expressions of mTOR and SREBP-1c, leading to increased milk protein and fat synthesis.
Though the physiological roles of Pur have not been fully studied, previous reports point that Pur family proteins may play a positive or negative role in modulating gene expression in a cell type-dependent specific manner (Daniel & Johnson, 2018; Johnson et al., 2013). For examples, PURB restricts the gene transcription of ATAT2 (the gene encoding SMA) in mouse embryo fibroblasts. PURB interacts with the 32 nt (−194 to −164) purine-rich sense strand of the promoter of ATAT2 via a sequential and cooperative binding mechanism to form high affinity 2:1 PURB:ssDNA complex (Rumora, Wang, Ferris, Everse, & Kelm, 2013). PURA plays a negatively regulatory role in controlling the heat shock protein HSP70 transcription in Crassostrea hongkongensis (Xu, Wang, Cui, & Zhang, 2018). In this study, we demonstrate that PURB binds to the promoter region (−1480 to −1266 nt) of mTOR and promoter regions (−217 to −27 nt) of SREBP-1c and promotes these two gene transcriptions in response to amino acid stimulation. The opposite results (positive or negative roles in modulating gene transcription) may be related to different cell and tissue backgrounds, and we speculated that this might be associated with the interaction of PURB with different transcriptional activators or inhibitors in different cells.
In this study, we found that PURB is located in the nucleus. We cannot exclude that PURB might also be in the cytoplasm due to the limitation of the primary antibody against PURB which cannot detect the possible modified molecular form of PURB. Our data that Met and Leu increased PURB nuclear expression is consistent with the regulatory role of PURB on gene transcription. We did not detect the possible role of PURA in the regulation of milk synthesis in BMECs, from literature (Kelm et al., 2018; Kelm et al., 2003), it is possible that PURB may form homo- and hetero-meric complexes (with PURA) to exert its function.
PI3K(s) are a family of related intracellular signal transducer enzymes, divided into four different classes: Class I, Class II, Class III, and Class IV. In these subtypes, Class I PI3Ks produce phosphatidylinositol (3,4,5)-trisphosphate (PI(3,4,5)P3) and activate downstream signaling pathways such as AKT-mTOR signaling (Dibble &Cantley, 2015; Leevers, Vanhaesebroeck, & Waterfield, 1999; Simioni et al., 2018). Amino acids can activate PI3K via the G protein-coupled receptors and some amino acid transporters (Goberdhan, Wilson, & Harris, 2016; Nie, He, Zhang, Zhang, & Ma, 2018; Zheng et al., 2016). Our experimental results demonstrated that Met and Leu increased PURB expression via the PI3K (Class I) signaling, in agreement with the previous results. In our previous study, we confirmed that Met regulates the gene expression of mTOR and SREBP-1c depending on the PI3K activity but independent of mTOR (Huang et al., 2017). Our data further support the idea that amino acids promote gene expression via the PI3K signaling to regulate cell physiology.
There is a possibility that amino acids not only promote PURB expression but also affect its possible modified molecular form for the regulation of gene expression. Previous reports show that Pur proteins have phosphorylated molecular forms. PURA is phosphorylated in vitro by casein kinase II at both the N- and C-terminus of the protein (de Oliveira et al., 2004). PURB is phosphorylated in short-term vasopressin-treated rat kidney collecting duct (Hoffert, Wang, Pisitkun, Shen, & Knepper, 2007). There is still no commercial antibody against phosphorylated PURB for detection. We speculate that the phosphorylated form of PURB might be an active form of PURB to regulate gene expression, and amino acids might increase this phosphorylation, which is needed for future study.
In summary, amino acids (Met and Leu) trigger PURB expression in the nucleus of BMECs via the PI3K signaling and binding to the promoters of mTOR and SREBP-1c, and PURB is required for amino acids to stimulate the gene expression of mTOR and SREBP-1c leading to milk protein and fat synthesis. Future studies need to explore the possible modified molecular form of PURB to deeply understand the molecular mechanism.
ACKNOWLEDGMENT
This study was supported by grants from the National Natural Science Foundation of China (31472162 and 31671473).
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.




