Inflammation and pericarditis: Are neutrophils actors behind the scenes?
Abstract
The morbidity of acute pericarditis is increasing over time impacting on patient quality of life. Recent clinical trials focused especially on clinical aspects, with a modest interest in pathophysiological mechanisms. This narrative review, based on papers in English language obtained via PubMed up to April 2018, aims at focusing on the role of the innate immunity in pericarditis and discussing future potential therapeutic strategies impacting on disease pathophysiology.
In developed countries, most cases of pericarditis are referred to as idiopathic, although etiological causes have been described, with autoreactive/lymphocytic, malignant, and infectious ones as the most frequent causes. Apart the known impairment of the adaptive immunity, recently a large body evidence indicated the central role of the innate immune system in the pathogenesis of recurrent pericarditis, starting from similarities with autoinflammatory diseases. Accordingly, the “inflammasome” has been shown to behave as an important player in pericarditis development. Similarly, the beneficial effect of colchicine in recurrent pericarditis confirms that neutrophils are important effectors as colchicine, which can block neutrophil chemotaxis, interferes with neutrophil adhesion and recruitment to injured tissues and abrogate superoxide production. Anyway, the role of the adaptive immune system in pericarditis cannot be reduced to a black or white issue as mechanisms often overlap. Therefore, we believe that more efficient therapeutic strategies have to be investigated by targeting neutrophil-derived mediators (such as metalloproteinases) and disentangling the strict interplay between neutrophils and platelets. In this view, some progress has been done by using the recombinant human interleukin-1 receptor antagonist anakinra.
1 INTRODUCTION
Acute pericarditis is included in a wide spectrum of pericardial disorders, such as pericardial effusion, cardiac tamponade, and constrictive pericarditis. These entities are included within the definition of “pericardial syndromes” (Adler et al., 2015). Pericarditis-related morbidity is increasing and can significantly impact on patient quality of life, especially for those cases referred to as chronic or complicated conditions (Cremer et al., 2016). Despite limited clinical research studies, pericarditis is often encountered by physicians. Current medical treatments for pericarditis have made little advances in recent years since nonsteroidal anti-inflammatory drugs (NSAIDs) and colchicine still represent two milestones, with corticosteroids to be used for selected cases (Adler et al., 2015). More recently, when NSAIDs and colchicine are not effective, the use of anakinra, an interleukin (IL)-1 receptor antagonist, has been explored as an alternative therapeutic option showing a reduced risk of relapse over a median follow-up of 14 months (Brucato et al., 2016; Buckley, Viscusi, Van Tassell, & Abbate, 2018).
Currently available trials investigated pericarditis from a merely clinical point of view, with a scarce interest in deepening our understanding of the pathophysiological mechanisms of the disease. Accordingly, recurrent pericarditis has been suggested to derive from an interplay involving environmental triggers and both innate and adaptive immune mechanisms (Cremer et al., 2016; van Kempen, Wenink, Leijten, Radstake, & Boes, 2015). Some progress has been also made for the etiology of pericarditis, since the term “idiopathic” has been reassessed and “previously unclear” causes have been defined, as reported by Maisch et al. (Maisch, Rupp, Ristic, & Pankuweit, 2013).
In this narrative review, we aimed at assembling current literature about the role of the innate immunity and discussing future investigation fields to better understand inflammatory pathways underlying pericarditis and its recurrences as well as potential therapeutic strategies. We searched on PubMed matching the following keywords: “pericarditis and neutrophils,” “inflammation and pericarditis,” and “inflammasome and pericarditis.” Articles have also been retrieved through searches of reference lists and authors’ files, whereas abstracts have not been taken into consideration. The search has been limited to papers published in English language and conducted without date limits up to April 2018.
2 ETIOPATHOGENESIS OF PERICARDITIS
In developed countries, 80% of cases of pericarditis are currently defined as “idiopathic” (Imazio et al., 2013, 2014), this reflecting poor knowledge of disease-related pathophysiology. In recent years, a greater attention has been paid to eliminate the term “idiopathic” and the most accepted scenario now shows a close interplay between infectious stimuli (especially viral agents) and the immune system (Cremer et al., 2016). In 2013, Maisch et al. identified etiological causes of pericarditis in patients with pericardial effusion great enough to undergo pericardiocentesis and pericardioscopy with epi- and pericardial biopsies. These specimens were then analyzed at both molecular and immunity-histological levels (Maisch et al., 2013), identifying that 35% of cases referred to as autoreactive/lymphocytic, 28% malignant, 14% infectious (both viral and bacterial), 15% traumatic, and the left 8% to other nonspecified causes (Maisch et al., 2013). Among patients with infectious etiology, main bacterial origin accounted for Mycobacterium tuberculosis and Borrelia burgdorferi infections, whereas viral infections especially included Parvovirus B19 and Epstein-Barr virus (EBV) as the most prevalent ones. In a few patients, coexisting infections with cytomegalovirus (CMV), influenza virus, hepatitis C virus, and human herpesvirus 6 were also reported (Maisch et al., 2013).
In developing countries, tuberculosis is the leading cause of pericardial diseases frequently associated with the human immunodeficiency virus infection (Ntsekhe & Mayosi, 2013).
3 PATHOPHYSIOLOGICAL MECHANISMS IN PERICARDITIS
3.1 Role of the adaptive immunity
Classically, pericarditis has been related to an impairment of the adaptive immunity, as indicated by both direct and indirect findings (Brucato et al., 2018). Indeed, pericarditis occurs in the natural history of autoimmune diseases, especially systemic lupus erythematosus, and has a good therapeutic response to immunomodulatory treatments. Moreover, in patients with pericarditis, the positivity for antinuclear antibodies and autoantibodies against specific cardiac antigens has been reported (Artom et al., 2005). Infections as well were supposed to stimulate an autoimmune response through the so-called “molecular mimicry,” a mechanism in which an external antigen sharing sequence similarities with self-peptides results in a cross-activation of autoreactive T or B lymphocytes by pathogen-derived peptides, thus causing inflammatory manifestations (Cusick, Libbey, & Fujinami, 2012). Besides, many microorganisms (especially bacteria or mycoplasma) and virus-infected cells can generate “super-antigens,” which bind T-cell receptor and then activate T lymphocytes with different antigenic specificity. Finally, in the so-called “epitope spreading,” an increased processing and presentation of self-antigens can stimulate the expansion or spreading of the immune response towards different self-antigens, as frequently occurs in the pathogenesis of different systemic autoimmune diseases (Sfriso et al., 2010).
3.2 Role of the innate immunity
Apart from these clear mechanisms of impaired response of the adaptive immune system, more recently evidence accumulated on the pivotal role of the innate immune system in the pathogenesis of recurrent pericarditis. This hypothesis arises from similarities between recurrent pericarditis and autoinflammatory diseases, which show an unprovoked multisystemic inflammation usually deriving from alterations of the innate immune system in the absence of antigen-specific T cells or high titers of autoantibodies (Cremer et al., 2016). It is important to remember that the innate immune response starts when pathogen recognition receptors (PPRs) recognize the so-called pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (PAMPs; Figure 1). The most known PPRs are toll-like receptors (TLRs), which can be found both on the cell membrane or within endosomes. Other important PPRs, located at the cytoplasmic level, include the nucleotide binding and oligomerization domain (NOD)-like receptor (NLR) family (Kawai & Akira, 2006). More information concerning the role and the influence in the immune response of TLRs and NLRs can be found elsewhere (Fukata, Vamadevan, & Abreu, 2009). When talking about innate immunity, the inflammasome has to be considered a central player (Figure 1). The term “inflammasome” has been used for the first time in 2002 to define a high-molecular-weight complex within the cytosol of stimulated immune cells composed of the adapter protein ASC, pro-caspase 1, and a sensor molecule, which contains the NLR family members (Martinon, Burns, & Tschopp, 2002). Currently, many distinct inflammasomes are known, each of which assembling after the interaction of a unique pattern-recognition receptor with PAMPs or other endogenous danger signals in the cytosol of the host cells (Broz & Dixit, 2016). Beside their role in the host immune defense, an impaired activation of the inflammasome has been shown to favor the development of cancer as well as autoimmune and metabolic diseases (Broz & Dixit, 2016). Hence, the strict control of inflammasome formation and signaling is now considered of potential relevance in different damages. Among different inflammasomes, the best characterized one owns an NLR pyrin domain-containing (NLRP3) or cryopyrin sensor molecule, which have been shown to respond to a wide array of stimuli, such as monosodium urate crystals, silica, asbestos, cholesterol, viruses, bacteria, fungi, and protozoa (Broz & Dixit, 2016; Duewell et al., 2010; Latz, Xiao, & Stutz, 2013; Paramel Varghese et al., 2016). Indeed, many viruses can be responsible for pericarditis, including adenovirus, influenza A virus, herpesvirus, CMV, Coxsackie and echovirus, EBV, and parvovirus B19 (Imazio, 2011). Once NLRP3 inflammasome is activated, it can stimulate the activation and release of IL-1β (from pro-IL-1β via caspase 1), which is a potent proinflammatory cytokine responsible for the recruitment of effector cells at the site of injury. This cytokine was also associated with the upregulation of prostaglandin production, the expression of leukocyte adhesion molecules, and thrombogenic mediators (Libby, 2017). Accordingly, the recently published Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) trial testing IL-1β inhibition in cardiovascular (CV) disease has shed some light on this pivotal cytokine and its therapeutic potential, as we will discuss later in the future perspectives paragraph.
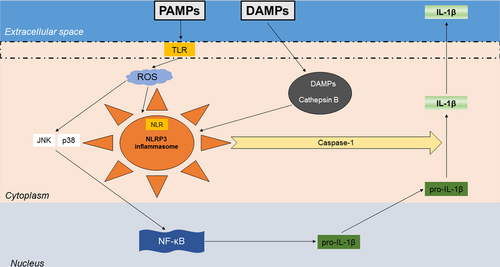
The role of the inflammasome in the pathogenesis of pericarditis. The innate immune system recognizes a microbial or sterile stimulus, for example, pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs), respectively, by receptors either at cell surface (toll-like receptor [TLR]) or within the cell (nucleotide-binding oligomerization domain-like receptor [NLR]), the former then included into the structure of the inflammasome (nucleotide-binding oligomerization domain-like receptor pyrin domain-containing 3 [NLRP3]). TLR signals can also activate the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) via reactive oxygen species (ROS), Jun amino-terminal kinase (JNK), and p38, finally leading to the production of pro-interleukin (IL)-1β, then activated by caspase-1 into IL-1β [Color figure can be viewed at wileyonlinelibrary.com]
In addition, the role of autoinflammatory processes in the pathophysiology of recurrent pericarditis is supported by the benefits induced by treatment with colchicine. Starting from crystal arthropaties, colchicine has been demonstrated to influence neutrophil function at different stages. As colchicine has the ability to concentrate intensively within leukocytes, it was shown to block neutrophil chemotaxis (Phelps, 2008). Colchicine can interfere with neutrophil adhesion and recruitment to injured tissues via microtubule polymerization (Cronstein et al., 1995). Colchicine-induced microtubule inhibition was shown to selectively block superoxide production by neutrophils in vitro (Roberge et al., 1993). Finally, colchicine is now an established therapy for familial Mediterranean fever as it inhibits pyrin by interacting with tubulin and colocalizing with microtubules (Moll & Kuemmerle-Deschner, 2013) and has modest effects on the NLRP3 inflammasome potentially influencing IL-1 release, thus representing an important therapeutic strategy both for autoinflammatory diseases and recurrent pericarditis (Brucato et al., 2016; Ozen et al., 2016).
To date, pathophysiological mechanisms of pericarditis can be classified as part of the autoimmune and autoinflammatory responses in the disease. This is clearly a simplified approach that does not take into account the real complexity of underlying biological processes. We also believe that these two inflammatory systems can overlap and be influenced by a genetic susceptibility to disease, whose elements have not been clarified yet.
4 THE ROLE OF NEUTROPHILS WITHIN THE PERICARDIUM
Although classically deemed as main effectors in the innate immune system due to their role in phagocytosis, mighty neutrophils still surprise researchers for their unique features, which are not fully understood. Apart from reactive oxygen species (ROS) production and release, these cells can also secrete a vast array of antimicrobial molecules that after storing in the granules can be quickly released on demand. Most of these mediators have been shown to play a role not only in infectious diseases, but also in noninfectious ones, such as atherosclerosis and diabetes (Bonaventura, Montecucco, & Dallegri, 2016; Liberale et al., 2017; Montecucco et al., 2017). Besides, neutrophil extracellular traps (NETs) have been recently recognized as a part of the wide defensive strategy of neutrophils and the neutrophil alternative death pathway, named NETosis, has been indicated as a further way to act in the innate immune system defense after their death (Bonaventura et al., 2018). Last but not least, the role of neutrophils is emerging in wound healing and repair in different systems (skin, lung, gut, and joints; Jones, Robb, Perretti, & Rossi, 2016). Accordingly, neutrophils have been reported to orchestrate myocardial healing in infarcted mice through neutrophil gelatinase-associated lipocalin release, thus polarizing macrophages towards a reparative phenotype and favoring an efficient clearance of cell debris (Horckmans et al., 2017).
The role of neutrophils has been only partially evaluated in patients suffering from pericarditis (Figure 2). Obviously, most data come from studies in which patients with different degrees of pericardial effusion have been enrolled, especially those with a malignant pericardial effusion (MPE; Vakamudi, Ho, & Cremer, 2017). Oyakawa et al. evaluated the composition of the MPE in 44 patients showing that neutrophils were the most represented white blood cells (WBCs; Oyakawa et al., 2018). Moreover, neutrophils, lymphocytes, and neutrophil-to-lymphocyte ratio (NLR) were significantly associated with effusion failure-free survival at 1 month, with NLR ≥ 3.5 predicting a shorter survival after pericardial drainage (p = 0.041; Oyakawa et al., 2018). Recently, Kim et al. reported about a cohort of patients with and without effusive-constrictive pericarditis (ECP), who underwent echocardiography before and after pericardiocentesis (Kim et al., 2018). When analyzing results of pericardial fluid analysis, there was no difference in total WBC count between groups, but the percentage of neutrophils was significantly higher (50% vs. 19%, p = 0.004) and that of monocytes was lower (11 vs. 25%, p = 0.007) among patients with ECP with respect to non-ECP subjects (Kim et al., 2018). This finding is very interesting and supports the hypothesis that the innate immune system plays a pivotal role in the pathogenesis of pericarditis with a likely proportion between the total number of neutrophils and the extent of pericardial effusion. Accordingly, Kim et al. found that fibrinous and loculated pericardial effusions were more common in the ECP group (Kim et al., 2018).
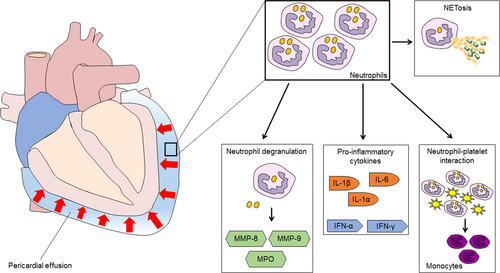
Putative role of neutrophils in pericardial effusion. Within the pericardium, neutrophils are found at high levels and can act as effectors of the inflammatory storm by releasing mediators during degranulation, producing proinflammatory cytokines, interacting with platelets and contribute for the switch from neutrophilic to monocytic recruitment, and providing NETosis. IL: interleukin; IFN: interferon; MMP: metalloproteinase; MPO: myeloperoxidase; NET: neutrophil extracellular traps; TLR: toll-like receptor [Color figure can be viewed at wileyonlinelibrary.com]
Pericardial interstitial cell (PIC) senescence has been postulated as a possible pathway driving pericardial structural remodeling (Liu et al., 2011, 2012). Accordingly, following a pericardial injury or disease, such as pericarditis, the normal steady state of PICs is impaired and quiescent PICs start to proliferate and release different mediators involved in repair, such as MMPs, leading to extracellular matrix (ECM) remodeling and finally to fibrosis (Han et al., 2017). These findings may explain the poor evolution of some cases of ECP, in which fibrosis tends to induce adhesions between the parietal and visceral pericardium. Indeed, this event can heal or progress until transient or permanent constrictive pericarditis. Hence, the effusive constriction should be considered as one hand of the evolution of pericarditis, typically characterized by effusion and epicardial involvement (Klein & Cremer, 2018).
Neutrophils have been described to interact with platelets to orchestrate a complex mechanism finely regulating the degree of tissue neutrophil infiltration and perfectly fits the newly coined term “thromboinflammation.” Indeed, platelets can work in coordination with neutrophils in the initiation of inflammatory processes following the interaction mediated by integrins and aggravate the pathophysiological course by microthrombosis and subsequent ischemia (Rossaint & Zarbock, 2015). Contrarily, the interaction between P-selectin and P-selectin glycoprotein ligand 1 during neutrophil-platelet interplay can act leading to the release of the pro-resolving lipid mediator maresin 1 (MaR1) reducing neutrophil recruitment and infiltration, as showed in a mouse model of acute brain ischemia/reperfusion injury (IRI; Xian et al., 2016). The close interplay between these two kinds of cells can culminate in the formation of heterotypic aggregates of platelets with neutrophils mediated by lipoxin mediators, that is lipoxin A4 (LXA4), which is likely to be responsible for IRI. Accordingly, the formyl peptide receptor (FPR) family has been described to be involved in the regulation and the resolution of IRI-related inflammation (Gavins, 2010). In 2013, Brancaleone et al. reported that neutrophil FPR2/3 activation was responsible for platelet/neutrophil aggregates and the quick generation of circulating LXA4, the latter modulating downstream vascular inflammatory responses during reperfusion (Brancaleone et al., 2013). Similarly, Ortiz-Muñoz et al. found that neutrophil-platelet aggregates are implicated in the acute lung injury, whereas aspirin decreased lung injury by reducing lung platelet sequestration and activation and concurrently neutrophil-platelet aggregation (Ortiz-Munoz et al., 2014). These events have been further confirmed by Vital et al. in the cerebral microcirculation following IRI showing that both annexin A1 and aspirin-triggered LXA4 activation of neutrophil Fpr2/3 regulated neutrophil-platelet aggregate formation in the brain and inhibited the reactivity of the cerebral microvasculature (Vital et al., 2016). Hence, in light of these findings, Fpr2/3-lipoxin A4 receptor might represent a promising therapeutic target to be tested in the resolution of pericarditis, as already done in other settings, which can in turn help the comprehensive understanding of the two component, platelets and neutrophils, in an integrated and generalized schema.
Observations from both animal and human models seem to confirm a role for neutrophils in mediating the recruitment of mononuclear cells at sites of inflammation (Soehnlein, Steffens, Hidalgo, & Weber, 2017). Indeed, patients with neutropenia show a reduced number of monocytes as well as patients with specific granule dysfunction present with an impaired monocyte chemotaxis (Gallin et al., 1982; Mokart et al., 2008). Recently, neutrophils have been described to provide a contribution for the switch from neutrophilic to monocytic recruitment since during their extravasation they release some granule proteins on the endothelium, which are chemotactic for monocytes (Soehnlein et al., 2017). Importantly, at the luminal side, the coordinated action of neutrophils with platelets and monocytes may explain the amplification of the inflammatory process, at least in the bloodstream; to date, no data about similar mechanisms are available for pericardial or pleural inflammation. Therefore, in ECP, concurrent variations in the number of neutrophils and monocytes and the switch from neutrophilic to monocytic recruitment is likely to be absent or actually still not demonstrated. In fact, in patients with ECP, those with high percentages of neutrophils showed a reduced percentage of monocytes and vice versa (Kim et al., 2018), hence this aspect deserves to be further investigated in future studies.
5 FUTURE PERSPECTIVES
To date, the blocking of IL-1-mediated pathways has been tested as an encouraging strategy in pericarditis (Buckley et al., 2018), as depicted in Table 1. Anakinra, a recombinant human IL-1 receptor antagonist, has been largely tested both in adults and children for recurrent idiopathic pericarditis (Camacho-Lovillo & Mendez-Santos, 2013; Finetti et al., 2014; Lazaros et al., 2014; Picco et al., 2009; Scardapane, Brucato, Chiarelli, & Breda, 2013), with one report for the treatment of recurrent pericarditis associated with the Myhre syndrome (Picco et al., 2013). Recently, in a pilot study enrolling 21 patients with recurrent pericarditis resistant to colchicine and corticosteroid treatments, anakinra (adults: 100 mg/day, children 2 mg/kg per day up to 100 mg) was added to reduce flares of pericarditis over a median of 14 months (Brucato et al., 2016). The CANTOS trial has now attracted great attention by demonstrating the clinical efficacy of selective IL-1β inhibition (e.g., canakinumab) in secondary prevention of CV adverse events after an acute coronary syndrome (Ridker et al., 2017). Canakinumab is a human monoclonal antibody, binding to IL-1β and blocking the interaction of IL-1β with its receptor, thus limiting further proinflammatory signaling events. Indeed, canakinumab has not yet been tested for patients suffering from recurrent pericarditis. Although anakinra is administered subcutaneously every day and blocks both IL-1α and IL-1β, thereby potentially altering host defense (Libby, 2017), canakinumab has been administered every 3 months in the CANTOS trial and is highly specific for IL-1β. In this view, new studies investigating more specifically the role of both the inflammasome and IL-1β in pericarditis are warranted as well as new trials investigating the role of the selective IL-1β inhibition in colchicine-resistant and steroid-dependent patients or those with recurrent pericarditis. Since sterile inflammation can generate both neutrophilic infiltration and IL-1β release (Dinarello, 2009), in our opinion neutrophils might represent a future target to be investigated among patients with pericarditis. Since neutrophils are abundant within the inflamed pericardium, especially in those cases with an autoinflammatory pathophysiological mechanism, it could be worth testing levels of neutrophil mediators, such as MMPs, within the pericardial fluid, which are expected to be at higher levels in patients experiencing recurrent pericarditis or ECP. MMPs are important mediators of neutrophil-driven inflammation after their degranulation, which are responsible for the breakdown of ECM both in physiological and disease processes (Quillard, Franck, Mawson, Folco, & Libby, 2017; Soehnlein et al., 2017). Among other inflammatory biomarkers, which can be considered as future therapeutic targets, NETs surely play a central role because they contribute to the inflammatory storm by attracting monocyte recruitment and trigger cytokine release (Soehnlein et al., 2017). More recently, osteopontin (OPN) has ensured an important place as inflammatory mediator in different settings, such as infections and CV diseases (Carbone et al., 2018; Zhao et al., 2018). Exogenous OPN has been described to have a role in the recruitment and migration of neutrophils, whereas the expression of OPN by neutrophils is not likely to be involved in their phagocytic, cytotoxic, or matrix degradative activities (Koh et al., 2007; Zhao et al., 2018). The role of OPN in serosa effusions has been partially evaluated among patients with lung cancer-related pleural effusion (Hsu et al., 2016), but not in those with pericarditis. For this reason, it is worth deepening this aspect to evaluate a potential role in the management and prognosis of patients with pericarditis. Furthermore, the investigation of neutrophil function can also focus on the so-called neutrophil priming (Soehnlein et al., 2017), by which their effector functions are increased via cytokines, TLR agonists, or chemoattractants, thus leading to a large ROS production, as already seen in the setting of other diseases (Araujo, Barbosa, Hsin, Maranhao, & Abdalla, 1995; Wong et al., 2015). Indeed, pericarditis, as a thriving inflammatory condition, can induce hyperglycemia and generate hyperinflammatory neutrophils, which are likely to contribute to a state of chronic inflammation.
| Author, year | Number of patients and treatment | Study design | Results |
|---|---|---|---|
| Finetti et al. (2014) | Fifteen patients (12 children and 3 adults) corticosteroid-dependent, 14 received colchicine | Case series | All patients treated with anakinra had a complete response (normalization of clinical manifestations, laboratory, and ECG/echocardiography) within a few days, thus stopping concomitant treatments. A 95% reduction of flares has been observed across a median follow-up of 39 months |
| Lazaros et al. (2014) | Ten patients with idiopathic, treatment-resistant recurrent pericarditis1 | Case series | One patient started anakinra 150 mg/day and two 100 mg/day sc, where seven were given 100 mg/day for 6 months followed by alternate day dosing for another 6 months. All patients experienced rapid symptom relief, normalization of CRP, and tapering/stopping of corticosteroids. Five patients relapsed shortly after anakinra discontinuation and were managed with anakinra and one with NSAIDs alone |
| Jain et al. (2015) | Thirteen patients with recurrent pericarditis, refractory to conventional therapy2 | Case series | Twelve patients had complete and one partial resolution treatment response, defined as improvement of symptoms. Eleven patients successfully stopped concomitant NSAID, colchicine, and glucocorticoids. Two patients required to continue a low-dose prednisone. Patients with baseline elevations of ESR and CRP showed normalized values 2–6 weeks after initiation of anakinra. Response to therapy was rapid (2–5 days) |
| D’Elia et al. (2015) | A 47-year-old woman with acute pericarditis then turned into constrictive pericarditis despite ibuprofen 1,800 mg/daily, colchicine 1 mg/daily, methylprednisolone 20 mg, and antibiotics | Case report | Anakinra 100 mg daily sc was added to colchicine with an improvement in echocardiographic parameters after 15 days and a complete resolution of constriction after 3 months at cRMI. After 6 months, anakinra was reduced of 100 mg/week every month, then stopped, carrying on with colchicine in absence of recurrence of constriction |
| Schatz et al. (2016) | A 39-year-old black female with RA with ECP undergoing pericardiocentesis and administered prednisone 40 mg daily, ibuprofen 600 mg tid, and colchicine 0.6 mg bid daily, with a recurrence after 4 weeks | Case report | Anakinra 100 mg daily sc resolved symptoms within 1 week as well as ECG and echocardiography normalized, with anakinra 100 mg sc daily at discharge and remained free of symptoms |
| Imazio et al. (2016) | Total of 110 consecutive pediatric patients with recurrent pericarditis (at least two episodes after the index attack) of any cause resistant to NSAIDs and corticosteroid-dependent | Retrospective, multicenter cohort study | Twelve children were administered anakinra (1 mg/kg/day sc) with a drop in the number of recurrences from 4.29 to 0.14 per year (p < 0.05) |
| Brucato et al. (2016) | Twenty-one consecutive patients enrolled at three Italian referral centers with recurrent pericarditis, elevation of CRP, colchicine resistance, and corticosteroid dependence | Double-blind, placebo-controlled, randomized withdrawal trial | Anakinra was administered at 2 mg/kg daily up to 100 mg for 2 months, then patients who responded were randomized to continue anakinra or switch to placebo for 6 months or until a pericarditis recurrence. Recurrent pericarditis occurred 2 of 11 patients (18.2%; incidence rate, 0.11% of patients per year) assigned to anakinra with an incidence rate difference of −1.95%. Median flare-free survival was 72 days after randomization in the placebo group and was not reached in the anakinra group (p < 0.001) |
| Camprubi et al. (2017) | A 35-year-old woman suffering from recurrent pericarditis with cardiac tamponade needing pericardiocentesis with a neutrophil exudate, finally diagnosed with TRAPS | Case report | Following NSAIDs, anakinra 100 mg/day sc was started two weeks after discharge with a favorable clinical and biological response and carried on for 1 year without flares |
- Note: bid: twice daily; cRMI: cardiac resonance magnetic imaging; CRP: C-reactive protein; ECG: electrocardiography; ECP: effusive-constrictive pericarditis; ESR: erythrocyte sedimentation rate; NSAID: nonsteroidal anti-inflammatory drug; RA: rheumatoid arthritis; sc: subcutaneously; tid: three times in day; TRAPS: tumor necrosis factor receptor-associated periodic syndrome.
- 1 Patients were resistant and/or intolerant to previous treatment with aspirin and/or nonsteroidal anti-inflammatory agents, colchicine, and corticosteroids, whereas two had failed also azathioprine therapy.
- 2 Maximally tolerated doses of nonsteroidal anti-inflammatory agents, colchicine, and prednisone administered for at least 6 months.
6 CONCLUSIONS
Since pericarditis is gaining some interest among physicians due its prevalence, new insights in its pathophysiology are required. A growing body of evidence demonstrates a central role of the innate immune system for those cases developing with an autoinflammatory-like mechanism. Accordingly, neutrophils might have a key role in this setting, particularly in patients with pericardial effusion, where they are found in large amount. Moreover, their classical products of degranulation (i.e., MMP-8, MMP-9, and MPO), along with NETs are claimed to influence the natural history of ECP. In this view, a further deepening of pathophysiological mechanisms linking neutrophil-mediated inflammation and pericarditis is of utmost importance to improve the management of patients suffering from this disease and precisely calibrate therapies capable of modifying its prognosis.
ACKNOWLEDGMENTS
This study was supported by a grant from the Rete Cardiologica from the Italian Ministry of Health to Fabrizio Montecucco.
CONFLICTS OF INTEREST
Authors declare that they have no conflicts of interest.
AUTHORS’ CONTRIBUTIONS
A. Bonaventura and F. Montecucco conceived the manuscript. A. Bonaventura wrote the manuscript draft. F. Montecucco read critically and improved the manuscript.




