Preventative tracheal administration of interleukin-27 attenuates allergic asthma by improving the lung Th1 microenvironment
Abstract
Background: Interleukin-27 (IL-27) modulates CD4+ T-cell differentiation and function. The aim of this study is to investigate the effect and molecular mechanisms of IL-27 on the development of asthma.
Methods: IL-27 was intranasally administered in an ovalbumin-induced asthma model, and lung mononuclear cells and different Th cell classes were detected by fluorescence-activated cell sorting. The effect and mechanisms of IL-27 on human bronchial epithelial (HBE) cells were investigated by measuring changes in chemotactic factors, cytokines, transcription factors, and signaling pathways.
Results: We found that intranasal administration of IL-27 could attenuate airway inflammation and hyperresponsiveness, upregulate the type 1 T helper (Th1)-T memory (Tm) cells and regulatory T (Treg) cells subgroups of lung tissue lymphocytes, and diminish the levels of type 2 T helper (Th2) cytokines. IL-27 upregulated the expression of C-C motif chemokine ligand 2 (CCL2), CCL3, and CCL4 in HBE cells and promoted the production of chemotactic factors to attract monocyte recruitment. Recruited monocytes secondarily secreted IL-27 to influence HBE cells in a positive feedback cycle. After IL-27 intervention, signal transducer and activator of transcription 1 (STAT1) phosphorylation increased, while STAT4 and STAT6 phosphorylation declined.
Conclusions: Preventative intranasal administration of IL-27 can recruit more IL-27-secreted monocytes to the airway and change the different T-cell classes in lung. The improved Th1 environment helps to alleviate Th2-mediated allergic asthma by repairing the STAT1 pathway but not the STAT4 pathway.
1 INTRODUCTION
In 2002, the interleukin-27 (IL-27) cytokine was newly identified as a member of the IL-12 family, which contains Epstein–Barr virus induced gene 3 (EBI3) and IL-27p28 (Pflanz et al., 2002). IL-27 is mainly produced by antigen presenting cells (APCs), including dendritic cells, monocytes, and macrophages following stimulation by microbial products or other immune stimuli. IL-27R-deficient (IL-27Rα−/−) mice infected with Toxoplasma gondii or Trypanosoma cruzi develop lethal inflammatory responses due to impaired type 1 T helper (Th1) responses.(Artis, Johnson, et al., 2004; Q. Chen et al., 2000; A. Villarino et al., 2003; Yoshida et al., 2001) Alternatively, IL-27Rα−/− mice are resistant to infection with Trichuris muris, which is a well-characterized helminth expelled by type 2 T helper (Th2)-mediated immune responses, suggesting the inhibitory effect of IL-27 on Th2 cytokine production (Artis, Villarino, et al., 2004; Lucas, Ghilardi, Li, & de Sauvage, 2003). Yoshimoto, Yoshimoto, Yasuda, Mizuguchi, and Nakanishi (2007) found that IL-27-treated Strongyloides venezuelensis -infected mice exhibited reduced levels of IgE and mMCP-1 in their sera and a decreased Th2 response but simultaneously increased Th1 response. Therefore, IL-27 may be a critical cytokine possibly involved in the regulation of human immunological diseases.
In allergic asthmatics, the profile of cytokines expressed by CD4+ T cells is biased toward Th2 cytokines such as IL-4, -5, and -13. In studies using mouse models, it has often been suggested that suppression of the Th2 response would lead to an enhancement of the Th1 response. Promoting a Th1 response might have therapeutic value(Kumar, Yang, Herbert, & Foster, 2012). In our previous study, we demonstrated that IL-27 inhibited the differentiation of naive CD4+ T cells into Th2 cells but not already differentiated Th2 cells. The discovery could be explained by the “Hygiene Hypothesis” theory, which is well known around the world. It might be the Th1 environment induced by IL-27 before stimulation of allergen that suppressed Th2 differentiation. Therefore, we conducted the following experiments in vivo and in vitro. We administered IL-27 to ovalbumin (OVA)-immunized mice before OVA sensitization and challenge. We observed that it can upregulate Th1–T memory (Th1–Tm) cells and regulatory T (Treg) cells subgroups of mouse lung tissue lymphocytes. It reversed signal transducer and activator of transcription 1 (STAT1) phosphorylation impairment of OVA-immunized lung cells. Thus, the improved Th1 environment helped to alleviate Th2-mediated allergic asthma.
2 MATERIALS AND METHODS
2.1 Animals
Six-week-old female C57/BL6 mice were purchased from Silaike Experimental Animal Limited Liability Company (Shanghai, China). All mice were maintained under pathogen-free conditions.
All the animal experiments were approved by the Animal Care and Use Committee at Zhongshan Hospital, Fudan University (Shanghai, China), and all experimental methods were performed in accordance with the National Institutes of Health Guidelines and Regulations.
2.2 OVA-induced allergic asthma model and prevention regimens
Mice were sensitized and challenged with OVA (Grade V; Sigma-Aldrich, St. Louis, Kansas, MO). Specific protocols for the mouse models are shown in Figures 1a and 3a. For the asthma model, 6-week-old female C57/BL6 mice were sensitized by intraperitoneal injection of 100 μl of phosphate-buffered saline (PBS; Invitrogen, Paisley, OR, Scotland, TX) solution containing 100 μg of OVA with 2 mg of Alum (Thermo Fisher Scientific, Waltham, MA) on Days 0 and 7. The mice were challenged on five consecutive days via intranasal administration of 100 μg of OVA in 50 μl of PBS under light isoflurane anesthesia from Days 14–18. For the prevention model, the mice were operated by IL-27 intranasal at two concentrations (25 and 50 ng/mouse) twice a day for 14 days before the second OVA sensitization. There was a minimum of eight mice in each group.
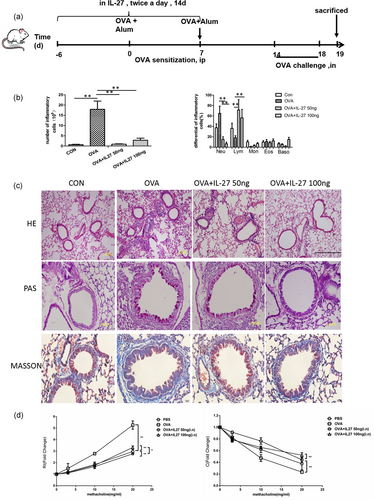
Preventative intranasal administration of IL-27 attenuates airway inflammation in an OVA-induced mouse model. (a) Protocol of OVA-induced allergic asthma and IL-27 administration. (b) The total cell number and the differential cell counts in BALF. (c) Representative photomicrographs of lung sections stained with HE, PAS, and Masson; examined at ×100 magnification for HE and ×400 magnification for Masson staining. (d) AHR was invasively measured by lung resistance and compliance in response to increasing concentrations of methacholine. The columns and error bars represent the mean and SEM (n = 6 per group). *p < 0.05, **p < 0.01. Similar results were obtained in at least six independent experiments. AHR: airway hyperreactivity; BALF: bronchoalveolar lavage fluid; HE: hematoxylin and eosin; IL-27: interleukin-27; OVA: ovalbumin; PAS: periodic acid–Schiff [Color figure can be viewed at wileyonlinelibrary.com]
2.3 Analysis of bronchoalveolar lavage fluid (BALF)
BALF was obtained within 24 hr after the last OVA challenge. The BAL was performed using 3 × 1 ml of 0.05 mM PBS-EDTA (Calbiochem, Darmstadt, Germany; Cataldo et al., 2002; Gueders et al., 2005). The cells were recovered through gentle manual aspiration. Differential cell counts were determined from stained cytospins by Wright–Giemsa. The percentages of Th1–Tm and Treg cells were determined by flow cytometry. The supernatant was collected and frozen at −80°C for protein assessment by enzyme-linked immunosorbent assay after centrifugation (1,200 rpm for 10 min, at 4°C).
2.4 Pulmonary histology and tissue processing
After the BALF, the left lung was excised with the right main bronchus clamped. The left lung sections were stained with hematoxylin and eosin (HE) or periodic acid–Schiff (PAS). The right lung sections were frozen at −80°C for protein assessment using western blot analysis. The peribronchial and inflammatory cell infiltration around the bronchi were estimated with a score calculation of the quantification of the peribronchial and perivascular inflammatory cells (Curtis, Byrd, Warnock, & Kaltreider, 1991). Value 0 represented no inflammatory cells around the bronchi; Value 1 represented occasional inflammatory cells; Value 2 represented that most bronchi were surrounded by a thin layer (1–5 cells) of inflammatory cells; Value 3 represented that most bronchi were surrounded by a thick layer (>5 cells) of inflammatory cells. The scores were expressed as a mean value per animal.
2.5 Measurement of airway hyperreactivity (AHR)
The mice were anesthetized for measurement of the pulmonary mechanics (Buxco Electronics, Wilmington, NC) 24 hr after the last OVA challenge (Kim et al., 2013). The mice were anesthetized with 50 mg/kg of pentobarbital and were wired for measurement of the pulmonary mechanics (Buxco Electronics). The mice were tracheostomized, intubated, and ventilated at the tidal volume of 0.2 ml and the frequency of 150 breaths/min (Akbari et al., 2003). The lung resistance (RL) and dynamic lung compliance (Cydn) were measured in response to increasing doses of aerosolized acetyl-β-methylcholine chloride (methacholine; 0–20 mg/ml; Sigma-Aldrich).
2.6 Cell surface and intracellular staining by FACS
To measure Th1–Tm cells and Treg cells from mouse lung mononuclear cells, we added fluorescein isothiocyanate (FITC)-labeled anti-mouse CD4, APC-labeled anti-mouse CD44, Cherry-labeled CD62L (BD Bioscience, San Jose, NJ), and the cells were incubated at 4°C for 20 min in a dark environment (all fluorescence-activated cell sorting [FACS] antibodies were used according to the product instructions). The cells were then permeabilized with prechilled Perm Buffer III (BD Bioscience) at 4°C for 30 min. After washing once with PBS, the cells were stained with phycoerythrin (PE)-labeled anti-mouse interferon γ (IFNγ; BD Bioscience) at 4°C for 20 min and ready for detection. For detection of the proportion of Treg cells in mouse lung mononuclear cells, FITC-labeled anti-mouse CD4 and PE-labeled anti-mouse CD25 (BD Bioscience) were added, and the cells were incubated at 4°C for 20 min in a dark environment. The cells were then permeabilized with prechilled Perm Buffer III (BD Bioscience) at 4°C for 30 min. After washing once with PBS, the cells were stained with APC-labeled anti-mouse Foxp3 at 4°C for 20 min. All samples were monitored using a FACS Aria II Machine (BD Bioscience) and analyzed using FlowJo 10.0 software (Tree Star, Inc., Ashland, OR).
2.7 Quantitative reverse-transcription polymerase chain reaction (RT-PCR) and western blot analysis
Lung tissue, or human bronchial epithelial (HBE) cells, and U937 cells were collected. The messenger RNA (mRNA) of these cells was extracted. PCR were carried out in 25 µl containing 1 µl complementary DNA, 1 mM of each forward and reverse primer, and 0.25* SyBr Green Mix. β-Actin was used as the internal control. Primer sequences included: β-actin, sense: 5′-CCTGTACGCCAACACAGTGC-3′, antisense: 5′-ATACTCCTGCTTGCTGATCC-3′; STAT1, sense: 5′-TATTCCAGACCAAAGGAAGCAC-3′, antisense: 5′-GAAGGGTGGACTTCAGACACAG-3′; C-C motif chemokine ligand 2 (CCL2), sense: 5′-CTCAGCCAGATGCAATCAAT-3′, antisense: 5′-AGCTTCTTTGGGACACTTGC-3′; CCL3, sense: 5′-GCAACCAGTTCTCTGCATCA-3′, antisense: 5′-TGCTCGTCTCAAAGTAGTCAGC-3′; CCL4, sense: 5′-GCACCAATGGGCTCAGAC-3′, antisense: 5′-CAGGATTCACTGGGATCAGCA-3′; IL-27p28, sense: 5′-CAGACGGCAGGCGACCTT-3′, antisense: 5′-TGACTGTGAACTCCCTCCGC-3′. Amplification data measured by fluorescence were collected in real time and analyzed by Rotor-Gene 6.0.14 Software (QIAGEN, Duesseldorf, Germany).
Mouse lung tissue was harvested, and total protein was extracted using a radioimmunoprecipitation assay kit (BioVision, San Francisco Bay Area, CA). Protein was electrophoresed on a polyacrylamide gel and transferred to Hybond-C nitrocellulose membrane (Amersham Pharmacia Biotech, Uppsala, Sweden). The membrane was incubated with anti-STAT1, anti-p-STAT1, anti-STAT4, anti-p-STAT4, anti-STAT6, and anti-p-STAT6 separately with concentrations of 1:1,000 at 37℃ for 1.5 hr and then incubated with peroxidase-conjugated goat anti-rabbit IgG at room temperature for 1 hr. Glyceraldehyde 3-phosphate dehydrogenase was used as the internal control. Protein was visualized by using enhanced chemiluminescence.
2.8 In vitro transwell assay
U937 cells and HBE cells were seeded in the upper portion or the lower portion of the chamber (BD Bioscience). HBE cells were immersed in RPMI-1640 with 10% fetal bovine serum, engaged with IL-27 (0.01 ng/ml) or tumor necrosis factor α (TNF-α; 1 ng/ml) or both. After incubation for 24 hr, cells on the membrane of the upper chamber were scrubbed, washed with PBS, fixed in 100% methanol, and stained with Giemsa dye. Images were visualized with a laser confocal microscope. For quantitative PCR (qPCR) measurement, U937 cells were collected from the upper chamber and mRNA of these cells was extracted. Real-time RT-PCR was performed as described above.
2.9 Statistical analysis
All of the error bars in this report represent SDs. Differences between the data sets were analyzed using one-way analysis of variance with a Bonferroni's post test to determine significance (Prism 5; GraphPad Software, San Diego, CA). A p < 0.05 was considered statistically significant.
3 RESULTS
3.1 Preventative intranasal administration of IL-27 attenuates airway inflammation and improves AHR in an OVA-induced mouse model
We administered IL-27 to OVA-immunized mice with two concentrations of 50 and 100 ng before OVA sensitization (Figure 1a). This kind of preventative intranasal administration of IL-27 attenuated airway inflammation. It was obvious that total cell numbers in BALF decreased dramatically. With regard to eosinophils, the proportion remained stable, but the absolute count decreased (Figure 1b). At the same time, both peribronchial and perivascular inflammatory infiltrates were reduced in observed HE-stained lung sections (Figure 1c), as well as a strikingly decreased accumulation of PAS-positive goblet cells was observed in the low-dose IL-27 treatment group (Figure 1c). We can also see that the collagen fibers decreased dramatically (Figure 1c). In the IL-27 preventative groups, there was significant protection from methacholine-induced AHR, a hallmark of asthma. The AHR from the IL-27 preventative groups were significantly different from both the OVA and PBS groups (Figure 1d). The results indicated that preventative administration of IL-27 can effectively alleviate airway inflammation and AHR.
3.2 Preventative intranasal administration of IL-27 upregulates Th1–Tm and Treg subgroups of mouse lung tissue lymphocytes and diminishes concentrations of Th2 cytokines
To test whether the above findings resulted from the induced Th1 environment and the suppression of Th2 environment, we explored the probable improved Th1 environment before asthma onset in the preventative group. We administered IL-27 intranasal to OVA-immunized mice before the OVA sensitization and killed the mice before the OVA challenge (Figure 2a). Th1–Tm cells were identified among various mouse lung lymphocytes by means of FACS analysis (Figure 2b). The proportion of Th1–Tm cells in the asthma group was much lower than that in the control group. However, in the two preventative IL-27 groups, the proportions increased remarkably, especially the high IL-27 concentration group (Figure 2c). Similarly, Treg cells were identified by FACS analysis (Figure 2d). The proportion of Treg cells in the asthma group was lower than that in the control group, and in the two preventative IL-27 groups, the proportions increased (Figure 2e). The results suggested that preventative intranasal administration of IL-27 before OVA challenge can upregulate Th1–Tm and Treg subgroups in mouse lung tissue lymphocytes, which can promote naïve CD4+ T-cell differentiation into Th1 cells and improve the pathologic symptoms of asthma. Thus, the improved Th1 environment helps to alleviate Th2-mediated allergic asthma (Figure 6).
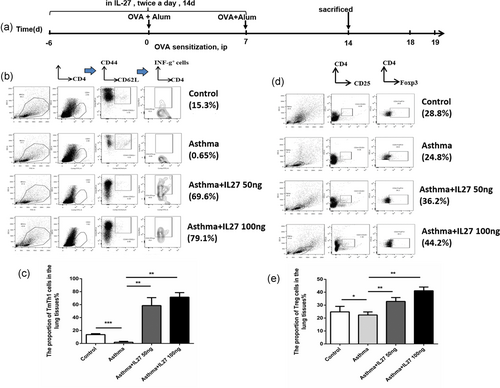
Preventative intranasal administration of IL-27 before OVA challenge upregulates Th1–Tm and Treg subgroups of mouse lung tissue lymphocytes. (a) Protocol of OVA-induced allergic asthma and IL-27 administration. (b) FACS analysis of Th1–Tm cells in various subsets of lung tissue lymphocytes. (c) The proportion of Th1–Tm cells in the lung tissue lymphocytes analyzed by flow cytometry. (d) FACS analysis of Treg cells in various subsets of lung tissue lymphocytes. (e) The proportion of Treg cells in the lung tissue lymphocytes analyzed by flow cytometry. Columns and error bars represent the mean and SEM (n = 6 per group). *p < 0.05, **p < 0.01, ***p < 0.001. Similar results were obtained in at least six independent experiments. FACS: fluorescence-activated cell sorting; IL-27: interleukin-27; OVA: ovalbumin; Th1: type 1 T helper; Tm: T memory; Treg: regulatory T [Color figure can be viewed at wileyonlinelibrary.com]
3.3 IL-27 upregulates the expression of CCL2, CCL3, and CCL4 in HBE cells
Next, we detected the effect of IL-27 on bronchial epithelial cells in vitro. HBE cells were treated under different concentrations of TNF-α, IL-27, and both (Figure 3a). It showed that CCL2, CCL3, and CCL4 mRNA increased under either varying doses of IL-27 or varying doses of TNF-α (Figure 3b,c). The concentration of 10 ng/ml IL-27 and 0.1 ng/ml TNF-α were optimal to induce chemokine production in the cell culture system. Lastly, we divided the HBE cells into four groups, according to different culture conditions as control, TNF-α, IL-27, and TNF-α + IL-27. Obviously, the HBE cells under both TNF-α and IL-27 conditions secreted much more CCL2, CCL3, and CCL4 chemotactic factors than did those under TNF-α only or IL-27 only conditions (Figure 3d). This result means that IL-27 did have an effect on the HBE cells and promoted the HBE cells to secrete more chemotactic factors. It also seemed that IL-27 and TNF-α had a synergetic effect.
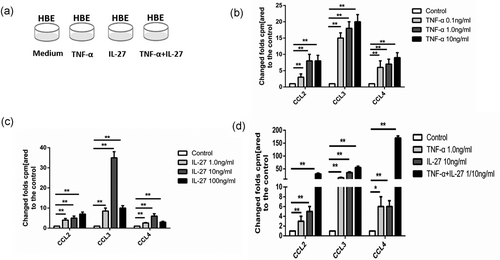
IL-27 upregulates the expression of CCL2, CCL3, and CCL4 of HBE cells. (a) HBE cells under different culture conditions. (b) CCL2, CCL3, and CCL4 mRNA expression of HBE cells induced by TNF-α in different concentrations. (c) CCL2, CCL3, and CCL4 mRNA expression of HBE cells induced by IL-27 in different concentrations. (d) CCL2, CCL3, and CCL4 mRNA expression of HBE cells induced by TNF-α, IL-27, or both. Columns and error bars represent mean and SEM (n = 6 per group). *p < 0.05, **p < 0.01, ***p < 0.001. Similar results were obtained in at least six independent experiments. CCL2: C-C motif chemokine ligand 2; HBE: human bronchial epithelial; IL-27: interleukin-27; mRNA: messenger RNA; TNF-α: tumor necrosis factor α
3.4 The promoted-secreted chemotactic factors recruit more U937 cells
In the last experiment, we found that IL-27 can promote HBE cells to secrete more chemotactic factors. Then, we further tested the chemotaxis of the promoted-secreted chemotactic factors. With the same previous protocol, the HBE cells were cultured under four kinds of conditions, which were medium, IL-27, TNF-α, and both. Then, the transwell assays were used to test chemotactic responses of U937 cells to the chemokines secreted by the HBE cells cultured under different conditions in vitro (Figure 4a). The U937 cell numbers were countered, respectively. Consistent with the last results, when the HBE cells were cultured under IL-27, there were more U937 cells due to higher concentrations of chemotactic factors. Similarly, IL-27 and TNF-α had a synergetic effect (Figure 4b).
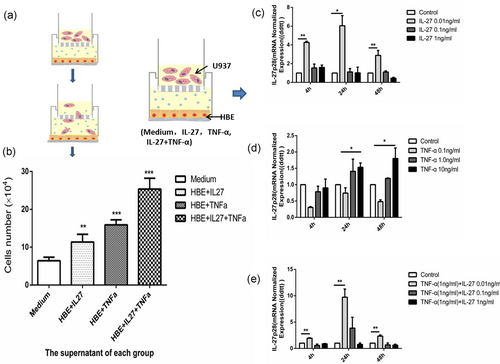
The chemotaxis of U937 cells and the positive feedback effect of IL-27 on the recruited U937 cells. (a) Protocol of the transwell assay. (b) The chemotaxis of U937 in transwells. (c) IL-27p28 mRNA content after engagement of different IL-27 concentration analyzed by real-time RT-PCR. (d) IL-27p28 mRNA content after engagement of different TNF-α concentration analyzed by real-time RT-PCR. (e) IL-27p28 mRNA content after IL-27 and TNF-α engagement analyzed by real-time RT-PCR. IL-27p28 mRNA contents were normalized using β-actin mRNA as internal reference. Columns and error bars represent the mean and SEM (n = 6 per group). *p < 0.05, **p < 0.01, ***p < 0.001. Similar results were obtained in at least six independent experiments. HBE: human bronchial epithelial; IL-27: interleukin-27; mRNA: messenger RNA; RT-PCR: reverse-transcription polymerase chain reaction; TNF-α: tumor necrosis factor α [Color figure can be viewed at wileyonlinelibrary.com]
3.5 Recruited U937 cells secrete IL-27 in a positive-feedback mechanism
To explore the effect of chemokines produced by HBE cells in the lower chamber on U937 cells located in the upper chamber, HBE cells were cultured under different conditions, as IL-27 alone, TNF-α alone, and TNF-α + IL-27. U937 cells were collected for evaluation of mRNA expression of IL-27p28 after 4, 24, and 48 hr of incubation. We examined the mRNA expression of IL-27p28 to test the real IL-27 secreted by U937 cells and to avoid interference of the exogenous IL-27 we added. The data demonstrated incubation with IL-27 alone or TNF-α alone could promote U937 cells to secrete IL-27 (Figure 4c,d). When HBE cells in the lower chamber were incubated with TNF-α and different doses of IL-27, the expression of IL-27p28 was higher in the TNF-α (1 ng/ml) group with a low dose of IL-27 (0.01 ng/ml), which was consistent with former results (Figure 4e).
The series of results implied that HBE cells cultured in the IL-27 environment can secrete more chemotactic factors to recruit the U937 cells. More important, the recruited U937 cells can, in return, secrete IL-27 to take effect on the HBE cells, which might form a cycle (Figure 6).
3.6 Preventative intranasal administration of IL-27 in mice before OVA challenge upregulates the STAT1 but not STAT4 pathway
Subsequently, possible mechanisms were probed. The STAT1 pathway is critical for IL-27 signaling, which can upregulate intercellular adhesion molecule 1 (ICAM-1) and T-bet expression in naive CD4+ T cells leading to Th1 differentiation (Kamiya et al., 2004; Owaki et al., 2005). In addition, IL-27 has been shown to activate STAT1 and STAT4 proteins (Jankowski, Kopinski, & Goc, 2010). The STAT1 and STAT4 pathways are considered to be critical for Th1 differentiation. The STAT6 pathway is the main mechanism for Th2 differentiation (Seki et al., 2003). Therefore, we investigated the possible role of these signaling pathways in the intervention of the preventative model. As per the same protocol previously, we obtained both mRNA and protein of STAT1, STAT4, and STAT6 from lung tissues of each group, which were primed for qPCR and densitometer measurement, respectively. It can be seen that, in the asthma model, the phosphorylation of STAT1 protein was impaired significantly (Figure 5a). In the preventative administration IL-27 group, the phosphorylation levels of the STAT1 protein were upregulated (Figure 5a). It seemed that the STAT4 pathway contributed little help for Th1 differentiation (Figure 5b). Interestingly, the STAT6 pathway decreased dramatically (Figure 5c), which implied that the downregulation of the STAT6 pathway may contribute to Th2 dedifferentiation and Th1 differentiation. These results indicated that the STAT1 pathway may have been repaired. The results not only revealed the relationship of preventative IL-27 and STAT1, but also suggested that preventative IL-27 can promote Th1 differentiation and alleviate Th2-mediated asthma by means of reversing the impairment of the STAT1 pathway but not the STAT4 pathway (Figure 5).
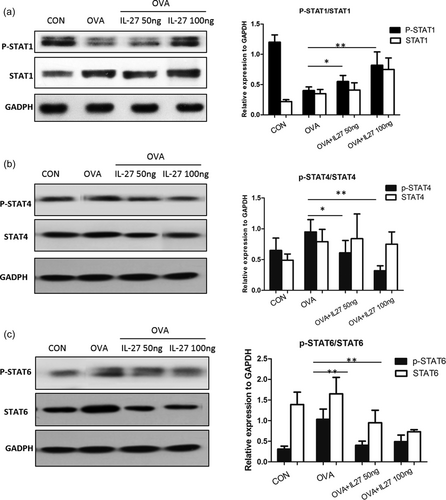
Preventative intranasal administration of IL-27 in mice before OVA challenge upregulates the STAT1 but not STAT4 pathway. (a) The protein expression of p-STAT1 and STAT1 of lung tissue by western blot analysis. (b) The protein expression of p-STAT4 and STAT4 of lung tissue by western blot analysis. (c) The protein expression of p-STAT6 and STAT6 of lung tissue by western blot analysis. Densitometer measurement of the protein expression relative to GAPDH are shown in the right panel, respectively. Data are representative of two independent experiments with similar results (*p < 0.05, **p < 0.01). GAPDH: glyceraldehyde 3-phosphate dehydrogenase; IL-27: interleukin-27; OVA: ovalbumin; STAT1: signal transducer and activator of transcription 1
4 DISCUSSION
In the past, experimental asthma, endogenous IL-27, or treatment with IL-27 could limit Th2 responses and protect against lung inflammation. Yoshida et al. (2001) reported that IL-27Rα−/− mice immunized with OVA exhibited enhanced asthmatic phenotypes in response to OVA challenge (Miyazaki et al., 2005). Yoshimoto et al. (2007) showed that intranasal administration of a mixture of IL-27 and OVA into OVA-immunized animals can inhibit OVA-induced allergic airway inflammation. We administered IL-27 to OVA-immunized mice before OVA sensitization. Consistently, this kind of preventative intranasal administration of IL-27 attenuated airway inflammation and AHR significantly.
Moro et al. (2016) used the model of intratracheal cytokine administration for their study. Y. Li et al. (2014) demonstrated that intranasal delivery of cytokine genes was a simple and efficient method to introduce human cytokines in mice, and concluded that IFN-λ1 might be applied as an immunotherapeutic agent for the treatment of allergic diseases. With the feasible nasal administration, we tried to investigate our hypothesis by intranasal delivery of IL-27. Additionally, we demonstrated that preventative intranasal IL-27 can inhibit OVA-induced allergic airway inflammation.
Large amounts of evidence suggest that alterations in the respiratory epithelium play a crucial role in both the development and persistence of asthma (Cardinale, Giordano, Chinellato, & Tesse, 2013; Holgate et al., 2003). A previous report suggested that IL-27 can also affect HBE cells (Cao, Wong, Yin, & Lam, 2010). Innovatively, we demonstrated the capacity of IL-27 to act on HBE cells and promoted the HBE cells to secrete more chemotactic factors. It showed that CCL2, CCL3, and CCL4 mRNA were increased under IL-27 conditions, which increased the mobility of mononuclear phagocyte system (MPS) toward the HBE cells observed in transwell systems. On the other hand, incubation with IL-27 alone or TNF-α alone could promote MPS cells to secrete more IL-27. The series of results implied that the HBE cells cultured in the IL-27 environment can secrete more chemotactic factors to recruit the MPS cells. More important, the recruited MPS cells can, in return, secrete IL-27, which might form a cycle. In a previous study, Behzadi, Behzadi, and Ranjbar (2016) summed up the hypothesis that the cooperation of IL-27 and IL-12 may trigger mast cells, monocytes, natural killer, and T cells to secrete IFN-γ. IFN-γ stimulates the production of IL-27 in a positive-feedback loop, which may suppress the inflammatory responses (Behzadi et al., 2016). We assumed that it may be the secreted IL-27, together with IFN-γ, stimulates the further production of IL-27.
It was reported that IL-27 can convert activated, inflammatory CD4+ T cells into IL-10-producing Th1 or type 1 regulatory T cells (Tr1 cells; Pot, Apetoh, & Kuchroo, 2011; Wojno & Hunter, 2012). Shimizu et al. (2005) found that mouse models of lupus deficient in IL-27 receptor α (WSX-1) showed a change in immunopathology from a Th1 type to a Th2 type. In contrast, overexpression of WSX-1, an IL-27 receptor, afforded protection against autoimmune lupus nephritis, validating the role of the IL-27-mediated Th1 response in regulation of autoimmunity in this disease model (Sugiyama et al., 2008). These results emphasized the role of IL-27 in Th1/Th2 balance and its impact on disease pathogenesis. Our experiments also showed that the proportion of Th1–Tm cells and Treg cells in the asthma group increased remarkably in the preventative IL-27 groups. The BALF concentrations of Th2 cytokines, IL-5, and IL-13 (Jia et al., 2016), diminished together with the increase in Th1 cytokines such as IFN-γ as well. The results proved our speculation that intranasal administration of IL-27 preventatively improved the Th1 environment, which helped to alleviate Th2-mediated allergic asthma.
Moreover, our experiments showed that the proportion of Treg cells of the asthma group increased dramatically in the two preventative IL-27 groups. Preventative intranasal administration of IL-27 before OVA challenge can upregulate Treg subgroups of mouse lung tissue lymphocytes. Our results are consistent with another study showing that IL-27 does not inhibit Treg generation, but rather endows them with IL-10 production and more effective suppression besides facilitating their survival and proliferation (Meka, Venkatesha, Dudics, Acharya, & Moudgil, 2015). However, there were also studies that showed mice that lack EBI3, IL-27p28, or IL-27Rα had normal Treg cell frequencies, and IL-27 inhibited the differentiation of Treg cells (Cox et al., 2011; A. V. Villarino et al., 2005). Thus, IL-27 may display a dual effect on Treg cells. It indicated that IL-27 had an effect on those associated with different classes of Th and Treg cells responses broadly. The different role for IL-27 may be model dependent and illustrates the heterogeneity of this cytokine.
Previous in vitro studies have suggested that STAT1 and STAT3 are the main signaling pathways controlling the effects of IL-27 on epithelial cells (Diegelmann, Olszak, Goke, Blumberg, & Brand, 2012). The STAT1 pathway is critical for IL-27 signaling, which can upregulate ICAM-1 and T-bet expression in naive CD4+ T cells leading to Th1 differentiation and inhibition of Th2 differentiation (Z. Chen et al., 2013, 2017; Kamiya et al., 2004; Owaki et al., 2005; Takeda et al., 2003). In addition, JAK/STAT independent, p38/MAPK signaling by IL-27 has been reported, which ultimately leads to IFN-γ secretion by activating T-bet. IL-27 also facilitates Th1 differentiation by inhibiting basal levels of the transcription factor GATA-binding protein-3 (GATA-3), which is a critical transcription factor for Th2 development. A T-bet-independent pathway has also been described, which involves IL-27-mediated enhanced surface expression and interaction of ICAM/LFA-1; downstream of this interaction lies ERK1/2, which leads to the differentiation of T cells into Th1 cells (Owaki, Asakawa, Fukai, Mizuguchi, & Yoshimoto, 2006). Phenotypic analysis of STAT4-deficient mice shows that activation of STAT4 is critical to Th1 differentiation. Previous studies suggested that STAT4 activation is particularly critical for the sustained production of Th1-type cytokines (Grogan et al., 2001). The precise mechanisms used by STAT4 to mediate its effects on Th1 differentiation have not been fully delineated. The reported probable mechanisms include T-bet (Finotto et al., 2002), IFN-γ (Xu, Sun, & Hoey, 1996), and GATA-3 (Ouyang et al., 1998) pathways. In our study, we showed the STAT1 pathway may have been repaired in the preventative group while the STAT4 pathway had no improvement. STAT6 appears to not only be necessary but also sufficient to drive Th2 differentiation (Zhu, Guo, Watson, Hu-Li, & Paul, 2001). In parallel with its induction of Th2-specific gene expression programs, STAT6 appears to suppress Th1-specific pathways (Pernis & Rothman, 2002). In our studies, we found that the STAT6 pathway increased in the OVA-induced asthma and decreased dramatically in the preventative groups, which indicated that the suppressed STAT6 pathway took part in the preventative IL-27 action. The results implied that preventative IL-27 can promote Th1 differentiation and alleviate Th2-mediated asthma by means of reversing the impairment of the STAT1 pathway, but not STAT4 pathway.
In our previous studies, we demonstrated that IL-27 inhibited the differentiation of naive CD4+ T cells into Th2 cells, but the already differentiated Th2 cells resisted the IL-27-mediated inhibition (Su et al., 2016). Furthermore, we found that preventative administration of IL-27 can effectively alleviate airway inflammation and AHR, but not in a therapeutic way if IL-27 were engaged after OVA challenge. Different from our results, Yoshimoto et al. (2007) thought intranasal administration of a mixture of IL-27 and OVA after OVA challenge can inhibit OVA-induced allergic airway inflammation. The inconsistency may be due to the differences between the protocols of our mouse asthma models, which may result in different severity of Th2 inflammation (Su et al., 2016) and different asthma phenotypes (J. J. Li et al., 2010). There is still a need for further understanding of the impact of IL-27 on asthma pathogenesis at different time points. Preventative intranasal administration of IL-27 can promote naïve CD4+ T cells to differentiate into Th1 cells and improve the pathologic symptoms of asthma. Thus, the improved Th1 environment helps to alleviate Th2-mediated allergic asthma by repairing the STAT1 pathway. The alteration of the local microenvironment could help us to detect a more effective treatment, or prevention against asthma. IL-27 could be a novel, promising preventative agent for asthma development or alleviate asthma exacerbation. However, the complication effects are there. Inhalation therapy of the cytokine may stimulate local mucosal tissue, causing airway smooth muscle irritation, and it is difficult to guarantee adequate and safe dosage. For diseased tissue, there may be little effect to reverse the pathology.
In conclusion, the HBE cells in the IL-27 environment can secrete more chemotactic factors to recruit MPS. The recruited MPS cells can, in return, secrete more IL-27, thus forming a positive feedback cycle. Preventative intranasal administration of IL-27 can promote naïve CD4+ T cells to differentiate into Th1 cells and improve the pathologic symptoms of asthma. Thus, the enhanced Th1 and Treg environment helps to alleviate Th2-mediated allergic asthma by repairing the STAT1 pathway, but not the STAT4 pathway. With more studies, IL-27 might be applied as an immunotherapeutic agent for asthma (Figure 6).
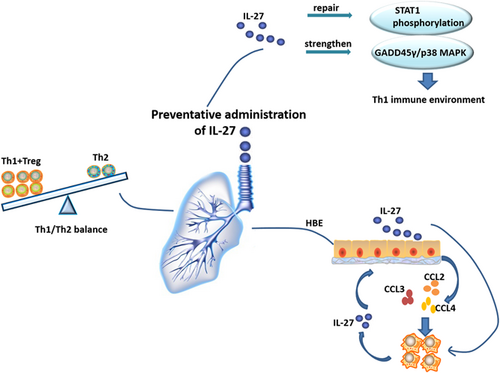
Hypothesis of preventative administration of IL-27 improving lung Th1 microenvironment. The HBE cells in the IL-27 environment can secrete more chemotactic factors to recruit the MPS. The recruited MPS cells can in return secrete more IL-27, thus forming a positive feedback cycle. The enhanced Th1 and Treg environment helps to alleviate Th2-mediated allergic asthma by repairing the STAT1 pathway and strengthening the GADD45γ/p38 MAPK pathway. CCL2: C-C motif chemokine ligand 2; HBE: human bronchial epithelial; IL-27: interleukin-27; MAPK: mitogen-activated protein kinase; Th1: type 1 T helper; Th2: type 2 T helper [Color figure can be viewed at wileyonlinelibrary.com]
ACKNOWLEDGMENTS
This study was supported by National Natural Science Foundation of China (81270078, 81470211 by Z. C.) and Shanghai Health Commission (201840288 by Z. C.) and research funds from Shanghai Respiratory Research Institute and Yang Scientists Training Program from Zhongshan Hospital; National Key Research and Development Program of China (2018YFC1313600 by LSH, 2016YFC0206401 by WXD).
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
Conceived and designed the study: Z. C. and X. W. Performed the biological experiments: X. L., S. L., T.Z., and J. J. Statistical analysis: X. L., S. L., and K. X. Wrote the paper: X. L. and Z. C. All authors read and approved the final manuscript.




