Helvolic acid attenuates osteoclast formation and function via suppressing RANKL-induced NFATc1 activation
Abstract
Excessive osteoclast formation and function are considered as the main causes of bone lytic disorders such as osteoporosis and osteolysis. Therefore, the osteoclast is a potential therapeutic target for the treatment of osteoporosis or other osteoclast-related diseases. Helvolic acid (HA), a mycotoxin originally isolated from Aspergillus fumigatus , has been discovered as an effective broad-spectrum antibacterial agent and has a wide range of pharmacological properties. Herein, for the first time, HA was demonstrated to be capable of significantly inhibiting receptor activator of nuclear factor-κB ligand (RANKL)-induced osteoclastogenesis and bone resorption in vitro by suppressing nuclear factor of activated T cells 1 (NFATc1) activation. This inhibition was followed by the dramatically decreased expression of NFATc1-targeted genes including Ctr (encoding calcitonin receptor), Acp5 (encoding tartrate-resistant acid phosphatase [TRAcP]), Ctsk (encoding cathepsin K), Atp6v0d2 (encoding the vacuolar H+ ATPase V0 subunit d2 [V-ATPase-d2]) and Mmp9 (encoding matrix metallopeptidase 9) which are osteoclastic-specific genes required for osteoclast formation and function. Mechanistically, HA was shown to greatly attenuate multiple upstream pathways including extracellular signal-regulated kinase (ERK) phosphorylation, c-Fos signaling, and intracellular Ca 2+ oscillation, but had little effect on nuclear factor-κB (NF-κB) activation. In addition, HA also diminished the RANKL-induced generation of intracellular reactive oxygen species. Taken together, our study indicated HA effectively suppressed RANKL-induced osteoclast formation and function. Thus, we propose that HA can be potentially used in the development of a novel drug for osteoclast-related bone diseases.
1 INTRODUCTION
Bone undergoes continuous remodeling that consists of a tight coupling of bone resorption and formation throughout life. Changes in either resorption or formation can lead to gross perturbations in the skeletal system, and potentially to life-threatening morbidity and mortality (Harvey, Dennison, & Cooper, 2010; Martin & Sims, 2005). Under normal age-related physiological conditions and certain pathological changes, osteoclasts act as key participants regulating bone loss. For instance, excessive osteoclastic resorption is commonly present in estrogen deficiency-induced osteoporosis (Horowitz, 1993; Kular, Tickner, Chim, & Xu, 2012) and osteolysis such as periprosthetic loosening after total joint arthroplasty (Abu-Amer, Darwech, & Clohisy, 2007; Xu et al., 2009).
Osteoclasts are large, multinucleated cells derived from hematopoietic cells of the monocyte/macrophage lineage (Roodman, 1999). Osteoclasts form through the fusion of mononuclear precursor cells and are unique in the ability to resorb mineralized bone matrix. Macrophage-colony-stimulating factor (M-CSF) and receptor activator of nuclear factor-κB ligand (RANKL) are two critical factors responsible for osteoclastogenesis and bone resorption. M-CSF is required for the survival, proliferation, and differentiation of osteoclasts via binding to its receptor c-Fms (Arai et al., 1999). It is also involved in the upregulation of receptor activatior of nuclear factor-κB (RANK), a transmembrane receptor which efficiently interacts with RANKL (Arai et al., 1999). The interaction between RANK and RANKL is indispensable for osteoclast development and function. Both RANK- (Dougall et al., 1999) and RANKL-deficient (Kong et al., 1999) mice were similarly characterized by severe osteopetrosis resulting from compromised osteoclast formation in bone.
The binding of RANKL to RANK induces the trimerization of an adapter molecule, tumor necrosis factor receptor (TNFR)-associated factor 6 (TRAF6), leading to the activation of nuclear factor-κB (NF-κB), extracellular signal-regulated kinase (ERK) phosphorylation (Boyle, Simonet, & Lacey, 2003), and Ca2+ signaling (Negishi-Koga & Takayanagi, 2009), which is then followed by the initial induction and activation of c-Fos and nuclear factor of activated T cells 1 (NFATc1). A number of osteoclast-specific genes such as Acp5 (encoding tartrate-resistant acid phosphatase [TRAcP]), Ctr (encoding calcitonin receptor), Ctsk (encoding cathepsin K), Atp6v0d2 (encoding the vacuolar H+ ATPase V0 subunit d2 [V-ATPase-d2]) and Mmp9 (encoding matrix metallopeptidase 9) are regulated by NFATc1 (Boyle et al., 2003; K. Kim, Lee, Ha Kim, Choi, & Kim, 2008). In addition, accumulating studies also demonstrated that RANKL-induced intracellular reactive oxygen species (ROS) generation plays a crucial role in osteoclast formation and activity (N. K. Lee et al., 2005; Wauquier, Leotoing, Coxam, Guicheux, & Wittrant, 2009; Yip et al., 2005).
Helvolic acid (HA), a mycotoxin originally isolated from Aspergillus fumigatus, was discovered as an effective broad-spectrum antibacterial agent (Luo et al., 2017; Qin, Li, Guan, & Zhang, 2009; Ratnaweera et al., 2014) and exerted enhanced antitumor efficacy in vivo when coadministrated with a well-known chemotherapeutic drug, cyclophosphamide (CTX; Xiao et al., 2017). HA was also shown to inhibit oxidized low-density lipoprotein (LDL) metabolism in macrophages (Shiao et al., 2008; Shinohara, Hasumi, & Endo, 1993). However, HA’s effects on RANKL-induced osteoclast formation and function still remain unknown. Given HA’s wide range of pharmacological properties, we investigated it’s in vitro effects on osteoclast development and its underlying molecular mechanisms. Our results demonstrated that HA effectively inhibited osteoclast formation and function through attenuation of NFATc1 activation via suppressing Ca2+ oscillation, intracellular ROS production, and c-Fos signaling. Taken together, HA is a potential candidate for the treatment of osteoclast-related bone diseases.
2 METHODS
2.1 Materials and reagents
HA was obtained from SHANGHAI ZZBIO CO., LTD (Shanghai, China) and dissolved in dimethyl sulfoxide (DMSO) at a stock concentration of 100 mM. It was further diluted to working concentrations with culture medium. DMSO of the same dilution was used as vehicle control in assays. Alpha modified minimal essential medium (a-MEM) and fetal bovine serum (FBS) were purchased from Thermo Fisher Scientific (Scoresby, Australia). The 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay kit and luciferase analysis system were purchased from Promega (Sydney, Australia). Primary antibodies for IκB-α, β-actin, phospho-ERK, ERK, NFATc1, and cathepsin K were purchased from Santa Cruz Biotechnology (Dallas, CA). Primary antibody for c-Fos was purchased from Cell Signaling Technology (Danvers, MA). Recombinant M-CSF was purchased from R&D Systems (Minneapolis, MN). Recombinant glutathione S-transferase-rRANKL protein was expressed and purified as previously described (Xu et al., 2000). Rhodamine phalloidin, fluo4, and 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) were obtained from Thermo Fisher Scientific and DAPI was purchased from Santa Cruz Biotechnology.
2.2 In vitro osteoclast differentiation
Primary bone marrow macrophages (BMMs) were isolated from the long bones of euthanized C57BL/6 mice using methods approved by the University of Western Australia Animal Ethics Committee (RA/3/100/1244) and then cultured in α-MEM supplemented with 10% (v/v) FBS, 1% (v/v) penicillin/streptomycin, and 50 ng/ml M-CSF. To induce osteoclast differentiation, BMMs were seeded into a 96-well plate at a density of 6 × 103 cells/well. After adhering to the bottom of the plate, BMMs were stimulated with M-CSF (50 ng/ml) and RANKL (50 ng/ml) in the presence of HA at a range of concentrations (0, 2.5, 5, 7.5, and 10 μM). The culture medium was changed every 2 days for 5 days until osteoclasts formed. The cells were then fixed with 2.5% glutaraldehyde solution and stained for TRAcP activity. Multinucleated cells with more than three nuclei were identified as osteoclasts.
2.3 Staining for actin ring formation
To observe actin rings, osteoclasts were induced as above and were fixed with 4% paraformaldehyde for 10 min, permeabilized with 0.1% (v/v) triton X-100 for 10 min, blocked with 3% bovine serum albumin (BSA), and subsequently stained with rhodamine phalloidin (1:300 dilution) in the dark for 2 hr. Cells were then washed with PBS twice, followed by incubation with DAPI for 10 min to visualize nuclei. After staining, cells were mounted in ProLong Gold antifade mountant and observed under NIKON A1Si confocal microscopy (Nikon Corporation, Tokyo, Japan). Three images were randomly captured for each group and the number of nuclei and the area of the actin rings were analyzed using ImageJ software.
2.4 MTS cell viability assay
To determine whether HA has cytotoxic effects on osteoclast precursors, MTS assay was performed following the manufacturer’s instructions. In brief, BMMs were plated in a 96-well plate at a density of 6 × 103 cells/well in triplicate. The following day cells were treated with a range of concentrations of HA (0, 2.5, 5, 7.5, and 10 μM) for 48 hr. Next, 20 μl MTS solution was added in each well and cells were then incubated at 37°C for 2 hr. The optical density was measured by spectrophotometric absorbance using a microplate reader (BMG LABTECH, Ortenberg, Germany) at a wavelength of 490 nm.
2.5 Hydroxyapatite resorption assay
The function of osteoclasts was evaluated by hydroxyapatite resorption assay. BMMs were seeded into six-well collagen-coated plates (Corning, Inc., Corning, NY) at a density of 1 × 105 cells/well and stimulated with M-CSF and RANKL to induce osteoclast differentiation. When osteoclasts started to form, cells were harvested gently by cell dissociation solution (Sigma–Aldrich, Sydney, Australia) and seeded onto hydroxyapatite-coated plates (Corning, Inc.). Cells were cultured in complete medium containing 50 ng/ml RANKL and M-CSF with or without HA (0, 5, and 10 μM). After mature osteoclasts formed, half of the wells in each group were stained for TRAcP to count the number of osteoclasts and the rest of wells were bleached to capture the images of hydroxyapatite resorption areas using a Nikon microscope (Nikon Corporation). The resorption area per osteoclast was analyzed to determine the osteoclastic resorption activity.
2.6 Intracellular Ca2+ oscillation
Calcium oscillations were measured using the calcium fluorophore Fluo4 as described previously (Song et al., 2016). Briefly, a total of 2 × 104 BMMs were seeded onto a 48-well plate and cultured with RANKL (50 ng/ml) and M-CSF (50 ng/ml) in the presence or absence of 10 µM HA for 48 hr. Cells were then rinsed with assay buffer (HANKS balanced salt solution, 1 mM probenecid, and 1% FBS), followed by incubation with 4 μM Fluo4 staining solution (Fluo4-AM dissolved in 20% pluronic-F127 [w/v] in DMSO diluted in assay buffer) for 45 min. The dye-loaded cells were washed twice with assay buffer and viewed on an inverted fluorescent microscope (Nikon) at an excitation wavelength of 488 nm, images were captured at 2 s intervals for 3 min and the results were analyzed using Nikon Basic Research Software. Cells showing two intensity peaks within the observed time frame were identified as oscillating cells and the oscillation intensity change was calculated by the maximum peak intensity minus the minimum intensity.
2.7 Determination of intracellular ROS generation
Intracellular generation of ROS was measured as described (N. K. Lee et al., 2005). In brief, after treatment with RANKL (50 ng/ml) and HA at different concentrations (0, 5, and 10 µM) for 60 min, BMMs were then washed and incubated with Hank’s balanced salt solution containing 5 mM H2DCFDA in the dark for 5 min. Upon oxidation, the acetate groups of the nonfluorescent H2DCFDA are cleavaged and then it is converted to the highly fluorescent 2’,7’-dichlorofluorescein (DCF). The DCF fluorescence was detected at an excitation wavelength of 488 nm and an emission wavelength of 515–540 nm using a NIKON A1Si confocal microscopy. The average fluorescence intensity was analyzed using ImageJ software.
2.8 NFATc1 luciferase reporter gene activity assay
NFATc1 transcriptional activation was evaluated using luciferase reporter gene assay as described previously (Zhou et al., 2016). Briefly, RAW264.7 cells transfected with an NFATc1 luciferase reporter (Cheng et al., 2018; van der Kraan et al., 2013) were seeded in 48-well plates and maintained overnight. Next, cells were pretreated with or without different concentrations of HA (0, 2.5, 5, and 10 μM) for 1 hr, followed by the addition of RANKL (50 ng/ml) for 24 hr. In the end, cells were then lysed, and luciferase activity was measured using a luciferase reporter assay kit (Promega). The results were normalized to that of vehicle control.
2.9 Western blot analysis
Signaling pathways were analyzed using western blot. For activation of early signaling pathways, BMMs were seeded into six-well plates at a density of 8 × 105 cells/well with complete α-MEM containing M-CSF. Cells were pretreated with or without HA (10 μM) for 2 hr before RANKL stimulation for the stated times (0, 10, 20, 30 or 60 min). For analysis of long-term effects on osteoclast formation BMMs (1 × 105 cells/well) were seeded into six-well plates and treated with RANKL with or without HA (10 μM) for 0, 1, 3, or 5 days. Protein was harvested by lysing cells using radioimmunoprecipitation lysis buffer, followed by centrifugation at 12,000g for 20 min. The supernatants were collected and total protein was separated on sodium dodecyl sulfate-polyacrylamide gels and transferred to a nitrocellulose membrane (GE Healthcare, Silverwater, Australia). Non-specific binding was blocked with 5% skim milk, and membranes were then incubated with primary antibodies (1:1,000) at 4°C overnight. The next day, membranes were incubated with secondary antibodies conjugated to horseradish peroxidase for 2 hr at room temperature. Antibodies were detected with enhanced chemiluminescence substrate (PerkinElmer, MA). The antibody reactivity was detected on an Image-quant LAS 4000 (GE Healthcare) and analyzed by the ImageJ software.
2.10 Quantitative polymerase chain reaction analysis
For real-time polymerase chain reaction (PCR), BMMs (1 × 105 cells/well) were cultured in six-well plates and induced to form osteoclasts as above with or without HA (0, 5, and 10 μM). Total RNA was prepared using an RNeasy Mini Kit (Qiagen, Chadstone VIC, Australia) and single-stranded complementary DNA was synthesized from 1 μg of total RNA template using Moloney murine leukemia virus reverse transcriptase with oligo-dT primer (Promega). SYBR Green PCR MasterMix was used to perform relative quantitative real-time PCR (qPCR). Cycling conditions were as follows: 94°C for 5 min, followed by 30 cycles of 94°C for 40 s, 60 °C for 40 s, and 72°C for 40 s, followed by an elongation step of 5 min at 72°C. The qPCR procedure was performed on a ViiA7 real-time PCR machine (Applied Biosystems, Warrington, UK). Hprt1 was utilized as the housekeeping gene and all reactions were performed in triplicate. The specific primers used are as follows: Atp6v0d2 (forward: 5′-GTGAGACCTTGGAAGACCTGAA-3′; reverse: 5′-GAGAAATGTGCTCAGGGGCT-3′), Acp5 (forward: 5′-TGTGGCCATCTTTATGCT-3′; reverse: 5′-GTCATTTCTTTGGGGCTT-3′), Ctr (forward: 5′-TGGTTGAGGTTGTGCCCA-3′; reverse: 5′-CTCGTGGGTTTGCCTCATC-3′), Nfatc1 (forward: 5′-CAACGCCCTGACCACCGATAG-3′; reverse: 5′-GGCTGCCTTCCGTCTCATAGT- 3′), c-Fos (forward: 5′-GCGAGCAACTGAGAAGAC-3′; reverse: 5′-TTGAAACCCGAGAACATC-3′), Ctsk (forward: 5′-GGGAGAAAAACCTGAAGC-3′; reverse: 5′-ATTCTGGGGACTCAGAGC-3′), Mmp9 (forward: 5′-CGTGTCTG GAGATTCGACTTGA-3′; reverse: 5′-TTGGAAACTCACACGCCAGA-3′), Hprt1 (forward: 5′- TCAGTCAACGGGGGACATAAA-3′; reverse: 5′-GGGGCTGTACTGCTTAACCAG-3′).
2.11 Data analysis
All quantitative data are presented as mean ± standard deviation obtained from at least three experiments. Statistical analyses were determined by Student’s t test and one-way analysis of variance. A p value of less than 0.05 was considered to be significant.
3 RESULTS
3.1 HA inhibits RANKL-induced osteoclastogenesis in vitro
The chemical structure of HA is demonstrated in Figure 1a. To determine the effects of HA on osteoclastogenesis, freshly isolated BMMs were induced to form osteoclasts under the stimulation of RANKL and M-CSF in the presence of HA at different concentrations. The results showed that HA was able to interfere with osteoclastogenesis in a dose-dependent manner as demonstrated by the significant reduction in the number of TRAcP-positive multinucleated osteoclasts (cells with more than three nuclei) at concentrations of 2.5 µM HA and higher (Figure 1b,c). To examine whether HA has cytotoxic effects on BMMs, an MTS assay was performed to assess cell viability. It was found that HA had no cytotoxicity on BMMs within the range of concentrations as indicated in our study (Figure 1d). Mature osteoclasts were also stained with rhodamine phalloidin to visualize any morphological changes information of F-actin structures (Figure 1e). F-actin ring formation is critical for osteoclast function and well-defined actin rings were observed in the control group. However, the area of actin rings per field and the number of nuclei per osteoclast in the HA-treated group were reduced (Figure 1f,g). To further evaluate which stage of osteoclast formation was most sensitive to HA treatment, cells were exposed to 10 µM HA at different time points (Figure 2a,b). We found that osteoclast formation was interrupted by treatment with HA at all three stages; however, treatment with HA at early stages (Day 1–3) inhibited osteoclastogenesis most significantly (Figure 2c). Taken together, these results indicate that HA inhibits RANKL-induced osteoclast differentiation without any obvious cytotoxicity in osteoclast precursors.
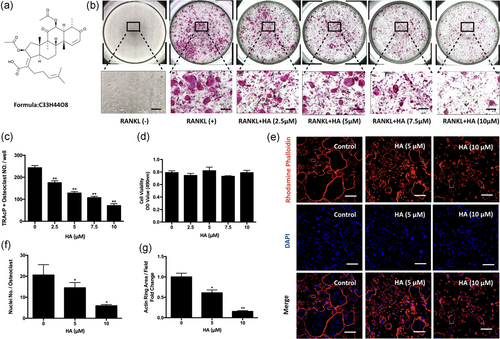
Helvolic acid (HA) inhibited RANKL-induced osteoclastogenesis in a dose-dependent manner, without cytotoxicity. (a) The chemical structure of HA. (b) Representative images of TRAcP staining. BMMs were cultured in the presence of M-CSF (50 ng/ml) and RANKL (50 ng/ml) with indicated concentrations of HA for 5 days. Cells were fixed and stained for TRAcP. (c) Quantification of TRAcP-positive osteoclasts. Multinucleated cells with more than three nuclei were identified as osteoclasts (n = 3). (d) BMMs were treated with a serial of concentrations of HA (0, 2.5, 5, 7.5, and 10 μM) for 48 hr. Cell viability was then measured using MTS assay (n = 3). (e) Representative confocal images of mature osteoclasts stained for actin ring and nuclei using Rhodamine Phalloidin and DAPI respectively. (f) The average number of nuclei per osteoclast (g) and the average of actin ring area per field were analyzed quantitatively (n = 3). *p < 0.05, **p < 0.01 relative to RANKL-induced control group. Scale bar = 200 μm. BMMs: bone marrow macrophages; M-CSF: macrophage-colony stimulating factor; MTS: 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; NFATc1: nuclear factor of activated T cells 1; RANKL: receptor activator of nuclear factor-κB ligand; TRAcP: tartrate resistant acid phosphatase [Color figure can be viewed at wileyonlinelibrary.com]
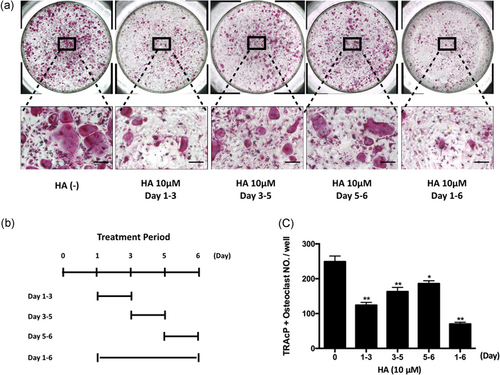
HA majorly inhibited RANKL-induced osteoclastogenesis at an early stage. (a) Representative images of the effect of 10 μM HA’s treatment on the different time periods. Osteoclast formation was induced by RANKL (50 ng/ml) and M-CSF (50 ng/ml), and the cells were treated by HA in the indicated time period. (b) The time periods of the treatment of HA. (c) TRAcP-positive multinucleated cells (nuclei >3) with the treatment of HA were counted (n = 3). *p < 0.05, **p < 0.01 relative to RANKL-induced control group. Scale bar = 200 μm. HA: helvolic acid; M-CSF: macrophage-colony stimulating factor; RANKL: receptor activator of nuclear factor-κB ligand [Color figure can be viewed at wileyonlinelibrary.com]
3.2 HA suppresses osteoclast hydroxyapatite resorption and osteoclast-specific gene expression
As HA inhibited osteoclastogenesis and F-actin ring formation, we hypothesized that HA might also affect osteoclastic resorption. Hydroxyapatite-coated plates were used to examine the function of osteoclasts. Relative to the control group the area of resorption per osteoclast was reduced substantially after treatment with 5 and 10 µM HA (Figure 3a–d). In addition, osteoclast-specific genes Atp6v0d2, Acp5, Ctr, and Mmp9 were assessed using qPCR. As demonstrated in Figure 3e–h, RANKL upregulated these genes expression of mature osteoclast whereas HA dramatically suppressed this upregulation. Therefore, these data showed that HA attenuated osteoclast hydroxyapatite resorption and messenger RNA (mRNA) expression of osteoclast-specific genes.
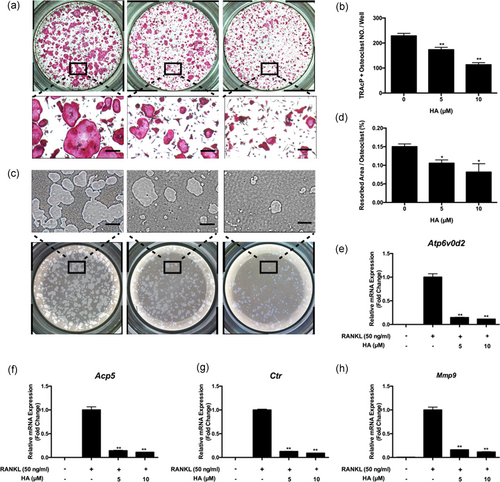
HA suppressed osteoclast hydroxyapatite resorption and osteoclast-specific genes expression. (a) Representative images and (b) quantification of TRAcP-positive osteoclasts (n = 3) on hydroxyapatite-coated plates after treatment of HA for 48 hr with the indicated concentrations. (c) Representative images of hydroxyapatite resorption. (d) Quantification of resorbed hydroxyapatite surface area per osteoclast (n = 3). (e-h) Real-time PCR results of osteoclast-specific genes Atp6v0d2, Acp5, Ctr, and Mmp9. The expression levels were normalized to the expression of Hprt1. *p < 0.05, **p < 0.01 relative to RANKL-induced control group. Scale bar = 200 μm. Ctr: calcitonin receptor; HA: helvolic acid; Mmp9: matrix metallopeptidase 9; PCR: polymerase chain reaction; RANKL: receptor activator of nuclear factor-κB ligand; TRAcP: tartrate-resistant acid phosphatase [Color figure can be viewed at wileyonlinelibrary.com]
3.3 HA abrogates RANKL-induced Ca2+ oscillation
Next, to explore the molecular mechanism by which HA inhibited osteoclast formation and activity, we investigated the effects of HA on RANKL-induced intracellular calcium oscillation. Activation of calcium oscillation is a well-defined process that is critical for the development of osteoclast via NFATc1 activation (Ishii et al., 2009; Negishi-Koga & Takayanagi, 2009). As is shown in Figure 4, compared with the control group, Ca2+ oscillation was enhanced by stimulation with RANKL. In contrast, after treatment with HA, RANKL-induced Ca2+ oscillation was significantly attenuated (Figure 4c,d).
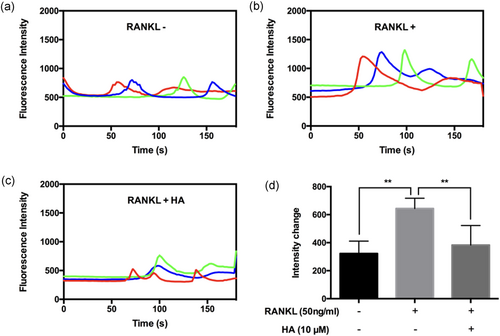
HA abrogated RANKL-induced Ca2+ oscillation. Representative images of fluorescence intensity waves of Ca2+ oscillation in (a) negative group, (b) positive group, and (c) HA (10 μM) treated group. Each color indicates a different cell. (d) Quantification of intensity change of Ca2+ oscillation in each group (n = 6). The values of intensity change were calculated by the maximum peak intensity minus the minimum intensity. *p < 0.05, **p < 0.01 relative to RANKL-induced control group. HA: helvolic acid; RANKL: receptor activator of nuclear factor-κB ligand [Color figure can be viewed at wileyonlinelibrary.com]
3.4 HA suppresses RANKL-induced ROS generation
ROS generation has been shown to increase in response to RANKL stimulation and they act as an intracellular mediator for osteoclast formation (N. K. Lee et al., 2005; Yip et al., 2005). Therefore, we tested whether HA would affect ROS generation in BMMs. Intracellular ROS generation was detected by the cell permeant, oxidation-sensitive dye H2DCFDA using confocal microscopy. We found that the addition of HA abolished the rise in DCF fluorescence by RANKL stimulation in a dose-dependent manner (Figure 5a). The intensity of DCF fluorescence per positive cell (Figure 5b) decreased significantly following HA treatment.
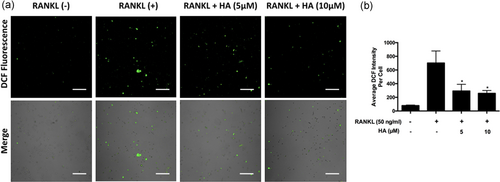
HA suppressed RANKL-induced ROS generation. (a) Cells were stimulated with RANKL and with or without the addition of HA. Intracellular ROS generation was detected by the cell permeant, oxidation-sensitive dye H2DCFDA and the DCF fluorescence was captured using confocal microscopy. (b) Quantification of DCF fluorescence intensity averaged on cells. Results are representative of three independent experiments. *p < 0.05, **p < 0.01 relative to RANKL-induced control group. Scale bar = 200 μm. DCF: 2’,7’-dichlorofluorescein; HA: helvolic acid; H2DCFDA: 2’,7’-dichlorodihydrofluorescein diacetate; RANKL: receptor activator of nuclear factor-κB ligand; ROS: reactive oxygen species [Color figure can be viewed at wileyonlinelibrary.com]
3.5 HA inhibits RANKL-induced NFATc1 activation
NFATc1 is well known as a master transcriptional regulator of osteoclast differentiation (Takayanagi et al., 2002), and thus the activation of NFATc1 may be a target of HA treatment. To determine NFATc1 activation, we first utilized an NFATc1 luciferase reporter gene construct in RAW264.7 cells to investigate the NFATc1 transcriptional activity. Cells pretreated with HA showed a dose-dependent impairment of NFATc1 activity (Figure 6a). Consistently, in western blot assay, the protein level of NFATc1 was dramatically upregulated on Day 3 and Day 5 in RANKL-stimulated cultures, but it was suppressed significantly when treated with HA (Figure 6b,c). This suppression was also found in the mRNA expression of Nfatc1 (Figure 6d). Cathepsin K is a crucial downstream protein of functional mature osteoclasts. Following HA treatment, the protein expression of cathepsin K exhibited a substantially decreased trend (Figure 6b,e). This result was consistently supported by real-time PCR, which demonstrated the attenuation of Ctsk mRNA expression after HA treatment (Figure 6e,f). These data strongly suggested that HA inhibited RANKL-induced NFATc1 activation during osteoclast formation.
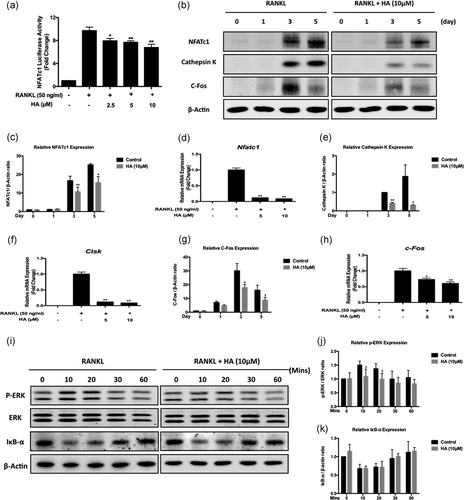
HA attenuated NFATc1 activation, c-Fos signaling, and ERK phosphorylation, but had no effect on NF-κB pathway. (a) HA affected luciferase activity of RAW 264.7 cells transfected with NFATc1 luciferase construct. Cells were pretreated with HA of different concentrations as indicated for 1 hr, followed by the stimulation of RANKL (50 ng/ml) for 24 hr. The luciferase activity was measured using luciferase reporter assay (n = 3). (b) Representative western blot images of the effects of HA on the protein expression of NFATc1, cathepsin K, and c-Fos during osteoclastogenesis. (c-h) Quantification of the ratios of band intensity and real-time PCR results of genes of NFATc1 (c and d), cathepsin K (e and f), and c-Fos (g and h). The band intensity was relative to β-actin (n = 3) and the mRNA expression levels were normalized to the expression of Hprt1 (n = 3). (i) Representative western blot images of the effects of HA on ERK phosphorylation and IκBα degradation and induced by RANKL. BMMs were treated with RANKL for the indicated time points with or without the addition of 10 μM HA. (j and k) Quantification of the ratios of band intensity of phosphorylated ERK (j) and IκBα (k) relative to total ERK and β-actin respectively (n = 3). *p < 0.05, **p < 0.01 relative to RANKL-induced control group. BMMs: bone marrow macrophages; ERK: extracellular signal-regulated kinase; HA: helvolic acid; mRNA : messenger RNA; NFATc1: nuclear factor of activated T cells 1; PCR: polymerase chain reaction; RANKL: receptor activator of nuclear factor-κB ligand
3.6 HA attenuates c-Fos signaling and ERK phosphorylation
To further elucidate the mechanisms through which HA-mediated the suppression of NFATc1 activation, we investigated upstream signaling pathways of NFATc1 such as c-Fos, ERK, and NF-κB. We studied the expression of c-Fos, an upstream component that binds to the promoter of NFATc1 to promote its transcriptional activities (Matsuo et al., 2004). C-Fos protein expression in BMMs increased on Day 3 after the stimulation with RANKL (Figure 6b). However, treatment with HA significantly inhibited the expression of c-Fos in both mRNA and protein level (Figure 6g,h). Due to the reduced expression of c-Fos by the interference of HA, we next determined whether its upstream of ERK phosphorylation had been affected. As shown in Figure 6i,k following the stimulation of RANKL, the phosphorylation of ERK increased particularly after 10 and 20 min whereas the HA treatment could significantly block this upregulation. The effect of HA on the RANKL-stimulated NF-κB pathway was also studied, the protein expression of IκBα, an inhibitor of NF-κB, was tested using western blot. After RANKL stimulation, IκBα degraded to activate NF-κB (Figure 6i). Treatment with HA had no effect on the degradation of IκBα (Figure 6k), indicating it does not impact the NF-κB pathway. Collectively, these data indicated that HA suppressed c-Fos signaling via the inhibition of ERK phosphorylation, but without effects on NF-κB activation(Figure 7).
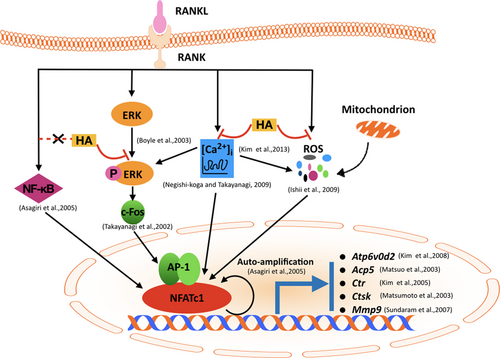
A proposed scheme depicting the inhibition of HA on RANKL-induced NFATc1 activation during osteoclast differentiation. The binding of RANKL to RANK activates NF-κB, ERK phosphorylation, Ca2+ oscillation, and ROS production, followed by the activation of c-Fos and NFATc1. A number of osteoclast-specific genes such as Atp6v0d2, Acp5, Ctr, Ctsk, and Mmp9 are then regulated by NFATc1 and resulted in the formation of mature osteoclasts. Our results indicated that the presence of HA exerted multiple inhibitions including attenuating ERK phosphorylation, diminishing Ca2+ oscillation, and reducing the production of ROS, but without effects on NF-κB pathway, thus eventually suppressing RANKL-induced osteoclast formation and function in vitro. Ctr: calcitonin receptor; Ctsk: cathepsin K; ERK: extracellular signal-regulated kinase; HA: helvolic acid; Mmp9: matrix metallopeptidase 9; NFATc1: nuclear factor of activated T cells 1; RANK: receptor activatior of nuclear factor-κB; RANKL: receptor activator of nuclear factor-κB ligand; ROS: reactive oxygen species [Color figure can be viewed at wileyonlinelibrary.com]
4 DISCUSSION
Osteoporosis is a highly prevalent disease characterized by low bone mass and increased susceptibility to bone fracture, which results in massive costs to both the individual and society (Harvey et al., 2010). Excessive osteoclast formation and function are considered as the main causes of bone lytic disorders such as osteoporosis and osteolysis (Charles & Aliprantis, 2014; Xu et al., 2009). Therefore, osteoclasts remain one of the potential therapeutic targets for the treatment of osteoporosis and osteoclast-related diseases. In this study, for the first time, HA was demonstrated to be capable of inhibiting osteoclast formation and function by suppressing RANKL-induced NFATc1 activation.
Our results indicated that HA effectively inhibited RANKL-induced osteoclast formation, particularly at early stages, in a dose-dependent manner but without exerting any obvious cytotoxicity. HA was also effective at inhibiting resorption suggesting that it might be a drug prototype for an alternative treatment of osteoclast-related bone disease.
We further studied the mechanisms behind HA’s inhibitory effects on osteoclasts. NFATc1 represents a master regulator of terminal RANKL-induced differentiation of osteoclasts (Takayanagi et al., 2002). The physiological significance of NFATc1 has been well explored. Embryonic stem (ES) cells were able to differentiate into osteoclasts in response to stimulation with RANKL and M-CSF whereas NFATc1-deficient ES cells failed (Takayanagi et al., 2002). In addition, osteoclast-specific conditional NFATc1-deficient mice developed osteopetrosis (Aliprantis et al., 2008). Growing evidence shows that a number of osteoclast-specific genes, including Acp5 (Matsuo et al., 2004; Takayanagi et al., 2002), Ctr (K. Kim et al., 2005; Takayanagi et al., 2002), ITGB3 (encoding integrin beta 3) (Crotti et al., 2006), Atp6v0d2 (K. Kim et al., 2008), and Mmp9 (Sundaram et al., 2007) are regulated by NFATc1. Ctsk is one of the targets regulated by NFATc1 (Matsumoto et al., 2004). It is found at the ruffled border of active mature osteoclasts and acts as an essential mediator of bone resorption (Troen, 2004). This evidence collectively demonstrates that NFATc1 has an indispensable function in osteoclast differentiation in vitro and in vivo. Therefore, we evaluated the expression level and transcriptional activity of NFATc1 during HA-treated osteoclast formation and demonstrated that NFATc1 activation was dramatically suppressed by HA treatment, followed by suppressing the expression of a number of NFATc1-targeted genes including Atp6v0d2, Acp5, Ctr, Ctsk, and Mmp9 leading to the compromised osteoclastogenesis and osteoclastic resorption.
The molecular mechanisms underlying activation of NFATc1 have also been extensively studied previously. NFATc1 is a master transcriptional switch for osteoclast differentiation (Takayanagi et al., 2002). NFATc1 activation is mediated by calcineurin, which is a specific phosphatase activated by calcium/calmodulin signaling (Negishi-Koga & Takayanagi, 2009). The evident and long-lasting Ca2+ oscillations observed during osteoclastogenesis are required to maintain the induction of NFATc1 (Negishi-Koga & Takayanagi, 2009). Chromatin immunoprecipitation experiments showed that NFATc1 was selectively recruited to the NFATc1 promoter in response to RANKL stimulation and this binding persisted during the terminal differentiation of osteoclasts, suggesting the mechanism of NFATc1 autoamplification (Asagiri et al., 2005). In this study, Ca2+ oscillation was induced by RANKL whereas diminished by the addition of HA, suggesting HA affected NFATc1 partly at least via suppressing Ca2+ oscillation.
C-Fos, a critical component of the transcription factor complex AP-1, is also known as a prerequisite for the robust induction of NFATc1 by functioning as a direct transcriptional regulator of NFATc1 (Takayanagi et al., 2002; Wagner & Eferl, 2005). C-Fos-deficient cells failed to induce NFATc1 activation following RANKL stimulation (Takayanagi et al., 2002) and overexpression of NFATc1 rescued osteoclast differentiation in c-Fos-deficient cells (Matsuo et al., 2004). Consistent with HA’s inhibition of NFATc1 activation, we also found that HA strongly reduced the mRNA expression and protein level of c-Fos. Because the expression and stabilization of c-Fos were shown to be linked to the ERK signaling pathway (M. S. Lee et al., 2009; Murphy & Blenis, 2006), along with inhibition of c-Fos by HA, we attempted to examine its upstream ERK phosphorylation. As expected, RANKL greatly stimulated the phosphorylation of ERK in BMMs, but BMMs pretreated with HA saw a decreased expression of phosphorylated ERK. In addition, Ca2+ signaling is also responsible for the initial induction of c-Fos in the early stage of osteoclast formation (Negishi-Koga & Takayanagi, 2009). We thus reasoned HA suppressed c-Fos via the suppression of ERK signaling and Ca2+ oscillation.
Recently, growing studies have shown that ROS are generated via nicotinamide adenine dinucleotide phosphate oxidase (Nox) in activated osteoclasts, which are thought to be highly associated with the process of osteoclastogenesis and bone resorption (H. Kim, Lee, Kim, Lee, & Kim, 2017; N. K. Lee et al., 2005; Rana, Schultz, Freeman, & Biswas, 2012). Furthermore, a variety of antioxidants were also found to be capable of inhibiting the formation and bone resorption of osteoclasts by reducing the production of intracellular ROS (Bartell et al., 2014; H. J. Kim et al., 2006; N. K. Lee et al., 2005). As Ca2+ oscillations mediated the production of mitochondrial ROS (H. Kim et al., 2013) and ROS was clearly demonstrated to be involved in NFATc1 induction (Ishii et al., 2009; Ke et al., 2006), we were interested to investigate whether HA’s inhibitory effects on NFATc1 were linked to its attenuation on ROS generation during RANKL-induced osteoclastogenesis. Interestingly, HA had a negative effect on RANKL-induced ROS production. These findings suggest that HA can also suppress NFATc1 via acting as a ROS scavenger or inhibitor. However, HA was previously reported to be ineffective in inhibiting phorbol myristate acetate-stimulated ROS generation in human polymorphonuclear leukocytes (Tsunawaki, Yoshida, Nishida, Kobayashi, & Shimoyama, 2004). This contradiction may lie in the different stimulation conditions and different type of cells used in the experiments.
NF-κB activation is a very early event in response to RANKL stimulation (Anderson et al., 1997) and it contributes to the initial induction of NFATc1 through binding to its promoter directly (Asagiri et al., 2005). In our study, we observed that the RANKL-induced degradation of IκBα was not affected by the treatment with HA, suggesting HA failed to inhibit the activation of NF-κB. However, whether HA can affect the downstream NF-κB complex, including p65/Rel A, to translocate to the nucleus and achieve its transcription of targeted genes still remains to be further investigated.
In summary, our studies provide evidence that HA exerted inhibitory effects on osteoclastogenesis via strongly suppressing RANKL-induced NFATc1 activation, the master regulator involved in osteoclast formation. Furthermore, we also clarified that the attenuated NFATc1 expression was related to the suppression of multiple pathways, including Ca2+ oscillation, intracellular ROS production, and c-Fos signaling. Considering its strong inhibitory effects, we propose that HA, or related molecules, could be potentially used in the development of an alternative drug for the osteoclast-related bone disease.
ACKNOWLEDGMENTS
This study was supported by grants from the Australian Health and Medical Research Council (NHMRC, No. 1107828, 1027932), and supported in part by Co-innovation Center for Bio-Medicine, Innovation Team of Tissue Repair and Reconstruction. This project was also supported by the National Natural Science Foundation of China (NSFC No. 81501910, 81702186). Kai Chen is supported by Australian Government Research Training Program Scholarship. We acknowledge the facilities and technical assistance of the Center for Microscopy, Characterization & Analysis, The University of Western Australia.
CONFLICTS OF INTEREST
All authors declare that they have no conflicts of interest.




