Curcumin induces concentration-dependent alterations in mitochondrial function through ROS in C2C12 mouse myoblasts
Abstract
Curcumin exhibits antioxidant properties in normal cells where the uptake is low, unlike in tumor cells where uptake is high and curcumin increases reactive oxygen species (ROS) production and cell death. Mitochondria are the main source and primary target of cellular ROS. We hypothesized that curcumin would regulate cellular redox status and mitochondrial function, depending on cell sensitivity and/or curcumin concentration in normal cells. We examined the differences between low and high concentrations of curcumin, with specific attention focused on ROS levels, mitochondrial function, and cell viability in mouse C2C12 myoblast under normal and simulated conditions of diabetes. Cells incubated with high concentrations of curcumin (10–50 μM) resulted in decreased cell viability and sustained robust increases in ROS levels. Mechanistic studies showed that increased ROS levels in cells incubated with 20 μM curcumin induced opening of mitochondrial permeability transition pores and subsequent release of cytochrome c, activation of caspases 9 and 3/7, and apoptotic cell death. Low concentrations of curcumin (1–5 μM) did not affect cell viability, but induced a mild increase in ROS levels, which peaked at 2 hr after the treatment. Incubation with 5 μM curcumin also induced ROS-dependent increases in mitochondrial mass and membrane potential. Finally, pretreatment with 5 μM curcumin prevented high glucose-induced oxidative cell injury. Our study suggests that mitochondria respond differentially depending on curcumin concentration-dependent induction of ROS. The end result is either cell protection or death. Curcumin may be an effective therapeutic target for diabetes and other mitochondrial diseases when used in low concentrations.
1 INTRODUCTION
Curcumin is a natural polyphenol and the major active component of turmeric (Curcuma longa). For hundreds of years, turmeric has been used as a traditional medicine in China and India for treating many human ailments such as diabetes, infectious, and autoimmune diseases (Hatcher, Planalp, Cho, Torti, & Torti, 2008). Modern medical research has identified many functions of curcumin, to include strong antioxidant properties. As a free radical scavenger, curcumin directly reacts with the superoxide anion, hydroxyl radicals, and hydrogen peroxide (Ak & Gulcin, 2008; de Oliveira, Jardim, Setzer, Nabavi, & Nabavi, 2016). It also inhibits the cleavage of hydrogen peroxide, which reduces the production of hydroxyl radicals and formation of other reactive oxygen species (ROS; Ak & Gulcin, 2008). Furthermore, curcumin activates antioxidant systems by increasing the expression of antioxidant enzymes such as glutathione peroxidase (Kunwar, Sandur, Krishna, & Priyadarsini, 2009; Panchatcharam, Miriyala, Gayathri, & Suguna, 2006), catalase (Kunwar et al., 2009; Panchal et al., 2008; Panchatcharam et al., 2006), superoxide dismutase (Barik et al., 2005; Kunwar et al., 2009; Panchatcharam et al., 2006), and heme oxygenase 1 (Kunwar et al., 2009; Motterlini, Foresti, Bassi, & Green, 2000).
Curcumin uptake is significantly higher in tumor cells than in normal cells (Kunwar et al., 2008). Studies in both cancer cells and animal models have revealed that curcumin has chemopreventive and chemotherapeutic activities (Perrone et al., 2015; Wilken, Veena, Wang, & Srivatsan, 2011). Over the past decade, more than one hundred clinical trials have used curcumin as a therapeutic target for a wide variety of cancers (Perrone et al., 2015; Wilken et al., 2011). Although the mechanism underlying the anticancer properties of curcumin is unclear, ROS has been implicated: curcumin increases ROS production in tumor cells, which suppresses tumor cell growth and induces cell death through mitochondria-dependent and -independent pathways (Kim et al., 2016; Moustapha et al., 2015).
Mitochondria are both the main source and a primary target of cellular ROS (Galluzzi, Kepp, Trojel-Hansen, & Kroemer, 2012; Yoon, Galloway, Jhun, & Yu, 2011; Youle & van der Bliek, 2012). Mitochondria generate ROS as the inevitable byproducts of oxidative phosphorylation in the electron transport chain (Yu, Wang, & Yoon, 2015). In addition, mitochondria contain several proapoptotic proteins, which serve key roles in regulating cell survival and death (Youle & van der Bliek, 2012). Indeed, mitochondrial dysfunction and complications have been associated with many human diseases, including diabetes (Yoon et al., 2011). We and others have shown that hyperglycemia-induced oxidative stress through the increased production of mitochondrial ROS and mitochondrial dysfunction serve a pivotal role in the development of diabetes and its associated complications (Giacco & Brownlee, 2010; Yoon et al., 2011; Yu, Sheu, Robotham, & Yoon, 2008). Although curcumin has been used for the treatment of diabetes (Meng, Li, & Cao, 2013; Zhang, Fu, Gao, & Liu, 2013), it is unclear whether it can preserve mitochondrial structure and function to prevent oxidative stress and mitigate cell injury in diabetic hyperglycemia.
In this study, we tested our hypothesis that curcumin regulates cellular redox status and mitochondrial function depending on cell sensitivity and/or curcumin concentration. We also tested the antioxidant activities of curcumin in simulated conditions of diabetes, namely incubation under a high glucose concentration. We used C2C12 mouse skeletal myoblasts in this study because skeletal muscle is a major insulin-responsive organ critical for maintaining glucose levels within the body. Moreover, skeletal muscle insulin resistance is the primary defect in diabetes mellitus (Petersen & Shulman, 2002; Shemyakin et al., 2010). Our recent data show that high glucose concentrations increase ROS production and alter mitochondrial function in C2C12 myoblasts in a similar way as it does in myofibers isolated from rat flexor digitorum brevis (Dohl et al, 2018).
2 MATERIALS AND METHODS
2.1 C2C12 cell culture
The mouse myoblast C2C12 cell line (ATCC® CRL-1772™) was cultured at 37°C in a 5% CO2 humidified incubator in Dulbecco's modified Eagle medium. The medium contains 25 mM glucose, 10% fetal bovine serum (FBS), 100 units/ml penicillin, and 100 μg/ml streptomycin. All the curcumin and high glucose treatments described below were added directly into the medium. The cells were passaged every 2–3 days to maintain confluency below 60% to avoid differentiation.
2.2 Curcumin treatments
Curcumin was purchased from Alfa Aesar (Haverhill, MA). Curcumin stock solutions were made by dissolving curcumin in dimethyl sulfoxide (DMSO) and stored at −20°C. On the day of treatment, curcumin stock solutions were directly added to the cells at 1:1,000 dilution so the cell culture medium contained the desired concentrations of curcumin and 0.1% DMSO. Controls were the cells treated with 0.1% DMSO only.
2.3 High glucose treatment
D-glucose were purchased from Sigma Chemical Co. (St. Louis, MO), dissolved in phosphate-buffered saline at a 2.5 M concentration, and sterile filtered. Normal Dulbecco's modified Eagle medium contains 25 mM glucose. For high glucose treatment, we added 4 µl of glucose stock solution per 1 ml Dulbecco's modified Eagle medium so the final concentration of glucose in culture was 35 mM. This treatment was used by us and others to mimic diabetic hyperglycemia in cultured cells (Jain et al., 2010; Yu et al., 2008). For the curcumin pretreatments, the desired curcumin concentrations were added in the medium 30 min before and maintained during the high glucose treatment.
2.4 Cell viability and cell death assays
Cell viability was determined by trypan blue exclusion test with Bio-Rad TC20 automated cell counter per manufacturer's instruction, or by counting living cells under microscope. Caspase 3/7 and caspase 9 activities were measured by CellEvent™ Caspase-3/7 Green detection reagent (Invitrogen, MA) and CaspGLOW™ Fluorescein Active Caspase-9 Staining Kit (Invitrogen), respectively. Dead cells were detected by using an Annexin V Alexa Fluor® 488 apoptosis kit (Invitrogen), as we reported previously (Yu, Deuster, & Chen, 2016).
2.5 Reactive oxygen species (ROS) measurement
Cellular ROS levels were measured by using the fluorescent probes dihydroethidium (DHE; Invitrogen, MA) and 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA, Invitrogen, MA). DHE can be oxidized and then intercalates within DNA, staining the nucleus a bright fluorescent red. ROS also oxidizes the nonfluorescent H2DCFDA and converts it to the highly fluorescent 2′,7′-dichlorofluorescein (DCF). Cells were loaded with 25 µM H2DCFDA or 5 µM DHE at 37°C for 30 min. Images were acquired at room temperature and H2DCFDA or DHE fluorescence intensity was measured using the ImageJ software (NIH, Bethesda, MD).
2.6 Mitochondrial mass and mitochondrial membrane potential measurement
Mitochondrial mass was measured as reported previously by staining live cells with MitoTracker Green (Invitrogen; Agnello, Morici, & Rinaldi, 2008). Mitochondrial membrane potential was evaluated by using tetramethylrhodamine ethyl ester (TMRE, Invitrogen) as described previously (Yu et al., 2016).
2.7 Cobalt quenching calcein assay
The mitochondrial permeability transition (MPT) was assayed by measuring calcein fluorescence quenched by cobalt ion in mitochondria as described previously (Yu et al., 2008). The cells were loaded with 1.0 µM calcein AM (Invitrogen) and 1.0 mM CoCl2 in Hanks’ balanced salt solution (HBSS) buffer at 37°C for 20 min. Cobalt ions under normal conditions do not cross the mitochondrial membrane and therefore only quench cytosolic calcein fluorescence. Upon MPT activation, cobalt ions move into mitochondria to thereby quench mitochondrial calcein fluorescence.
2.8 Fluorescence microscopy
Fluorescence images were viewed and acquired with a Nikon Eclipse Ti epifluorescence microscope equipped with a digital camera. Excitation/emission wavelengths were 358/461 nm for blue fluorescence 4′,6-diamidino-2-phenylindole (DAPI), 480/535 nm for green fluorescence (MitoTracker Green, annexin V, Alexa 488, calcein, and caspase-3/7 Green), and 555/613 nm for red fluorescence (MitoTracker red, TMRE, ethidium, and Alexa 594).
2.9 Subcellular fractionation
Subcellular fractionation was performed as described previously (Yu et al., 2014). Briefly, the cells were suspended in cold isolation buffer (10 mM Hepes pH 7.2, 1 mM EDTA, 320 mM sucrose) containing protease inhibitors and homogenized in a Dounce homogenizer. The homogenate was centrifuged at 700 × g for 8 min. The first supernatant was saved, and the pellet was homogenized and centrifuged again. The two supernatants were pooled and centrifuged together at 17,000 × g for 15 min to obtain the mitochondrial pellet, whereas the supernatant was kept as the cytosolic fraction.
2.10 Western blot analysis
A total of 10 μg of protein from cell lysate was loaded to gels and separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis. Western blot analysis was performed with mouse anticytochrome c (1:1,000 dilution, Santa Cruz Biotech, TX) and mouse antiactin (1:2,000 dilution, Santa Cruz Biotech, TX) as the primary antibodies. Horseradish peroxidase-conjugated antimouse antibodies (1:5,000 dilution, Cell Signaling Technology, MA) were used as secondary antibodies. The bands were visualized with western ECL blotting substrates (Bio-Rad, CA) and images were acquired using a Bio-Rad ChemiDoc MP Imaging System. Western blot densitometry analysis was performed using the ImageJ software (NIH, MD).
2.11 Statistical analysis
Data are expressed as mean ± standard deviations. The significance was evaluated using student's t test when comparing two groups or by analysis of variance when comparing three or more groups. The results were considered significant at p ≤ 0.05.
3 RESULTS
3.1 Concentration- and time-dependent effects of curcumin on cell viability
We first examined cell viability in cultured C2C12 mouse myoblasts incubated with DMSO (control, CTL) and six different concentrations of curcumin (CUR). As shown in Figure 1a,b, curcumin incubation at 1, 2, and 5 μM concentrations for up to 24 hr did not change cell morphology or viability. However, in cells treated with higher concentrations of curcumin (10, 20, and 50 μM), they lost their shape, became round, and detached from the culture surface (Figure 1a right), an indication of cell death. Cell viability assay confirmed that curcumin caused decreased viability in time- and concentration-dependent manners: about 80%, 70%, and 60% cells survived after 24 hr incubation with 10, 20, and 50 μM curcumin, respectively (p < 0.001 vs. control, Figure 1b).
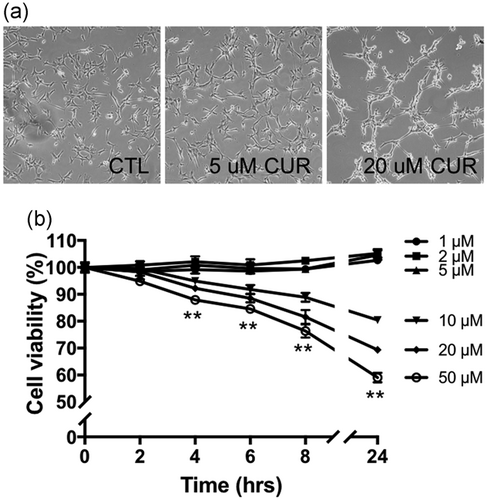
Concentration- and time-dependent effect of curcumin on cell viability. (a) Representative bright field microscopy images of C2C12 cells incubated with DMSO (CTL), 5 μM or 20 μM curcumin (CUR) for 24 hr. (b) cell viability of C2C12 myoblasts incubated with different concentrations of curcumin for indicated time. Values are mean ± SD, **p < 0.01 in cells treated with 10, 20, and 50 µM curcumin for indicated times versus 0 hr; experiments were repeated at least three times independently. DMSO: dimethyl sulfoxide
3.2 Effect of curcumin on cellular ROS levels and mitochondrial mass
Since ROS have been implicated in curcumin-induced cell death (Kim et al., 2016; Moustapha et al., 2015) and mitochondria serve a central role in regulating cell survival (Yu et al., 2015), we doubly labeled C2C12 myoblasts with MitoTracker Green and the ROS probe DHE (Figure 2a). Cells were incubated with 5 or 20 μM curcumin, which represent low and high concentration of curcumin, respectively. The 5 μM curcumin significantly increased MitoTracker Green fluorescence, whereas 20 μM curcumin had little effect (Figure 2a,b). MitoTracker Green stains mitochondria independent of the membrane potential. Therefore, our results indicate that curcumin increases mitochondrial mass at a low concentration (5 μM), but not at a high concentration (20 μM). ROS levels were measured by increased ethidium fluorescence resulting from oxidation of DHE (Figure 2a, bottom). Both 5 and 20 μM curcumin concentrations increased ethidium fluorescence. The increase of ROS in 20 μM curcumin was significantly higher than that in 5 μM curcumin (Figure 2a,c). Both 5 and 20 μM curcumin concentrations-induced ROS production were further confirmed by using another ROS indicator, H2DCFDA (Figure 2d,e).
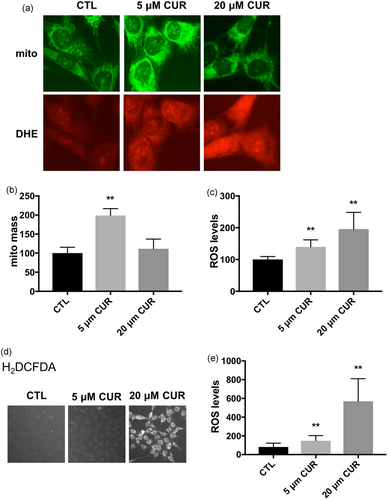
Effect of curcumin on mitochondrial mass and cellular ROS levels. (a) Representative fluorescent microscopy images of C2C12 cells double-labeled with MitoTracker Green (top) and DHE (bottom). The cells were incubated with DMSO (CTL), 5 or 20 μM curcumin (CUR) for 4 hr. Quantification of MitoTracker fluorescence (b) and ethidium fluorescence (c). Values are mean ± SD, **p < 0.01 versus CTL. n = 40 in each treatment, experiments were repeated at least three times independently. (d) Representative fluorescent microscopy images of C2C12 cells labeled with H2DCFDA. The cells were incubated with DMSO (CTL), 5 or 20 μM curcumin (CUR) for 4 hr. (e) Quantification of H2DCFDA fluorescence. **p < 0.01 versus CTL. n = 40 in each treatment, experiments were repeated at least three times independently. DHE: dihydroethidium; DMSO: dimethyl sulfoxide; ROS: reactive oxygen species [Color figure can be viewed at wileyonlinelibrary.com]
3.3 Curcumin-induced ROS increases are time- and concentration-dependent
To further investigate the role of ROS in cell survival and mitochondrial functions, we performed time lapse experiments measuring cellular ROS levels every 2 hr in C2C12 cells incubated with 5 or 20 μM curcumin. The 5 μM curcumin increased ROS levels by 48% (p < 0.01) at 2 hr of incubation. During extended periods of 5 μM curcumin incubation, the ROS levels remained high but no further increase was noted (Figure 3a,b). In contrast, 20 μM curcumin incubation caused more robust and continued increases in ROS levels. Compared with control, the cellular ROS levels were increased by 64%, 85%, 123%, and 148% at 2, 4, 6, and 8 hr (p < 0.001), respectively, with 20 μM curcumin incubation (Figure 3a,b). Pretreatment with N-acetyl l-cysteine (NAC), a glutathione precursor, suppressed both 5 and 20 μM curcumin-induced ROS increase in C2C12 myoblasts (Figure 3c).
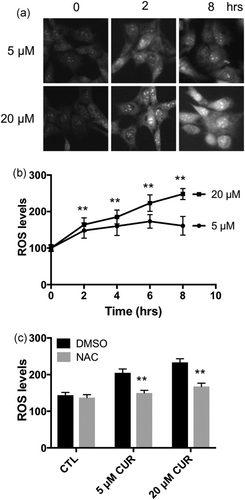
Concentration- and time-dependent effects of curcumin on cellular ROS levels. (a) Representative fluorescent microscopy images of C2C12 cells labelled with DHE and incubated with 5 or 20 μM curcumin for indicated times. (b) ROS levels were measured by quantifying ethidium fluorescence intensity and presented as % to 0 hr of 5 or 20 μM curcumin treatment; **p < 0.01 versus 5 μM curcumin treatment. (c) NAC pretreatment prevented both 5 and 20 μM curcumin-induced ROS increase in C2C12 myoblasts. Values are mean ± SD, **p < 0.01 versus DMSO. n = 40 in each treatment. Experiments were repeated at least three times independently. DHE: dihydroethidium; DMSO: dimethyl sulfoxide; NAC: N-acetyl l-cysteine; ROS: reactive oxygen species
3.4 Increased ROS levels in 20 μM curcumin incubation caused mitochondrial permeability transition and cell death
We observed sustained robust increases in ROS levels and cell death with 20 μM curcumin incubation. High ROS levels could cause mitochondrial permeability transition (MPT) that leads to apoptosis (Yu et al., 2008). Therefore, we examined MPT by the cobalt quenching calcein assay with 20 μM curcumin incubation. In control cells, cobalt ions only quenched cytosol calcein fluorescence whereas mitochondrial calcein fluorescence remained intact. In cells incubated with 20 μM curcumin, mitochondrial calcein fluorescence was significantly decreased, indicating opening of MPT pores as cobalt ions moved into the mitochondria through the pores and quenched mitochondrial calcein fluorescence (Figure 4a). NAC, which abolished curcumin-induced ROS increase (Figure 3c), also prevented MPT (Figure 4a), which suggests that increased ROS levels are likely the cause of MPT upon 20 μM curcumin incubation. Consistent with the MPT data, 20 μM curcumin incubation induced release of cytochrome c from the mitochondria into the cytosol (Figure 4b,c), activation of caspase 3/7 (Figure 4d) and caspase 9 (Figure 4e), and apoptotic cell death (Figure 4f,g). Our results are consistent with previous studies by Moustapha et al. and Oelkrug et al., which curcumin concentrations of 20–25 μM induced apoptosis in Huh-7 human liver cell line and C2C12 myoblasts (Moustapha et al., 2015; Oelkrug et al., 2014). NAC pretreatment blocked all these intrinsic (mitochondrial) pathways of apoptosis. Taken together, these data indicate that 20 μM curcumin incubation induces cell death through elevated ROS production and activation of MPT.
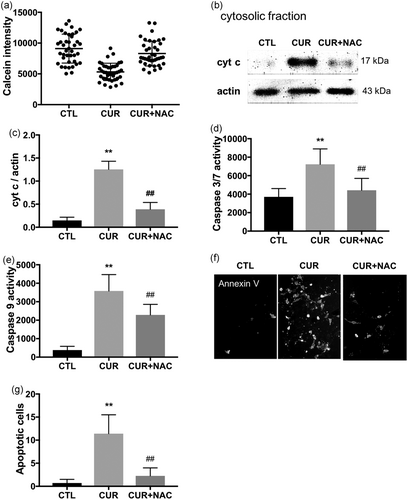
The 20 μM curcumin-induced ROS-dependent MPT and apoptotic cell death. (a) MPT was evaluated by quantifying mitochondrial calcein fluorescence in 40 randomly selected cells. The 20 μM curcumin incubation for 4 hr decreased calcein fluorescence, which was blocked by NAC pretreatment. (b) Representative western blots showing that cytochrome c (cyt c) was released into the cytosol; NAC pretreatment prevented the cyt c release. (c) Quantified data of the west blot. Experiments were repeated at least three times independently. **p < 0.01 versus CTL, ##p < 0.01 versus CUR. Caspase 3/7 (d) and caspase 9 (e) activities were also increased in cells incubated with 20 μM curcumin for 4 hr; NAC pretreatment prevented caspase 3/7 activation. n = 40 in each group, **p < 0.01 versus CTL, ##p < 0.01 versus CUR. (f) Representative fluorescent microscopy images of C2C12 cells labelled with Annexin V and incubated with 20 μM curcumin for 4 hr. (g) apoptotic cell death was detected by Annexin V cell surface labeling. Annexin V positive cells were counted in 20 fields in each group, **p < 0.01 versus CTL, ##p < 0.01 versus CUR; experiments were repeated three times. CTL: control; CUR: curcumin; MPT: mitochondrial permeability transition; NAC: N-acetyl l-cysteine
3.5 The 5-μM curcumin-induced ROS-dependent increases in mitochondrial mass and membrane potential
Curcumin at low concentrations (5 μM) induced a mild increase in ROS levels (Figure 3a,b). We next examined the relationship between increases in ROS levels and mitochondrial mass in C2C12 myoblasts incubated with 5 μM curcumin. As shown in Figure 5a, 5 μM curcumin incubation induced a greater than 2-fold increase in mitochondrial mass (p < 0.001); inhibition of ROS production by NAC prevented the 5 μM curcumin-induced increase in mitochondrial mass. We also examined mitochondrial function by staining the cells with tetramethylrhodamine ethyl ester (TMRE), a preferred fluorescent dye for quantitative measurement of membrane potential (Δψm). To eliminate possible artefactual effect of the increase in mitochondrial mass on membrane potential, we also stained the cells with MitoTracker Green and Δψm was calculated by the ratio of TMRE-to-MitoTracker. The 5 μM curcumin incubation caused a ~20% increase in Δψm (p < 0.001), and the increase of Δψm was blocked by the NAC pretreatment (Figure 5b,c). In summary, our data show that curcumin at low concentrations (5 μM) increases mitochondrial mass and membrane potential, which is dependent on the ROS production.
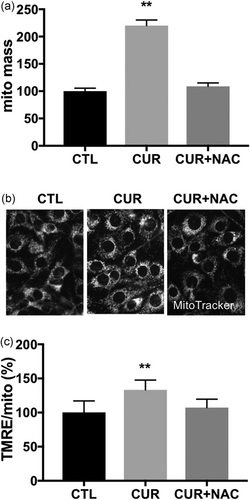
The 5 μM curcumin-induced ROS-dependent increase in mitochondrial mass and membrane potential (Δψm). (a) Mitochondrial mass was quantified by MitoTracker fluorescence. (b) Representative fluorescent microscopy images of C2C12 cells labelled with MitoTracker Red (c) Δψm was calculated by the ratio of TMRE-to-MitoTracker. C2C12 myoblasts were incubated with DMSO (CTL), 5 μM curcumin (CUR), 5 μM curcumin plus NAC for 8 hr. Values are mean ± SD, n = 40. **p < 0.01 versus CTL. CTL: control; DMSO: dimethyl sulfoxide; ROS: reactive oxygen species
3.6 5 μM curcumin prevented high glucose-induced ROS production and cell death
Curcumin is a well-known antioxidant and we found that curcumin at low concentrations increases mitochondrial mass and function (Figure 4). Oxidative stress and mitochondrial dysfunction have been suggested to serve roles in diabetes complications (Yoon et al., 2011; Yu et al., 2008). We tested whether curcumin could prevent high glucose-induced ROS production and cell death. C2C12 myoblasts were initially maintained in media containing 25 mM glucose. For high glucose concentrations (HG), cells were incubated in 35 mM glucose for 24 hr. HG significantly increased ROS levels (Figure 6a) and decreased cell viability (Figure 6b). In our previous studies, we used L-glucose and mannitol as osmotic controls for glucose, none of them induced ROS production or mitochondrial alterations (Yu et al., 2008; Yu, Robotham, & Yoon, 2006).
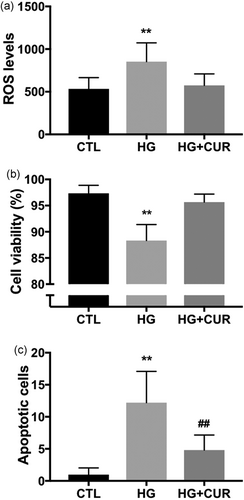
The 5 μM curcumin prevents high glucose-induced ROS production and cell injury. C2C12 cells were maintained in media containing 25 mM glucose (CTL), or transferred to media containing 35 mM glucose (HG) for 24 hr. The 5 μM curcumin treatment prevented HG-induced ROS production (a), decrease in cell viability (b), and apoptotic cell death (c). Experiments were repeated at least three times, curcumin was added to the medium 30 min before adding high glucose. **p < 0.01 versus CTL. CTL: control; HG: high glucose; ROS: reactive oxygen species
Annexin V binding assay showed increased numbers of positive cells in high glucose conditions (Figure 6c), which suggests that HG-induced cell death includes apoptosis. The 5 μM curcumin treatment not only normalized ROS levels (Figure 6a), but also prevented decreases in cell viability (Figure 6b) and apoptotic cell death (Figure 6c) under 35 mM high glucose conditions. Since the antioxidant effects of curcumin may depend upon intracellular redox conditions (Giordo et al., 2013), we also pretreated cells incubated in 35 mM high glucose conditions with 20 μM curcumin and found that curcumin at high concentration did not prevent ROS increases (data not shown). Taken together, our results suggest that mitochondria respond differentially depending on curcumin concentration-dependent induction of ROS: a low concentration of curcumin induces slight increases in ROS, which in turn preserves mitochondrial integrity and function, and ameliorates hyperglycemia-induced oxidative stress and cell injury.
4 DISCUSSION
In this study, we observed time- and concentration-dependent effects of curcumin on cellular ROS levels, mitochondrial function, and cell viability. Curcumin at low concentrations induced a mild increase in ROS levels, which was associated with an increase in mitochondrial mass and membrane potential. At high concentrations, curcumin produced a more pronounced increase in ROS levels, which was associated with opening of the MPT pore, release of cytochrome c from mitochondria into the cytosol, activation of caspase, and eventual apoptotic cell death. Interestingly, under conditions of simulated hyperglycemic conditions, 5 μM curcumin prevented high glucose-induced oxidative cell injury.
Because curcumin is lipophilic and has poor intestinal permeability, its bioavailability is very low (Moustapha et al., 2015; Prasad, Tyagi, & Aggarwal, 2014). The literature remains controversial as to the availability of curcumin in plasma after administration, and most studies report only negligible or small amounts of curcumin can be detected in the circulation, based on animal and human studies (Prasad et al., 2014). Numerous studies over the past decades have been performed to develop different formulations of curcumin and improve its bioavailability (Begum et al., 2008; Prasad et al., 2014). Recent data show that oral administration of curcumin can increase blood curcumin concentrations to as high as 4 µM in rodents (Shoba et al., 1998), and 2 µM in humans (Cheng et al., 2001), which are within the range of the low concentration of curcumin (1–5 µM) used in the current study. Nevertheless, uptake of curcumin is significantly higher in tumor cells compared with normal cells (Kunwar et al., 2008).
A recent study reported that curcumin increases ROS levels in cervical cancer cells, but not in normal epithelial cells (Kim et al., 2016). In the current study, we found that both low and high concentrations of curcumin increase cellular ROS levels in cultured C2C12 myoblasts (Figure 3). The low concentration (5 µM) of curcumin-induced ROS production was mild; the high concentration (20 μM) of curcumin caused a more robust and constant increase in ROS levels during the extended period of incubation, which was associated with cell injury. Together, our findings as well as others support the hypothesis that curcumin regulation of cellular redox status is dependent on the concentrations.
Cellular ROS levels reflect the balance between ROS production and antioxidant defenses. The mechanism by which curcumin induces ROS production is unclear. Intracellular ROS sources include NADPH oxidase, nitric oxide synthase, xanthine oxidase, peroxisome beta-oxidation of fatty acids, endoplasmic reticulum (ER) stress, and the mitochondrial electron transport chain (ETC; Holmstrom & Finkel, 2014; Zorov, Juhaszova, & Sollott, 2014). Cells uptake curcumin and accumulate it at the plasma membrane (Kunwar et al., 2008), nucleus (Kunwar et al., 2008), ER (Moustapha et al., 2015), and lysosomes (Moustapha et al., 2015), with the least being found in mitochondria (Kunwar et al., 2008). Indeed, we have performed additional experiments and found that mitochondria are not the site of curcumin-induced ROS production (data not shown).
Curcumin accumulates in the ER and releases calcium from the ER to the cytosol, which is then taken up by mitochondria and can lead to mitochondrial dysfunction and more ROS production (Moustapha et al., 2015). In our study, the low concentration (5 µM) of curcumin-induced ROS production was mild and peaked at 2 hr after the treatment (Figure 3). Because curcumin increases the expression of antioxidant enzymes such as glutathione peroxidase (Kunwar et al., 2009; Panchatcharam et al., 2006), catalase (Kunwar et al., 2009; Panchal et al., 2008; Panchatcharam et al., 2006), superoxide dismutase (Barik et al., 2005; Kunwar et al., 2009; Panchatcharam et al., 2006), and heme oxygenase 1 (Kunwar et al., 2009; Motterlini et al., 2000), the ROS production in cells treated with low concentration of curcumin is balanced by the antioxidant defense system: intracellular ROS is maintained at physiological levels for cell signaling pathways (Holmstrom & Finkel, 2014). On the other hand, the high concentrations of curcumin continue increasing ROS production, and when the ROS levels exceed a certain threshold, they appear to perturb the cellular redox balance and shift cells into a state of oxidative stress, and eventual cell death (Figures 3 and 4).
We observed that the low concentration of curcumin increased mitochondrial mass and activated mitochondrial function; the high concentration of curcumin opened the MPT pore and released cytochrome c, thereby promoting cell death. It is noteworthy that the low and high concentrations of curcumin induced opposite changes in mitochondria and these changes appear to be tightly correlated with cellular ROS levels. Mitochondria are a major target for ROS, which as a group of free radicals can cause protein oxidation, DNA damage and lipid peroxidation at pathological levels (Holmstrom & Finkel, 2014; Schieber & Chandel, 2014). Excessive ROS levels directly oxidize MPT components and induce MPT pore opening (Bernardi, Rasola, Forte, & Lippe, 2015; Halestrap, 2009). As discussed previously, curcumin also releases calcium from the ER (Moustapha et al., 2015); calcium is taken into mitochondria where it can bind to the matrix side of the MPT pore to cause conformational changes and opening of the pores (Brookes, Yoon, Robotham, Anders, & Sheu, 2004; Ichas & Mazat, 1998). Nevertheless, our studies show that inhibition of ROS by NAC prevented the decrease in mitochondrial calcein fluorescence in cells incubated with 20 μM curcumin (Figure 4a), which strongly suggests that ROS participate in curcumin-induced opening of the MPT pores. Sustained opening of the MPT pores results in release of cytochrome c from the mitochondrial intermembrane space into the cytosol and subsequent caspase activation, which ultimately leads to cell death (Figure 4b–d).
In contrast to the effects of excessive ROS on cell function, a small increase at physiological levels activates signaling pathways for regulating cell function, where redox signaling activates transcription factors involved in mitochondrial biogenesis (Yoboue & Devin, 2012). In rat hepatoma cells, ROS increase nuclear expression of mitochondrial transcription factor A (TFAM) through phosphorylation of nuclear respiratory factor 1 (NRF-1; Piantadosi & Suliman, 2006). In skeletal muscle, H2O2 treatment activates AMPK and enhances expression of PGC-1alpha, the master regulator of mitochondrial biogenesis (Irrcher, Ljubicic, & Hood, 2009). These findings are consistent with our results that low concentrations of curcumin induce mild increases in ROS production which in turn increases mitochondrial mass and activity (Figure 2). Inhibition of ROS by NAC abolished these changes in mitochondria (Figure 5), further confirming that curcumin increases mitochondrial mass and activity through a mild increase in ROS production.
Oxidative stress and mitochondrial dysfunction are hallmarks of many human diseases, including diabetes (Rochette, Zeller, Cottin, & Vergely, 2014; Yoon et al., 2011). Mitochondria are the main source of ROS in diabetes, and mitochondrial destruction contributes to hyperglycemia-induced oxidative stress (Yoon et al., 2011; Yu et al., 2006; Yu et al., 2008). Curcumin as an antioxidant agent has been used for the treatment of diabetes (Meng et al., 2013; Zhang et al., 2013). To further explore the role of curcumin on mitochondrial function, we tested the effects of low dose curcumin under altered physiological conditions, specifically under simulated conditions of diabetes: high cell glucose. Importantly, curcumin (5 µM) treatment prevented high glucose-induced ROS production and cell death (Figure 6), potentially through preserving mitochondrial integrity as noted above. Therefore, our study provides insights into how curcumin might contribute to the relief of oxidative stress and cell injury.
In summary, a concentration-dependent regulation of ROS production and mitochondrial function by curcumin was noted in cultured mouse C2C12 myoblasts (Figure 7). ROS levels increased in cells incubated in both high and low concentrations of curcumin, but high concentrations induced a sustained and robust increase in ROS, which led to opening of the MPT pores and subsequent cytochrome c release, caspase activation, and cell death. In contrast, a small increase in ROS at low concentrations of curcumin incubation activated signaling pathways to promote mitochondrial health and protect cells from oxidative damage. Our study shows that ROS balance in cells differentially responds to curcumin concentrations: cell protection or death. New or alternate formulations should be developed to manipulate curcumin concentrations within cells. Overall, curcumin may be an effective therapeutic target for cancer, diabetes, and other mitochondrial diseases.
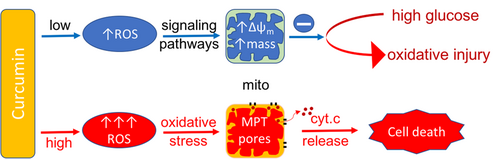
Concentration-dependent regulation of ROS levels and mitochondrial function. ROS levels increase in cells incubated in both high and low concentrations of curcumin, but a small increase in ROS at low concentrations of curcumin incubation activates signaling pathways to promote mitochondrial health and protect cells from hyperglycemia-induced oxidative damage. In contrast, high concentrations induce a sustained and robust increase in ROS, which leads to opening of the MPT pores and subsequent cytochrome c release, caspase activation, and cell death. MPT: mitochondrial permeability transition; ROS: reactive oxygen species [Color figure can be viewed at wileyonlinelibrary.com]
ACKNOWLEDGMENTS
This study was supported by the Defense Heath Program Center Alliance for Nutritional and Dietary Supplement Research HU0001-14-1-003.
CONFLICTS OF INTEREST
The authors declare no conflict of interest. The views expressed are those of the authors and do not reflect the official position of the USUHS, United States Department of Defense.




