Autophagy participates in cyst breakdown and primordial folliculogenesis by reducing reactive oxygen species levels in perinatal mouse ovaries
Abstract
The reserve of primordial follicles, which serves all oocytes for the female reproductive lifespan, is established a few days after birth in mice. During this process, more than half of the oocytes are primarily eliminated by apoptosis. Autophagy, the conserved intracellular process maintaining cellular homeostasis, serves as a protective mechanism for oocyte survival. In the current study, we speculate a new role for autophagy during primordial folliculogenesis. Active autophagy was observed in perinatal ovaries from 16.5 days post coitus to 3 days post parturition. The inhibition of autophagy by 3-methyladenine (3-MA) increased the number of cyst oocytes and delayed follicle formation in vivo and in organ cultures. Furthermore, the reactive oxygen species (ROS) level was elevated in ovaries treated with 3-MA, while N-acetylcysteine, an oxidant, alleviated the inhibitory effect of 3-MA on primordial folliculogenesis. Additionally, the expression of growth differentiation factor 9 and transforming growth factor β1, which regulates follicle activation, was decreased after 3-MA treatment. These data suggest that the physiological level of autophagy in perinatal ovaries regulates germ cell cyst breakdown and primordial follicle assembly by ROS clearance and exerts extensive effects on further follicular development.
1 INTRODUCTION
In mammals, a fixed population of primordial follicles, which are formed during early life, lasts the entire female reproductive lifespan to serve as the source of oocytes. The primordial folliculogenesis occurs in a stepwise fashion. Primordial germ cells synchronously proliferate to form germ cell cysts, in which germ cells are connected by intercellular bridges due to the incomplete cytokinesis of oogonial division and oocyte aggregation (Lei & Spradling, 2013). Following the germ cell entry of meiosis and arrest of the first meiotic division at the diplotene stage, the oocytes break apart from each other, which is called cyst breakdown, and become encapsulated in primordial follicles consisting of one oocyte and a single layer of squamous pregranulosa cells. In mice, the breakdown of cysts initiates as early as 17.5 days post coitus (dpc), and by 5 days postparturition (dpp), the endowment/reserve of primordial follicle is established (Pepling & Spradling, 2001).
Germ-cell cyst breakdown and primordial follicle assembly are accompanied by a loss of approximately two-thirds of the oocytes. Such oocyte loss may trigger cyst breakdown, mediated by developmentally programmed cell death primarily through apoptotic pathways (Pepling & Spradling, 2001). However, the mechanisms governing oocyte loss during cyst breakdown are not well understood. Recent studies have implicated autophagy as another cell death mechanism during cyst breakdown.
Macroautophagy (hereafter referred to as autophagy) is a highly conserved mechanism that maintains cellular homeostasis through degrading and recycling unneeded proteins, damaged cytoplasmic organelles and other macromolecules. In addition to the primary function to survive nutrient starvation conditions by recycling cellular components, autophagy participates in numerous development and differentiation processes, including cell growth, intracellular quality control, and degradation for subsequent biogenesis of organelles such as cilia and acrosome (Mizushima & Levine, 2010; Tang et al., 2013; H. Wang et al., 2014). Canonically, autophagy is initiated from an isolated membrane, followed by the formation of a double-membrane autophagosome, which encloses cytoplasmic components and organelles. Then, the autophagosome moves and fuses with lysosomes, forming an autolysosome, which is subsequently degraded and recycled by the lysosome (Mizushima & Komatsu, 2011). In the initiation stage of autophagosome formation, autophagy-related protein 7 (ATG7) activates light chain 3 (LC3) and facilitates the formation of the ATG12–ATG5–ATG16L1 complex. Both the complex and activated LC3 conjugate to the membrane and drive membrane expansion and vesicle completion to form an autophagosome. Beclin-1 (BECN1), the mammalian counterpart of the yeast ATG6 protein, is also integral to the vesicle nucleation phase of autophagosome formation. The last step is the fusion of the autophagosome with the lysosome for subsequent complete degradation (Kanninen, De Andrade Ramos, & Witkin, 2013; Mizushima, Yoshimori, & Ohsumi, 2011).
Autophagy is considered as an oocyte survival mechanism against apoptosis to maintain the endowment of primordial follicle in the ovary (Pepling, 2012). Lysosomal amplification has been observed in oocytes at birth (Rodrigues, Limback, McGinnis, Plancha, & Albertini, 2009), and LC3 is transiently induced in the neonatal ovaries (Song et al., 2015), indicating that active autophagy and massive oocyte loss concurrently occur. With autophagy compromised by the disruption of Becn1 or Atg7, perinatal female mice exhibit the premature loss of oocytes (Gawriluk et al., 2011). Moreover, the germ cell-specific knockout of Atg7 also leads to female subfertility, whereby severe germ cell over-loss occurs postnatally, and autophagy protects oocytes from over-loss by apoptosis in starved neonatal ovaries culture (Song et al., 2015). Since there is basal level of cellular autophagy for the physiological turnover of damaged organelles and long-lived proteins, it is difficult to determine whether autophagy is involved in the regulation of primordial folliculogenesis in a knockout mice model, as the overall dysfunction of autophagy disrupts cellular physiological activity.
In the current study, activated autophagy was observed in prenatal and postnatal ovaries during cyst breakdown and primordial follicle formation. The inhibition of autophagy by 3-MA induces the persistence of germ cell cysts in vivo and in ovary cultures. Further investigation revealed that autophagy reduces accumulated reactive oxygen species (ROS) in the ovary, and disrupted autophagy also disrupted the expression of key factors regulating postnatal folliculogenesis. Their results suggest that autophagy plays another role in the regulation of perinatal primordial folliculogenesis under physiological conditions.
2 MATERIALS AND METHODS
2.1 Animals and ethics statement
The Kunming White mice were purchased from Medical and Laboratory Animal Center of Chongqing, China (Certificate No. SCXK (YU) 20070001), and maintained in the Chongqing Medical University Animal Care Facility with free access to water and food under a light–dark cycle. Female mice 6–8-week old) were mated with fertile adult male mice to induce pregnancy. Mice with a vaginal plug at noon were considered to be at 0.5 dpc.
All experiments were performed in accordance with the Institutional and National Guidelines and Regulations and were approved by the Chongqing Medical University Animal Care and Use Committee. All surgeries were performed under sodium pentobarbital anesthesia, and all the efforts were made to minimize suffering.
2.2 3-Methyladenine (3-MA) administration
Fetuses were normally delivered at 19.5 dpc and were designated as 0 dpp; female pups were separated and littermates were randomly divided into two groups. To examine the effect of autophagy on primordial follicle formation, female pups were treated on 0–2 dpp with autophagy inhibitor 3-MA (15 µg·g body weight (bw)−1·day−1, dissolved in phosphate buffered saline [PBS]) by intraperitoneal injection (3-MA group), and pups in the other group were given PBS with the same volume (control group). Each group used five pups. The neonatal ovaries were collected on 3 dpp and processed for histological and morphological evaluation.
2.3 Ovary organ culture and chemicals
Ovaries from fetal mice were dissected carefully on the desired day, and transferred into individual wells of a six-well plate with 1 ml serum-free Dulbecco’s modified Eagle’s medium (DMEM)/F12 media (Hyclone, GE Healthcare Life Sciences, UT) as we described previously (Mu et al., 2015). Media was equilibrated for 2 hr before use in a 37°C incubator with 5% CO2 and was half changed every other day.
The autophagy inhibitor 3-MA and mammalian target of rapamycin complex 1 (mTORC1)-specific inhibitor rapamycin were purchased from Sigma Aldrich (Saint Louis, MO). The oxidant N-acetyl l-cysteine (NAC) was purchased from Beyotime Institute of Biotechnology (Jiangsu, China). All the chemicals are first dissolved in PBS and kept at −20℃, and then redissolved in DMEM/F12 to add into the media before use.
2.4 Histological procedures and immunofluorescence
Ovaries were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned to a thickness of 5 μm. After dewaxing and rehydration, hematoxylin staining was carried out according to standard laboratory procedures.
For immunofluorescence, after antigen unmasking with 1 mM EDTA (pH 8.0), the sections were immunostained overnight at 4°C with primary antibodies: LC3 (1:50; Cell Signaling Technology Danvers, MA), lysosome-associated membrane protein 2 (LAMP2, 1:50; Abcam, Cambridge, UK), mouse Vasa homolog (MVH, 1:500; Abcam). Subsequently, the sections were incubated with FITC-conjugated secondary antibodies at 37°C for 90 min. Propidium iodide staining was performed to visualize cellular nucleus. Finally, vectashield mounting medium (Beyotime Institute of Biotechnology, Jiangsu, China) was applied to the slides.
2.5 Germ-cell counts
Ovarian germ cells and follicles were quantified according to a widely used approach (Flaws, Hirshfield, Hewitt, Babus, & Furth, 2001). In brief, 5 μm serial sections across the entire ovary were stained with hematoxylin and every fifth section was analyzed for the presence of oocytes and primordial follicles. Three to six different ovarian samples from each group were analyzed. Oocytes were characterized by the cellular size and morphology. A primordial follicle was defined as an individual oocyte surrounded by several flattened pregranulosa cells. Single oocytes were unassembled germ cells that pregranulosa cells did not completely surround them.
2.6 RNA extraction and real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA of 20 fetal ovaries was extracted with the RNAiso Plus (Takara Biotechnology, Dalian, China) according to the manufacturer’s protocol. OD260/OD280 of RNA was 1.8–2.0. Reverse transcription (RT) was conducted with oligo (dT) using PrimeScript RT Master Mix (Takara Biotechnology, Tokyo, Japan) from 1 µg RNA. Real-time PCR was performed using SYBR Premix EX Tag Kits (Takara Biotechnology, Tokyo, Japan) on a Bio-Rad real-time system (CFX Connect, Hercules, CA). The PCR primers used are provided in the Supporting information Data. The relative quantity of each gene was calculated by the method, and normalized to the endogenous β-actin reference gene.
2.7 Western blot analysis
Total protein from 20 fetal ovaries was extracted and protein concentrations were measured using a bicinchoninic acid (BCA) procedure. Proteins of 30 μg were separated with 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then electrophoretically transferred onto polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA). After blockage by 5% nonfat dry milk, membranes were incubated overnight at 4°C with the primary antibodies: LC3 (1:1,000), ATG5 (1:750; Cell Signaling Technology, USA), BECN1 (1:1,000; Cell Signaling Technology), p62 (1:1,000, Cell Signaling Technology), LAMP2 (1:1,000). After three times washing in TBST, the membranes were incubated for 1 hr at room temperature with the appropriate secondary antibody. Finally, the membranes were visualized using an enhanced chemiluminescence detection system (Millipore, Burlington, MA). Levels of β-actin were measured at the same time as an internal control.
2.8 Measurement of ROS levels
The fetal ovaries were collected after the culture, and the dispersed ovarian cells from each group were obtained by trypsin treatment of 3 min. And then, the reactive oxygen species (ROS) levels of the samples were analyzed by Reactive Oxygen Species Assay Kit (Beyotime Institute of Biotechnology, Shanghai, China) according to the manufacturer’s instructions. In brief, ovarian cells from 15 ovaries were collected in each group, washed by PBS and incubated with fluorescent probes, 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA), for 20 min with 5% CO2 at 37°C. Then, cells were washed by serum-free cell culture medium. Ovarian cells with ROS-related fluorescence intensity were first viewed directly using a fluorescence microscope (BX43; Olympus, Tokyo, Japan). Finally, ROS levels were detected by the microplate reader (Bio-Rad, Hercules, CA).
2.9 Statistical analysis
Experiments were repeated at least three times and the data are presented as the mean ± SEM. There were three to six ovaries per group for the germ cell counting. An analysis of variance was used for comparison of mean values across the groups and multiple comparisons were made by LSD t test. SPSS version 17.0 software (Chicago, IL) was used for statistical analysis. Values of p < 0.05 were regarded as statistically significant.
3 RESULTS
3.1 Autophagy is activated in oocytes during primordial folliculogenesis
We examined the expression of several mediators of autophagy in the pooled ovaries from 16.5 dpc to 3 dpp to determine whether autophagy occurs during germ-cell cyst breakdown and primordial follicle assembly. ATG5 and BECN1 are integral in the vesicle nucleation and expansion phases for autophagosome formation, and p62 and LAMP2 are involved in the degradation and retrieval phases in the final stage of autophagy.
In neonates, autophagy is induced in organs during the early neonatal starvation period, and reaches its maximal level in the ovaries during 3–6 hr after birth (Kuma et al., 2004; Song et al., 2015). By comparing prenatal and postnatal messenger RNA (mRNA) expression using qRT-PCR, all the autophagy marker genes were strongly upregulated at 17.5 dpc, reached the highest level at approximately 19.5 dpc, and subsequently decreased after that (Figure 1a). This finding was further supported by western blot analysis to analyze protein expression in developing ovaries (Figure 1b). Similarly, the protein expression levels of ATG5, BECN1, p62, and LAMP2 remained high from 17.5 to 19.5 dpc. The activation of LC3 is critical for autophagy, and the active form of LC3 (13 kDa) was also upregulated from 17.5 to 19.5 dpc.
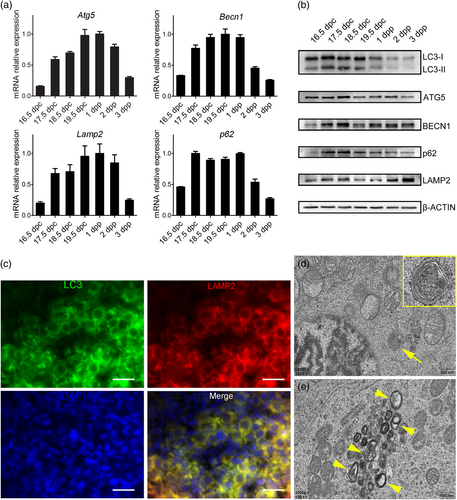
Active autophagy is observed in perinatal ovaries. (a) mRNA expressions of autophagy mediators in ovaries from 17.5 dpc to 3 dpp by qRT-PCR. (b) Protein expressions of autophagy mediators in ovaries from 17.5 dpc to 3 dpp by western blot analysis. (c) The immunofluorescence analysis for autophagosome marker LC3 and lysosome marker LAMP2. The colocalization of LC3 and LAMP2 represents the formation of autolysosome. Oocytes can be recognized by the larger cell size and round nucleus, while somatic cell nucleus is flattened. Scale bar = 20 μm. (d) A TEM image from an 18.5 dpc ovary, showing the isolated membrane is expanding to enclose one disorganized mitochondria to form an autophagosome in an oocyte (yellow arrowhead). Scale bar = 500 nm. (e) A TEM image from an 18.5 dpc ovary, showing numerous autophagosomes containing mitochondria-like contents in an oocyte (yellow arrows). Framed areas were enlarged at the top right corner. Scale bar = 500 nm. LC3: light chain 3; dpc: days post coitus; dpp: days postparturition; LAMP2: lysosome-associated membrane protein 2; mRNA: messenger RNA; qRT-PCR: quantitative reverse transcription polymerase chain reaction; TEM: transmission electron microscope [Color figure can be viewed at wileyonlinelibrary.com]
LC3 and LAMP2 are membrane constituents of functional autophagosomes and lysosomes, respectively. The colocalization of LC3 and LAMP2 represents the formation of the autolysosome, which degrades the enclosed materials. By immunofluorescence, we observed that both LC3 and LAMP2 were expressed in the cytoplasm of oocytes in the germ-cell cysts (cells with round nucleus and connected cytoplasm) in 18.5 dpc ovaries (Figure 1c), while somatic cells only showed background expression (cells with flattened nucleus). Additionally, TEM analysis revealed that numerous autophagosomes or lysosomes containing the segregation and degradation of organelles, primarily mitochondria-like, were ultrastructurally recognized in the oocytes of 18.5 dpc ovaries (Figure 1d).
These findings suggest that autophagy is activated in oocytes during the initial germ-cell cyst breakdown and follicle histogenesis.
3.2 Inhibition autophagy impairs primordial folliculogenesis by neonatal injection of 3-MA
To address the function of autophagy in the neonatal mouse ovary, we blocked autophagy by the injection of the autophagy inhibitor 3-MA into neonatal mice. Mice of 0 dpp (at birth) were administered a daily injection of 3-MA (15 µg·g bw−1·day−1) for 3 days and the 3-dpp ovaries were collected for further analysis. The dosage of 3-MA was determined in a preliminary study (data in press). The mice in the control group were injected with equal volumes of PBS. Western blot analysis showed that the autophagy mediators ATG5 and BECN1 were downregulated in the pooled ovaries of 3-MA treated group, indicating that the initial process of autophagy was inhibited, and the accumulated p62 manifested the failed autolysosome formation by 3-MA injection (Figure 2e).
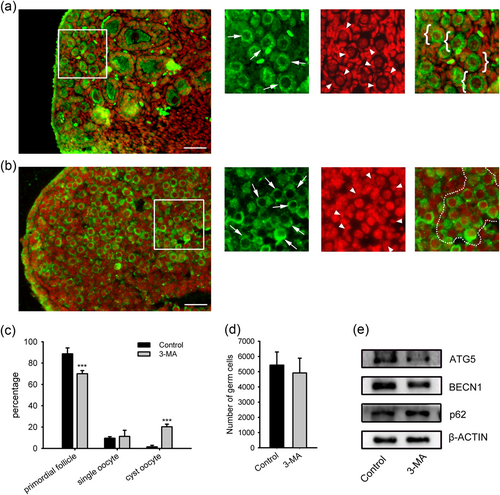
The effect of 3-MA injection to neonates on primordial folliculogenesis. Neonatal mice on 0 dpp were given a daily injection of 15 μg/g bw 3-MA for 3 days. (a) Paraffin sections from a control mouse of 3 dpp. (b) Section from a mouse injected with 3 MA on 3 dpp. Enlarged images of the framed area were on the right side. MVH antibody (green) to visualize oocytes, PI (red) to visualize nucleus. Oocytes are indicated by arrows, and pregranulosa cells are indicated by arrowheads. White circles indicate primordial follicles which are defined as oocytes individually enclosed in pregranulosa cells, and white brackets indicate germ-cell cyst. Scale bar = 40 μm. (c) Counting analysis of primordial follicles and single oocytes. (d) Total oocytes number. *p < 0.05; ***p < 0.01, n = 3. 3-MA: 3-methyladenine; MVH: mouse Vasa homolog; PI: propidium iodide [Color figure can be viewed at wileyonlinelibrary.com]
Immunofluorescence staining for the MVH (a cytoplasmic germ cell molecular marker) demonstrated that the neonatal injection of 3-MA leads to delayed folliculogenesis. In littermate control ovaries at 3 dpp, most MVH-positive cells had already assembled into individual primordial follicles (Figure 2a). However, in the 3-MA injection group, most MVH-positive cells, particularly near the cortical surface of the ovary, remained in germ-cell cysts (Figure 2b). To quantify primordial folliculogenesis in 3-MA-treated and control littermate mice, we counted serial sections of ovaries isolated at 3 dpp, and primordial follicles, unassembled single oocytes, and oocytes in the germ-cell cysts were presented as the percentage of the total germ cells. The 3-MA injected neonatal mouse ovaries contained significantly fewer follicles than that of the control (68.00 ± 4.95% vs. 90.35 ± 3.92%), and the cyst oocytes were increased (20.02 ± 2.73% vs. 1.66 ± 1.21%; Figure 2c). However, the total oocyte numbers between groups were not statistically significant (Figure 2d). These data indicate that the inhibition of autophagy by the neonatal injection of 3-MA disrupts the breakdown of germ cell cysts and the assembly of primordial follicles.
3.3 3-MA inhibits germ-cell cysts breakdown and primordial follicles assembly in ovary organ cultures
Since autophagy mediators were abundantly expressed prenatally (Figure 1), we adjusted the autophagy inhibition period in ovary organ cultures accordingly. The ovaries at 17.5 dpc were isolated and placed in culture with or without 3-MA at different concentrations. Cyst breakdown, primordial follicle formation, and oocytes survival were analyzed after the 5-day culture (3 dpp, equally). The downregulation of ATG5 and BECN1 and upregulation of p62 by immuneblotting showed that autophagy in the cultured ovaries was inhibited by 3-MA treatment (Figure 3e).
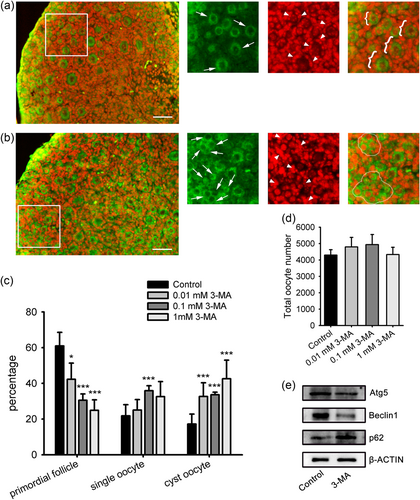
The effect of 3-MA on primordial folliculogenesis in ovary organ culture. Ovaries of 17.5 dpc were cultured with 3-MA at different concentrations for 5 days. (a) Paraffin section from the control group. (b) Section from an ovary cultured with 0.1 mM 3-MA. MVH antibody (green) to visualize oocytes, PI (red) to visualize nucleus. Scale bar = 40 μm. (c) Counting analysis of primordial follicles and single oocytes. (d) Total oocytes number. (e) Protein expression analysis of ATG5, BECN1 and p62 by western blot analysis. ***p < 0.01, n = 4. ATG5: autophagy-related protein 5; BECN1: beclin 1; dpc: days post coitus; 3-MA: 3-methyladenine; MVH: mouse Vasa homolog; PI: propidium iodide [Color figure can be viewed at wileyonlinelibrary.com]
In accordance with the in vivo model, 3-MA treatment inhibited germ cell cyst breakdown and primordial follicle assembly in the ovary cultures. Representative images of the ovary cultured with 0.1 mM 3-MA contained larger cysts compared with the control (Figure 3a,b). Germ-cell counts showed that the 3-MA treatment dose dependently decreased the percentage of primordial follicles (0.01 mM, 42.29 ± 9.01%; 0.1 mM, 30.53 ± 3.52%; 1 mM, 24.86 ± 5.89%; control, 60.96 ± 7.58%), and increased the proportion of cysts oocytes (0.01 mM, 32.66 ± 7.68%; 0.1 mM, 33.59 ± 1.46%; 1 mM, 42.54 ± 10.46%; control, 17.22 ± 5.54%; Figure 3c). However, there were no significant differences between the groups in the proportion of single oocytes and total oocyte number (Figure 3c,d). Collectively, these data revealed that the inhibition of autophagy by 3-MA leads to remnants of germ-cell cysts in ovary, suggesting that autophagy is involved in the physiological progress of germ-cell cyst breakdown and primordial follicle assembly.
3.4 Inducing autophagy by rapamycin has no effect on primordial folliculogenesis
Autophagy is downregulated by mTOR signaling, thus the mTOR inhibitor rapamycin is widely used as an autophagy inducer. To further investigate the role of autophagy in the developing ovaries, rapamycin was added to the culture to determine whether autophagy induction accelerates primordial folliculogenesis. We collected 17.5 dpc ovaries cultured with or without rapamycin at 1, 10, and 100 nM and examined the cyst breakdown, primordial follicle formation and oocytes survival after 3 days of culture (equal to 1 dpp, when cysts breakdown and follicles start to assemble under physiological condition).
As shown in Figure 4, rapamycin at different concentrations slightly promoted primordial follicle formation with normal morphology (1 nM, 16.21 ± 4.95%; 10 nM, 18.64 ± 2.88%; 100 nM, 18.28 ± 2.50%; control, 13.01 ± 2.94%), but there were no statistically significant differences in the percentages of either primordial follicles, single oocytes, cyst oocytes or total oocytes number. Meanwhile, the increased expression of ATG5 and the decreased expression of p62 by western blot analysis confirmed that autophagy was induced by the treatment of 100 nM rapamycin on the fetal ovary (Figure 4b). These results suggested that the induction of autophagy does not accelerate germ-cell cysts breakdown and primordial follicles assembly. This result is inconsistent with that of another report showing that rapamycin delays primordial follicle formation (J. Zhang et al., 2017), for different treatment periods (17.5 dpc to 1 dpp vs. 0 to 1 dpp) and at higher concentrations (100 vs. 250 nM). Since rapamycin is an mTOR inhibitor, the role of mTOR in primordial folliculogenesis merits further investigation.
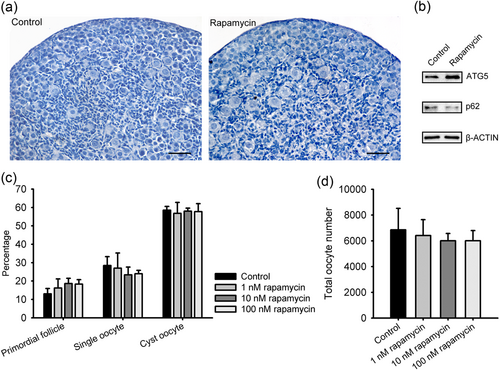
The effect of rapamycin on primordial folliculogenesis in ovary organ culture. Ovaries of 17.5 dpc were cultured with rapamycin at different concentrations for 3 days. (a) Paraffin sections from the control ovary and the ovary treated with 100 nM rapamycin. Scale bar = 30 μm. (b) Protein expression analysis of ATG5 and p62 by western blot analysis in the control ovary and the ovary treated with 100 nM rapamycin. (c) Counting analysis of primordial follicles and single oocytes. (d) Total oocytes number. n = 3. ATG5: autophagy-related protein 5; dpc: days post coitus [Color figure can be viewed at wileyonlinelibrary.com]
3.5 Autophagy helps ROS clearance to contribute to primordial folliculogenesis
Considering the numerous autophagosomes with enclosed mitochondria-alike organelles and abundant mitochondria accumulation in oocytes during primordial folliculogenesis (Lei & Spradling, 2016), we speculated that autophagy reduces ROS during this process. We collected 17.5 dpc ovaries cultured with or without 0.1 mM 3-MA for 5 days (= 3 dpp), and examined the ROS levels. Cellular ROS oxidizes the DCFH-DA probe to produce DCF, which displays green fluorescence when excited at 488 nm. The cells from ovaries treated with 3-MA had stronger fluorescence intensity (Figure 5a). By chemiluminescence analysis, the optical density of 3-MA group was significantly increased compared to the control (Figure 5b), suggesting that the inhibition of autophagy increases ROS levels in the ovary.
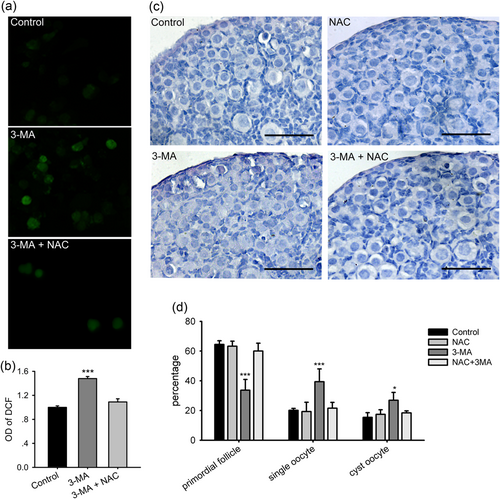
The 3-MA treatment increases ROS levels in the perinnial ovary, and NAC reverses the inhibitory effect of 3-MA on primordial folliculogenesis. Ovaries of 17.5 dpc were cultured for 5 days with different treatments. (a) Dispersed ovarian cells incubated with DCFH-DA. (b) The analysis of fluorescence intensity of DCF by a microplate reader. (c) Paraffin sections from the control ovary and the ovary treated with NAC, 3-MA, and NAC plus 3-MA. Scale bar = 30 μm. (d) Counting analysis of primordial follicles and single oocytes. *p < 0.05; ***p < 0.01, n = 3. DCF: dichlorofluorescein; DCFH-DA: 2′,7′-dichlorofluorescein diacetate; dpc: days post coitus; NAC: N-acetylcysteine; 3-MA: 3-methyladenine; ROS: reactive oxygen species [Color figure can be viewed at wileyonlinelibrary.com]
To investigate whether the increased ROS level was involved in the disruption of primordial folliculogenesis, NAC, a wide spreading oxidant, was added to the culture. The ovaries were collected on 18.5 dpc and cultured for 4 days (equal to 3 dpp). The ovaries were treated with 10 μM NAC, 0.1 mM 3-MA, and 10 μM NAC plus 0.1 mM 3-MA. By chemiluminescence analysis, the addition of NAC reversed the ROS levels increased by 3-MA in ovaries (Figure 5a,b). Furthermore, serial sections of the ovaries were analyzed for cyst breakdown after culture. 3-MA alone significantly inhibited primordial follicles formation (only 33.71 ± 7.22% compared with 64.47 ± 2.46% in control, p < 0.01), and increased the percentage of single oocytes (39.39 ± 8.60% vs. 20.11 ± 1.28%, p < 0.01) and cyst oocytes (26.90 ± 5.27% vs. 15.41 ± 3.15%, p < 0.05), while NAC alone had no effect on primordial folliculogenesis and cyst breakdown (63.27 ± 3.42% in primordial follicle, 21.57 ± 3.94% in single oocytes, 18.41 ± 1.38% in cyst oocytes; Figure 5c,d). However, when 3-MA and NAC were added to the ovary culture in combination, NAC reversed the inhibitory effect of 3-MA on cyst breakdown and primordial folliculogenesis (Figure 5c,d). Specifically, the percentage of primordial follicles was increased to 60.02 ± 5.31%, single oocytes were reduced to 21.57 ± 3.94%, and cyst oocytes were decreased to 18.41 ± 1.39% in the presence of NAC and 3-MA. Moreover, no significant differences were observed in total oocytes counts among the groups.
Since autophagy inhibition by 3-MA increased ROS levels in the ovary, and its effect on cyst breakdown was blocked by the addition of the oxidant, these data demonstrate that physiological autophagy in the ovary reduces oxidative stress to regulate cyst breakdown and primordial follicle formation.
3.6 Autophagy regulates expressions of growth factors of oocytes
Several signaling factors have been well characterized and demonstrated as important for postnatal ovarian development and primordial follicle recruitment. FIGLA, NOBOX, and LHX8 are key oocyte transcription factors, and oocytes in these loss-of-function mutants ultimately undergo cell death throughout the ovaries after birth (Jagarlamudi & Rajkovic, 2012). Growth differentiation factor 9 (GDF9), transforming growth factor β1 (TGFβ1), and anti-Müllerian hormone (AMH), members of the TGFβ family, govern oocytes-pregranulosa interactions in primordial follicle activation (Trombly, Woodruff, & Mayo, 2009; Z. P. Wang et al., 2014). We further detected the expression of these genes to explore whether perinatal autophagy affects key factors to regulate postnatal follicle development.
On 17.5 dpc, the ovaries were isolated and placed in culture with or without 0.1 mM 3-MA for 3 days. The expression levels of the key factors involved in postnatal ovarian development were analyzed via qRT-PCR and western blot analysis after culture. When autophagy was inhibited by 3-MA, the mRNA of Amh, Gdf9, Nobox, and Tgfb1 was remarkably reduced compared with control ovaries, while the mRNA levels of Figla and Lhx8 were constant over the groups (Figure 6a). Furthermore, the disrupted protein expression of GDF9 and TGFb1 was confirmed by western blot analysis (Figure 6b). This finding indicates that perinatal physiological autophagy also exerts extensive effects on further follicular development.
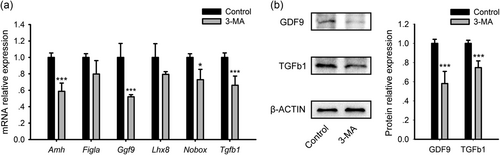
The effect of 3-MA on signaling factors regulating follicle development. Ovaries of 17.5 dpc were cultured with 0.1 mM 3-MA for 3 days. (a) mRNA expressions analysis of key signaling molecules in folliculogenesis by qRT-PCR. (b) Protein expressions analysis of key signaling molecules in folliculogenesis by western blot analysis. *p < 0.05; ***p < 0.01, n = 3. dpc: days post coitus; 3-MA: 3-methyladenine; mRNA: messenger RNA; qRT-PCR: quantitative reverse transcription polymerase chain reaction
4 DISCUSSION
The process of primordial folliculogenesis extends over 3–5 days of development in the neonatal mouse, during which neonates adapt to postparturition starvation. Programmed cell death, including autophagy, can be triggered under stress conditions such as nutrient deprivation. The results of the current study are in contrast with those of previous studies that have used autophagy null mice to illustrate early ovary development abnormalities, which have been attributed to overall oocyte loss, as opposed to employing autophagy inhibitor to explore the role of autophagy in primordial folliculogenesis. In the current study, we observed that autophagy was physiologically activated in prenatal mouse ovaries, specifically in cyst oocytes. The inhibition of autophagy by 3-MA interrupted cyst breakdown and primordial follicle formation in vivo and in vitro. In addition to elevated ROS levels in the ovary after autophagy inhibition, NAC, an efficient oxidant, mitigated the inhibitory effect of 3-MA on primordial folliculogenesis. Moreover, the blockage of autophagy reduced the expression of key signaling molecules to regulate follicle activation. These observations demonstrated the complex and essential nature of autophagy during primordial folliculogenesis.
In mice, massive female germ cell loss occurs postnatally, which initiates cyst breakdown and establishes a fixed primordial follicle reserve. The fate of germ cells to either form primordial follicles or undergo apoptosis is still difficult to anticipate. Previous studies have indicated that through the intercellular bridges within the cysts, oocytes receive the organelles from sister cyst (nurse-like) germ cells and form primordial follicle, whereas the other nurse-like germ cells die (Lei & Spradling, 2016; Pepling & Spradling, 2001). Specifically, the number of mitochondria in the oocytes with Balbiani body, a candidate marker for the survival oocytes (C. Wang, Zhou, & Xia, 2017), increased abundantly from 17.5 dpc. Finally, primary oocytes in primordial follicle acquire approximately five times as that of centrosomes, Golgi, and mitochondria and four times as that of cytoplasmic volume. The enriched mitochondria produce more ROS, while aged organelles require recycling. Thus, the activated autophagy observed in prenatal ovaries may be a response to increased ROS levels or a strategy for the elimination of dying nurse-like germ cells.
In the process of perinatal oocyte loss, apoptosis accounts for the bulk of germ cell loss in fetal mouse ovaries (Pepling & Spradling, 2001), while autophagy plays a protective role for oocytes survival (Song et al., 2015). However, several studies have indicated that autophagy can be a mechanism of caspase and apoptosis-independent cell death, and there is a strong causal relationship in which one process controls the other (Gump & Thorburn, 2011). Autophagy recycles damaged mitochondria and caspases and provides membranes for caspase processing in apoptosis (Mukhopadhyay, Panda, Sinha, Das, & Bhutia, 2014). Two autophagy proteins involved in autophagy–apoptosis interactions are BECN1 and p62. When BECN1 and B-cell lymphoma 2 (Bcl-2), an anti-apoptotic protein, are bound, BECN1 is incapable of initiating autophagy, while apoptosis also requires BAX dissociation from the same Bcl-2/Bcl-xL complex. Cleaved BECN1 by apoptotic protein caspase-3 promotes autophagy, indicating another BECN1-dependent mechanism by which apoptosis can inhibit autophagy (Gump & Thorburn, 2011; Mukhopadhyay et al., 2014). However, the direct interaction of p62 with caspase-8 is critical for the efficient activation of the apoptotic protein caspase-8, while autophagy reversely degrades caspase-8 (Gump & Thorburn, 2011), suggesting that the prenatal increase of p62 (Figure 1a,b) may be a preparation of active apoptosis for subsequent oocytes loss. Nevertheless, the mutual relationship between autophagy and apoptosis during primordial folliculogenesis remains elusive and requires further study.
As a major regulator of autophagy and a master regulator of cellular metabolism, mTOR responds to dynamic changes in cellular nutrients and energy level. mTOR forms two distinct signaling complexes, mTORC1 and mTORC2, and mTOC1 is responsible for autophagy regulation. Notably, mTORC1 inhibits the formation of autophagy-initiating ULK complex by phosphorylating complex components, such as ATG13 and ULK1/2 (Kim & Guan, 2015). Thus, rapamycin, the selective inhibitor of mTORC1, is widely used as an autophagy inducer. However, as a master regulator of metabolism, mTORC1 integrates a multitude of extracellular signals and intracellular cues to drive growth and proliferation, in addition to autophagy. In folliculogenesis, mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles, as the premature activation of primordial follicles was observed in mouse ovaries with the oocyte-specific deletion of Tsc1 and Tsc2, two negative regulators of mTORC1 (Adhikari et al., 2009; Adhikari et al., 2010; H. Zhang & Liu, 2015). A recent study reported that the inhibition of mTORC1 by rapamycin delays primordial follicle formation through decreased somatic cell proliferation and the reduced expression of a growth factor KIT ligand (KITL) (J. Zhang et al., 2017). Given the delicate regulatory mechanisms of mTORC1 in early follicular development, the reason for the incapability of rapamycin to reverse the effect of 3-MA and on primordial follicle formation, as shown in Figure 4, is unresolved.
Intriguingly, many growth factors are regulators of mTORC1 activity. In particular, the signaling pathway of insulin/insulin-like growth factor (IGF) and epidermal growth factor (EGF) to activate mTORC1 represents the best understood mechanism (Huang & Fingar, 2014). Insulin/IGF activates PI3K/Akt signaling which phosphorylates TSC2 to suppress the inhibitory effect of the TSC complex towards mTORC1, and EGF activates c-Raf, MAPK, and p90 ribosomal S6 kinase to phosphorylate TSC2. In the current study, GDF9 and TGFβ1, two members of the TGFβ family, were reduced in the ovaries by 3-MA treatment. In the ovary, oocyte-derived GDF9 suppresses the expression of Ddit4l, a negative regulator of mTOR, to activate mTOR in cumulus cells, and this oocyte-dependent activation of mTOR signaling in cumulus cells controls the development and survival of cumulus-cell-oocyte complexes (Guo et al., 2016). Additionally, TGFβ activates the mTORC1 pathway in fibroblasts via a PI3K-Akt-TSC2-dependent mechanism (Rahimi et al., 2009). However, in neonatal ovaries, TGFβ1 signaling regulates primordial follicle growth in an Akt-independent pathway, in which TGFβ1 suppresses the activation of p70 S6 kinase-1–ribosomal protein S6 to inhibit mTORC1 signaling (Z. P. Wang et al., 2014). The interaction of growth factors and mTORC1 may be the underlying mechanism for the autophagy response to growth factors. The reduced expression of GDF9 and TGFβ1 by 3-MA treatment might reversely regulate autophagy activation in the current study.
In conclusion, we showed that autophagy is activated in perinatal ovaries and plays a role in regulation of germ cell cyst breakdown and primordial follicle assembly. Furthermore, the physiological level of autophagy cleared the excess ROS in ovarian cells and promoted the expression of GDF9 and TGFβ1, which govern the subsequent follicle activation. Thus, these observations may provide another mechanism underlying the physiological role of autophagy in primordial folliculogenesis.
ACKNOWLEDGMENTS
This study was supported by grants from the National Natural Science Foundation of China (31571554 and 31601200), and Basic and Frontier Research Program of Chongqing (Grant No. cstc2016jcyjA0318).
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.




