Downregulation of microRNA-592 protects mice from hypoplastic heart and congenital heart disease by inhibition of the Notch signaling pathway through upregulating KCTD10
Abstract
Evidence has demonstrated that the microRNA (miR) may play a significant role in the development of congenital heart disease (CHD). Here, we explore the mechanism of microRNA-592 (miR-592) in heart development and CHD with the involvement of KCTD10 and Notch signaling pathway in a CHD mouse model. Cardiac tissues were extracted from CHD and normal mice. Immunohistochemistry staining was performed to detect positive expression rate of KCTD10. A series of inhibitor, activators, and siRNAs was introduced to verified regulatory functions for miR-592 governing KCTD10 in CHD. Furthermore, the effect of miR-592 on cell proliferation and apoptosis was also investigated. Downregulated positive rate of KCTD10 was observed in CHD mice. Downregulation of miR-592 would upregulate expression of KCTD10 and inhibit the activation of Notch signaling pathway, thus promote cell proliferation. This study demonstrates that downregulation of miR-592 prevents CHD and hypoplastic heart by inhibition of the Notch signaling pathway via negatively binding to KCTD10.
1 INTRODUCTION
Congenital heart disease (CHD) is considered as one of the universal birth defects and also the major reason for morbidity and mortality in neonatal age (Guo et al., 2015). There are approximately 1% neonates suffering from CHD, of which one-third present with severe symptoms (Bertoletti, Marx, Hattge Junior, & Pellanda, 2014). CHD mainly causes arrhythmia, cardiomyopathies, and abnormality in morphology, which can finally lead to structural impairment (McCulley & Black, 2012). Many factors have been found out to be responsible for the relatively high incidence of CHD, including genetic abnormal mutation, epigenetic modifications, and environmental impacts (Ko, 2012015; Xu et al., 2014). The diagnostic approach of CHD is fetal ultrasound echocardiography, however, the diagnostic efficiency is lower than 35% due to the high misdiagnosis rate in regular antenatal care of cardiac abnormalities (Gu et al., 2016). Patients are usually arranged to undergo surgery or cardiopulmonary bypass as conventional treatments in clinical practices, which may be conducive to enhancing survival rate to 85%; however, due to the potential shortcoming, a combined approach has also been developed to achieve a better outcome (Holoshitz, Kenny, & Hijazi, 2014). Considering the limited therapeutic tools, herein, we attempt to discover some other possible regimens for CHD.
As a member of an miRNA family, miR-592 has been revealed for its role in relieving injury caused by human colorectal cancer by suppressing the proliferation and anchorage-independent growth of human colorectal cancer cells (Liu et al., 2015). Several miRNAs have been reported to be associated with cancer, hematopoietic disease, and skeletal disorders (Calin et al., 2002; Michael, O’Connor, van Holst Pellekaan, Young, & James, 2003; Waki et al., 2015). Furthermore, it is associated with heart disease. Particularly, Piotr Fic et al. have pointed out that miRNAs may play significant roles in cardiac development and cardiovascular system, for example, heart failure and cardiac arrhythmia (Fic, Kowalczuk, Grabarska, & Stepulak, 2014). Potassium channel tetramerization domain containing 10 (KCTD10) belongs to the polymerase delta interacting protein 1 (PDIP1) gene family along with the other two members, PDIP1 and TNFAIP1 (Liu et al., 2009; Sun et al., 2016). Moreover, a published assay has covered that KCTD10 is highly expressed in skeletal muscle, human heart, and placenta while cooperating with proliferating cell nuclear antigen, indicating its potential role in cell proliferation of human heart (Sun et al., 2016). In addition, the Notch signaling pathway is regarded as a crucial pathway in vascular differentiation, consisting of cell proliferation, migration, invasion, and apoptosis (Ren et al., 2014). Therefore, it is reasonable to build up a hypothesis that KCTD10 may be conducive to CHD through the Notch signaling pathway, which can help clinicians to effectively plan surgery and medical management and their follow-up. Hereby, this study aims to investigate the underlying effects of miR-592 on CHD and heart development through the Notch signaling pathway by binding to KCTD10.
2 MATERIALS AND METHODS
2.1 Ethical statement
This study was approved by the Ethics Committee of the Ethics Committee of the First Hospital of China Medical University. All animal experiments were in accordance with the principles of the management and use of local experimental animals.
2.2 Animal grouping and model establishment
Forty healthy and clean mice from Institute of Cancer Research were provided by Shanghai SLAC Laboratory Animal Co., Ltd., Shanghai, China (0042561; weight: 25−30 g; age: 7−9 weeks; no limitation with gender). Female and male mice in heat were chose at a ratio of 3:1 in a cage for 2 hr. The day that vaginal plug was found was recorded as the first day (D1) of pregnancy. Twenty female mice at D7 were selected for the experiment, 10 of which were administered with 700 mg/kg valproic acid (H20010595, Guangdong Kangaiduo Chain Drugstore Co., Ltd., Guangdong, China) by intraperitoneal injection and served as the CHD group, another 10 with 0.9% NaCl by intraperitoneal injection served as the normal group.
2.3 Hematoxylin-Eosin staining
Hearts of fetus mice were obtained by uterine-incision delivery at D19. Subsequently, the sections were stained using hematoxylin (H8070–5 g, Beijing Solarbio Life Sciences Co., Ltd., Beijing, China) and e stained by ammonia followed with eosin solution (PT001, Shanghai Bogoo Biotechnology Co., Ltd., Shanghai, China) for another 2 min. The optical microscope (DMM-300D, Shanghai Caikon Optical Instrument Co., Ltd., Shanghai, China) was used to observe the pathological changes of cardiac tissues after the sections were mounted with neutral balsam in the fume hood.
2.4 Immunohistochemistry
After adding rabbit anti-mouse KCTD10 monoclonal antibody (bs-16925R, Beijing Bioss Antibodies Co., Ltd., Beijing, China) as primary antibody, the samples were incubated overnight at 4°C. Phosphate buffer saline (PBS) was used to rewash samples for three times (3 min each time). Goat anti-rabbit IgG (1:1000, ab6789, Abcam, Inc., Cambridge, MA) labeled by biotin was used as a secondary antibody. Incubation at 37°C for 30 min later, the sections were washed with PBS for another three times (3 min each time). Streptomycin avidin and peroxidase solution (Beijing Zhongshan Biotech Co., Ltd., Beijing, China) was added. The sections were colored by diaminobenzidine (DA1010–3 ml, Beijing Solarbio Life Sciences Co., Ltd.) for 5−10 min. The degree of coloring was controlled under the microscope. The sections were dehydrated with gradient ethanol, cleaned by xylene, and mounted by neutral resin. Five visual fields were randomly chosen from each section with the positive staining area, ratio of positive staining area, and average integral optical density measured.
2.5 Reverse transcription quantitative polymerase chain reaction
Total RNA was extracted using Ultrapure RNA Isolation Kit (D203-01, Beijing GenStar BioSolutions, Co., Ltd., Beijing, China). Primers of miR-592, KCTD10, Jagged1, Hes1, Hey2, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were designed and then synthesized by TaKaRa Biotechnology Ltd., Dalian, Liaoning, China (Table 1). The reverse transcription system was set as 20 μl. The total RNA was reversely transcribed into cRNA according to the instructions of TaqMan MicroRNA Assays Reverse Transcription Primer (4366596, Thermo Fisher Scientific, Waltham, MA). Reaction system contained 25 μl of SYBR® Premix Ex Taq™ II (2×), 2 μl of polymerase chain reaction (PCR) forward primer, 2 μl of PCR reverse primer, 1 μl of ROX Reference Dye (50×), 4 μl of template DNA, and 16 μl of ddH2O. The reverse transcription quantitative PCR (RT-qPCR) was performed using ABI PRISM® 7300 (Prism® 7300, Shanghai Kunke Co., Ltd., Shanghai, China). Reaction conditions were as follows: predenaturation for 10 min at 95°C, 40 cycles of denaturation for 15 s at 95°C, annealing for 30 s at 60°C, and extension for 1 min at 72°C. U6 gene was served as the internal control of miR-592 with GAPDH (abs830032, Absin Bioscience Inc., Shanghai, China) as the internal control of KCTD10, Jag1, Hes1, and Hey2. Relative expression of the target gene was calculated based on the 2 (-Delta Delta C(T)) method, in which -Delta Delta C(T) =Delta C(T)experiment group - Delta C(T)control group and Delta C(T) =C(T)target gene – C(T)U6/GAPDH.
| Gene | Primer sequence |
|---|---|
| miR-592 | F: 5′-CCATGACATTGTGTCAATATGCGA-3′ |
| R: 5′-CGTCATGATGTTGCGTCACC-3′ | |
| KCTD10 | F: 5′-CACCCGCACTACTTCCTTCAA-3′ |
| R: 5′-GTGAGCACTTCCATGCGTC-3′ | |
| Jag1 | F: 5′-ATGCAGAACGTGAATGGAGAG-3′ |
| R: 5′-GCGGGACTGATACTCCTTGAG-3′ | |
| Hes1 | F: 5′-TCAACACGACACCGGACAAAC-3′ |
| R: 5′-ATGCCGGGAGCTATCTTTCTT-3′ | |
| Hey2 | F: 5′-CGCCCTTGTGAGGAAACGA-3′ |
| R: 5′-CCCAGGGTAATTGTTCTCGCT-3′ | |
| U6 | F: 5′-TCCGACGCCGCCATCTCTA-3′ |
| R: 5′-TATCGCACATTAAGCCTCTA-3′ | |
| GAPDH | F: 5′-AGGTCGGTGTGAACGGATTTG-3′ |
| R: 5′-GGGGTCGTTGATGGCAACA-3′ |
- Note. F: forward; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; Hes1: hairy and enhancer of split-1; Hey2: hairy/enhancer-of-split related with YRPW motif protein 2; Jag1: Jagged1; KCTD10, potassium channel tetramerisation domain containing 10; miR-592, microRNA-592; R: reverse; RT-qPCR: reverse transcription quantitative polymerase chain reaction
2.6 Western blot analysis
Total protein was extracted using ProteoPrep® Total Extraction Sample Kit (C0418, Sigma-Aldrich Chemical Company, St Louis, MO). Protein bands on the gel were transferred to nitrocellulose membrane in a semidry way at 15 V for 18 min. Then, the membrane was washed with Tris-buffered saline with Tween 20 (TBST) for 5 min and blocked with 5% bovine serum albumin at room temperature for 1 hr. After rewashed with TBST for three times (each for 5 min), the membrane was incubated with the following antibodies (all purchased from Abcam Inc., Cambridge Science Park, Cambridgeshire, England) at 4°C overnight: rabbit-anti-mouse KCTD10 (1: 100, ab94867), Jagged1 (1: 1000, ab109536), Hes1 (1: 1000, ab108937), Hey2 (1: 1000, ab86010), and horse radish peroxidase (HRP) labeled rabbit-anti-mouse GAPDH (1: 2500, ab9485, served as the internal control). TBST washing was repeated for three times (5 min each time), the membrane was incubated with secondary antibody, HRP labeled goat-anti-rabbit (diluted to 1: 5000 using TBST) for 1 hr at room temperature with sealing fluid added. Analysis of developed images was exposed. The method was suitable for the subsequent cell experiment.
2.7 Cell culture, grouping, and transfection
Cardiac tissue was cut from hearts of fetus mice and then cultured. Embryonic endocardial cells (EECs) in the logarithmic growth were seeded into 6-well plates, and cells were transfected in accordance with the instructions of liPofectamin 2000 (11668-019, Shanghai HengFei Biological Science and Technology Co., Ltd., Shanghai, China) when the cells reached upon 30%−50% confluency. The cells were grouped into normal group, blank group (without any transfection of sequence), negative control (NC) group (blank plasmid transfected), miR-592 mimic group (cells transfected with miR-592 mimic), miR-592 inhibitor group (cells transfected with miR-592 inhibitor), miR-592 inhibitor + siRNA-KCTD10 group (cells transfected with miR-592 inhibitor and siKCTD10), and siRNA-KCTD10 group. All needed materials for transfection were provided by Shanghai GenePharma Co., Ltd., Shanghai, China.
2.8 Dual-luciferase reporter gene assay
On the basis of online bioinformatics analysis software available at microRNA.org, dual-luciferase reporter gene assay was used to testify whether KCTD10 is a direct target of miR-592 or not. Endonuclease sites Spe I and Hind III were introduced to pMIR-reporter. Mutant sites on complementary sequence of seed sequence were designed on KCTD10 wild type (WT). Target sequence was inserted in plasmid of pMIR-reporter using T4 DNA ligase after cleaved by restriction enzymes. The correctly identified sequence luciferase reporter plasmids WT and mutant type (MUT) was cotransfected with miR-495 respectively, into HEK-293T cells (CRL-1415, Shanghai Xinyu Biotechnology Co., Ltd., Shanghai, China). Renilla luciferase was set as the internal control with relative luciferase activity (RLU) measured. Luciferase activity was calculated in conform with the formula that RLU (luciferase activity) = RLU (Renilla luciferase)/RLU (firefly luciferase) (Collin, 1989).
2.9 3-(4,5-Dimethylthiazol-2-yl)−2,5-diphenyltetrazolium bromide (MTT) assay
When cell confluence reached about 80%, single cell suspension was obtained after washing with PBS twice and treated with 0.25% trypsin. Then, it was seeded in a 96-well plate with 3 × 103 − 6 × 103 cells in each well (0.2 ml/well) after cell number was counted. Each group repeated six wells. Afterward, the cells were cultured and replaced with medium containing 10% MTT solution (5 g/l) (GD-Y1317, Guduo Biotechnology Co., Ltd., Shanghai, China) at 24 , 48 and 72 hr. Optical density (OD) at 490 nm was measured using a microplate reader (Nanjing Detie Laboratory Equipment Co., Ltd., Nanjing, Jiangsu, China) for each well. Each experiment was performed three times. The curve showing cell viability was drawn with time point as the horizontal axis and OD as the vertical axis.
2.10 Flow cytometry
Annexin V-FITC/PI double staining was adopted to assess cell apoptosis. After culture at 37°C in a humidified atmosphere of 5% CO2 for 48 hr, the cells were collected and washed twice with PBS. Then, centrifugation was conducted followed by cells re-suspended in 200 μl binding buffer with 10 μl Annexin V-FITC and 5 μl propidium lodide (PI) mixed evenly. The reaction took place at room temperature away from light for 15 min. Another 300 μl binding buffer was added into cells. Flow cytometry (6HT, Wuhan Cellwar Bio-technology Co., Ltd., Wuhan, Hubei, China) was conducted to detect cell apoptosis at 488 nm of excitation wavelength.
2.11 Statistical analysis
Data were analyzed using SPSS 21.0 software (IBM Corp. Armonk, NY). Measurement data were expressed as mean ± standard deviation. Comparison between two groups was conducted by t test. Comparisons among multiple groups were assessed by one-way analysis of variance (ANOVA). p < 0.05 was considered to be statistically significant.
3 RESULTS
3.1 Pathological changes in cardiac tissues of the normal and CHD groups
Initially, Hematoxylin-Eosin (HE) staining was applied to observed pathological changes in cardiac tissues. According to the results of HE staining, mice in the normal group showed well-developed interventricular septal and great vessels with mature atrioventricular valve and aorta valve. As for mice in the CHD group, interventricular septal defect, aortic overriding, and hypoevolutism were observed (Figure 1).
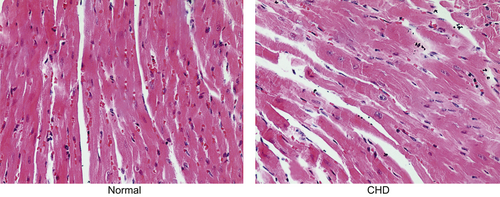
Cardiac structure in the normal and CHD groups using HE staining (×200); as for mice in the CHD group, interventricular septal defect, aortic overriding, and hypoevolutism were observed. Note. CHD: congenital heart disease; HE: hematoxylin-eosin [Color figure can be viewed at wileyonlinelibrary.com]
3.2 CHD mice had an elevated expression of Jagged1, Hes1, and Hey2 whereas a declined expression of KCTD10 in cardiac tissues
Subsequently, the positive expression rate of KCTD10 in cardiac tissues of normal and CHD mice was determined by the immunohistochemistry assay. The results of immunohistochemistry showed that positive staining of KCTD10 was mainly expressed in cytoplasm of tan-colored to brown-colored while positive cells were distributed in a sheet, diffuse, or focal way. As shown in Figure 2a, KCTD10 protein was positively expressed in cardiac tissues of normal mice with darker stained whereas negatively expressed in the CHD mice with lighter or no stained. As statistically analyzed, the positive expression rate of KCTD10 protein was much higher in the normal group than that in the CHD group (p < 0.05) (Figure 2b).
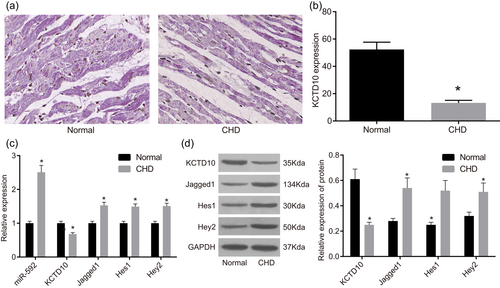
MiR-592, Jagged1, Hes1, and Hey2 increased whereas KCTD10, decreased in cardiac tissues of CHD mice according to immunohistochemistry, RT-qPCR, and western blot analysis. Note: Panel A, the positive expression rate of KCTD10 decreased in cardiac valve tissues of CHD mice (×400) according to immunohistochemistry assay; Panel B, the positive expression rate of KCTD10 in the normal and CHD groups; Panel C, miR-592, Jagged1, Hes1, and Hey2 expression increased whereas the mRNA expression of KCTD10, decreased in cardiac tissues of CHD mice according to RT-qPCR; Panel D, the protein expression of KCTD10, decreased, while protein expression of Jagged1, Hes1, and Hey2 increased in cardiac tissues of CHD mice according to western blot analysis. The gray value of KCTD10, Jagged1, Hes1, Hey2, and GAPDH in the normal and CHD groups was showed. CHD: congenital heart disease; Hes1: hairy and enhancer of split-1; Hey2: hairy/enhancer-of-split related with YRPW motif protein 2; KCTD10: potassium channel tetramerisation domain-containing 10; MiR-592: microRNA-592; RT-qPCR: reverse transcription quantitative polymerase chain reaction; arrow refers to the positive cells; *p < 0.05 compared with the normal group [Color figure can be viewed at wileyonlinelibrary.com]
Besides, the expression of KCTD10, miR-592, Jagged1, Hes1, and Hey2 was measured by RT-qPCR and western blot analysis. The results of RT-qPCR (Figure 2c) demonstrated that miR-592 expression, and mRNA expression of Jagged1, Hes1, and Hey2 was highly expressed in CHD cardiac tissues than those in normal cardiac tissues whereas mRNA expression of KCTD10 was lowly expressed (all p < 0.05). Results of western blot analysis (Figure 2d) demonstrated that protein expression of KCTD10 was declined remarkably whereas protein expression of Jagged1, Hes1, and Hey2 was elevated in CHD cardiac tissues than those in normal cardiac tissues (all p < 0.05).
3.3 KCTD10 is a target gene of miR-592
KCTD10 was verified to be a target gene of miR-592 (Figure 3a) because the specific-binding sites existed between 3′UTR of KCTD10 gene and sequences of miR-592. Results of dual-luciferase reporter assay revealed that in comparison with the NC group, luciferase activity of KCTD10 wt-3′-UTR was markedly inhibited by miR-592 (p < 0.05) whereas of mut-3′-UTR was not inhibited by miR-592 (Figure 3b). All these came to a demonstration that miR-592 could specifically bind to KCTD10-3′-UTR, and downregulate the expression of KCTD10 after transcription.
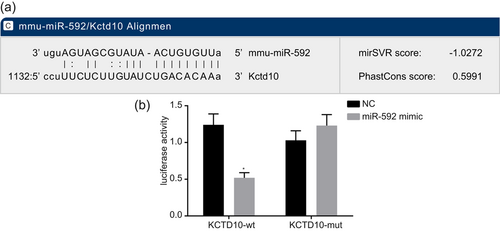
KCTD10 was target gene of miR-592. Note. Panel A, predicted miR-592 binding sites on the KCTD10 3′ UTR; Panel B, miR-592 downregulates the expression of KCTD10; miR-592: microRNA-592; NC: negative control; *p < 0.05 compared with the NC group [Color figure can be viewed at wileyonlinelibrary.com]
3.4 Downregulation of miR-592 inhibits the activation of the Notch signaling pathway via upregulation of KCTD10 in EECs
The expression of miR-592, KCTD10, and Notch signaling pathway-related genes in EECs after transfection was assessed by RT-qPCR and western blot analysis. Compared with the normal group, miR-592 expression significantly increased whereas the mRNA and protein expression of KCTD10 decreased in EECs of CHD mice in each group (all p < 0.05). In comparison with the blank and NC groups, the miR-592 expression increased whereas the mRNA and protein expression of KCTD10 decreased in the miR-592 mimic group (all p < 0.05), and an opposite trend was revealed in the miR-592 inhibitor group (all p < 0.05) (Figure 4a,b).
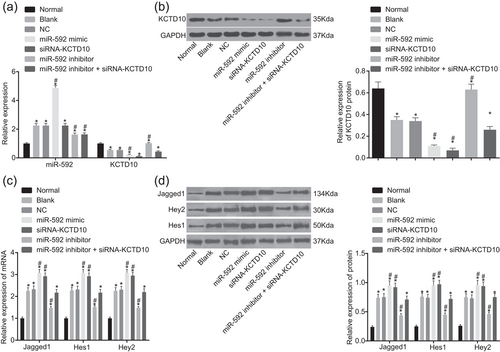
Inhibition of miR-592 increased the expression of KCTD10 and blocked the Notch pathway according to RT-qPCR and western blot analysis. Note. Panel A, decreased expression of miR-592 elevated the mRNA expression of KCTD10 in EECs of CHD mice according to RT-qPCR; Panel B, decreased expression of miR-592 elevated the protein expression of KCTD10 in EECs of CHD mice according to western blot analysis. The gray value of KCTD10 and GAPDH was showed; Panel C, decreased expression of miR-592 inhibited the mRNA expression of Jagged1, Hes1, and Hey2 in EECs of CHD mice according to RT-qPCR; Panel D, decreased expression of miR-592 inhibited the protein expression of Jagged1, Hes1, and Hey2 in EECs of CHD mice according to western blot analysis. The gray value of Jagged1, Hes1, Hey2, and GAPDH was showed. EECs: embryonic endocardial cell; Hes1: hairy and enhancer of split-1; Hey2: hairy/enhancer-of-split related with YRPW motif protein 2; KCTD10: potassium channel tetramerisation domain containing 10; miR-592: microRNA-592; NC: negative control; RT-qPCR: reverse transcription quantitative polymerase chain reaction; siRNA: short interfering RNA; *p < 0.05 compared with the normal group; #p < 0.05 compared with the blank and NC groups. The one-way ANOVA was applied for comparison and the experiment was repeated three times
In contrast to the normal group, the expression of Jagged1, Hes1, and Hey2 was distinctly upregulated in EECs of CHD mice in each group (all p < 0.05). In comparison with the blank and NC groups, the mRNA and protein expression of Jagged1, Hes1, and Hey2 increased in the miR-592 mimic and siRNA-KCTD10 groups, and an opposite trend was observed in the miR-592 inhibitor group (all p < 0.05). However, no significant difference was revealed between the blank and NC groups and the miR-592 inhibitor + siRNA-KCTD10 group (p > 0.05) (Figure 4c,d). Planned comparisons revealed that downregulated miR-592 inhibited the activation of the Notch pathway through upregulation of KCTD10.
3.5 Downregulated miR-592 promotes proliferation of EECs
The effect of miR-592 on proliferation ability of with or without transfection was measured by MTT assay. According to the results of MTT assay (Figure 5), in comparison with the normal group, the relative cell proliferation rates reduced remarkably in the other groups (all p < 0.05) whereas no significant difference observed between the blank and NC groups (p > 0.05). Compared with the blank and NC groups, cell proliferation was inhibited in the miR-592 mimic and siRNA-KCTD10 groups (both p < 0.05) and enhanced in the miR-592 inhibitor group (p < 0.05). However, the cell proliferation had no obvious changes in the miR-592 inhibitor + siRNA-KCTD10 group compared with the blank and NC groups (p > 0.05). From these results, it was cleared that downregulation of miR-592 promoted EEC proliferation of CHD mice.
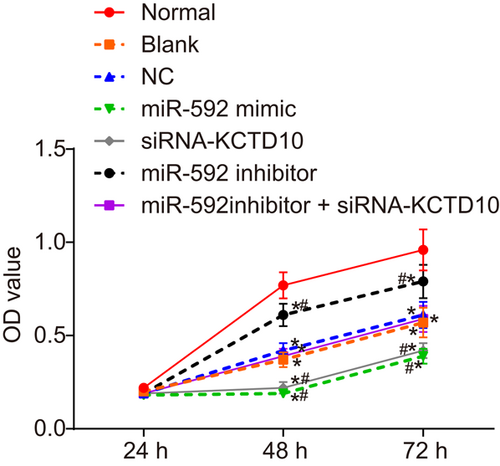
EEC proliferation was enhanced by downregulation of miR-592. Note. EECs: embryonic endocardial cells; miR-592: microRNA-592; NC: negative control; siRNA: short interfering RNA; *p < 0.05 compared with the normal group; #p < 0.05 compared with the blank and NC group. The two-way ANOVA was used for statistical analysis and the experiment was repeated three times [Color figure can be viewed at wileyonlinelibrary.com]
3.6 Downregulated miR-592 inhibits apoptosis of EECs
Furthermore, Annexin V-FITC/PI double staining was also conducted to investigate the effect of miR-592 on apoptosis rate of EECs. As shown in Figure 6a,b, the results of Annexin V-FITC/PI double staining demonstrated that the proportion of early and late apoptotic cells increased in CHD mice of each group compared with the normal group (all p < 0.05), whereas no significant difference found between the blank group and the NC group (p > 0.05). However, the proportion of early and late apoptotic cells was higher in the miR-592 mimic and siRNA-KCTD10 groups whereas lower in the miR-592 inhibitor group than those in the blank and NC group (all p < 0.05). The proportion of early and late apoptotic cells did not differ significantly in the miR-592 inhibitor + siRNA-KCTD10 group compared with the blank and NC groups (p > 0.05). Together, the present results confirmed that upregulation of miR-592 and silencing of KCTD10 promoted EECs apoptosis of CHD mice, which could be suppressed by downregulation of miR-592.
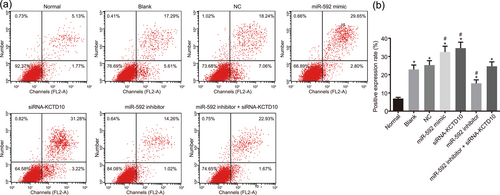
EEC apoptosis was inhibited by downregulation of miR-592. Note. Panel A, EEC apoptosis mapping measured by flow cytometry. Quadrants: lower left, proportion of living cells; right lower, proportion of early apoptotic cells; right upper, proportion of advanced apoptotic cells; left upper, dead cell and its fragments; Panel B, the inhibition of miR-592 suppressed the apoptosis of EECs; EECs: embryonic endocardial cells; miR-592: microRNA-592; *p < 0.05 compared with the normal group; #p < 0.05 compared with the blank and NC groups. The one-way ANOVA was used for comparison and the experiment was repeated three times [Color figure can be viewed at wileyonlinelibrary.com]
4 DISCUSSION
The current study is designed to investigate the effect of miR-592 on EEC proliferation and apoptosis in CHD mouse models as well as heart development. Our findings provide available evidence proving that miR-592 inhibits EEC proliferation and induces apoptosis in CHD through the Notch signaling pathway via targeting KCTD10.
Above all, it was found that CHD eventually would result in hypoplastic heart as implied by cardiac pathological changes of interventricular septal defect, aortic overriding, and hypoevolutism. A significant finding of the current study is that KCTD10 is a target of miR-592. Wang et al. have explored in their paper that miRNAs carries out their functions via binding site in 3′UTR after transcription (W. Wang et al., 2017). MicroRNA.org, a biology prediction site, demonstrates that the mRNA of miR-592 has the binding site of KCTD10 in its 3′UTR. Therefore, results from our study are reasonable enough to indicate that miR-592 specifically binds KCTD10-3′-UTR and reduces the expression of KCTD10 at the post-transcriptional level.
Moreover, our study also suggested that EEC proliferation tends to be enhanced and apoptosis tends to be inhibited with downregulated miR-592 expression and upregulated KCTD10 expression. Since that KCTD10 is a direct target of miR-592, it is reasonable to infer that the ectopic expression of miR-592 led to downregulation of KCTD10. The effect of downregulated KCTD 10 on cell proliferation has ever been documented to be inhibitory via its BTB/POZ domain (Y. Wang et al., 2009). The similar role of miR-592 has been demonstrated that downregulated miR-592 inhibits tumor cell growth by targeting DEK in the 3′-UTR binding site in human hepatocellular carcinoma (Li, Zhang, Zhou, Yue, & Su, 2015). What is more, a few years ago, Krithi Irmady along with his colleagues conducted transferase-mediated deoxyuridine triphosphate-biotin nick end labeling assay on primary hippocampal neurons of neuronal ischemic injury, which declared that the number of apoptotic cells was reduced sharply in the group treated with miR-592 (Irmady et al., 2014). There was a study revealed that miR-592 inhibitor had the ability to suppress migration, proliferation, and colony formation of colorectal cancer cells (Liu et al., 2015). A likely explanation for the results we observed from our study is that downregulated miR-592 induces proliferation and inhibits EEC apoptosis.
Furthermore, another key result of our study lies in that elevated mRNA and protein expression of KCTD10 and declined expression of Jagged1, Hes1, and Hey2 were found in cells treated with miR-592 inhibitor. Thereinto, Hes1, summarized by a previous study, is able to exert protective effects on cardiomyocytes through various signaling pathway including the Notch signaling pathway (Huang, Lai, Wan, Qi, & Liu, 2016). Several researchers have considered the Notch signaling pathway in heart development, arriving at the conclusion that this pathway may response to healing process of the damaged heart through modulating the proliferation of cardiomyocytes and formation of valve (Grochowski, Loomes, & Spinner, 2016; Nemir et al., 2014). Also, the activation mechanism of Notch signaling pathway on cardiomyocytes has been discovered to achieve mainly through Jagged1 expression (Nemir et al., 2014). Jagged1 has been described to express within vascular structure in mammalian heart in relation to heart development from an introductory article composed of Christopher M. Grochowski et al. (Grochowski et al., 2016). Besides, Hu et al. have ever conducted experiment on zebra fish, which is reported to share similar KCTD10 function with human’s, offering well-proven statement that downregulated KCTD10 prevents cell from proliferation thus causing impairment in heart development directly (Li et al., 2014). A reporter written by Ren et al. has also documented that KCTD10 regulates the Notch signaling pathway in a negative manner (Ren et al., 2014). The idea is consistent with the observation results using MTT assay and flow cytometry in the current study that downregulated KCTD10 inhibits EEC proliferation and induces apoptosis.
As stated above, the current study suggests that miR-592 suppresses EEC proliferation and induces apoptosis through the activation of the Notch signaling pathway by downregulating KCTD10 expression in CHD. These findings may open novel avenues for future therapies. Notwithstanding, it should be noted that this study has examined only 40 samples, therefore, a relative larger sample shall be used for further study.
ACKNOWLEDGMENT
We would like to thank sincerely the reviewers for critical comments on this study.
CONFLICTS OF INTEREST
The author declare that they have no confilcts of interest.




