Tumor-associated macrophages and epithelial–mesenchymal transition in cancer: Nanotechnology comes into view
Abstract
Tumor-associated macrophages (TAMs) are an important component of the leukocytic infiltrate of the tumor microenvironment. There is persuasive preclinical and clinical evidence that TAMs induce cancer inanition and malignant progression of primary tumors toward a metastatic state through a highly conserved and fundamental process known as epithelial–mesenchymal transition (EMT). Tumor cells undergoing EMT are distinguished by increased motility and invasiveness, which enable them to spread to distant sites and form metastases. In addition, besides becoming resistant to apoptosis and antitumor drugs, they also contribute to immunosuppression and get a cancer stem-cell like phenotype. Here, we will focus on selected molecular pathways underlying EMT—in particular, the role of TAMs in the induction and maintenance of EMT—and further discuss how the targeting of TAMs through the application of nanotechnology tools allows the development of a whole new range of therapeutics.
1 INTRODUCTION
Macrophages are the first line of defense in the immune system against invading pathogens, phagocytose microbes, and antigens present for T cells. They also play a central role in tissue growth, repair, homeostasis, and remodeling by the production of numerous molecules; such as cytokines, chemokines, growth factors, proteolytic enzymes, and scavenger receptors (De palma & Lewis, 2013; Wynn, Chawla, & Pollard, 2013). For example, macrophages are involved in developmental processes, such as tissue morphogenesis and vascular and neuronal patterning, and also in pathophysiological responses, such as inflammation and organ healing or regeneration (Cieslewicz et al., 2013; De palma & Lewis, 2013; Mantovani, Biswas, Galdiero, Sica, & Locati, 2013). Although with some exceptions, such as for the intestine and the dermis, arising from progenitor cells in the yolk sac, they differentiate into macrophages in the tissues and can subsequently be shifted to diverse functional phenotypes by individual environmental signals (Franklin et al., 2014; Wynn et al., 2013). These polarization states are generally referred to as classically activated M1 macrophages and alternatively activated M2 macrophages, which parallel Th1/Th2 programming of adaptive immune cells (Biswas & Mantovani, 2010; Mantovani, Sozzani, Locati, Allavena, & Sica, 2002). The M1 macrophages are induced by toll-like receptor (TLR) ligands and (IFN)-γ, resulting in a proinflammatory and a microbicidal functional phenotype (Allavena & Mantovani, 2012; Mantovani et al., 2013; Mosser & Edwards, 2008). In contrast, Th2 cytokines, like IL-4 and IL-13, stimulate monocytes or macrophages to express an M2 activation state, which limits inflammation and tissue repair (Mantovani et al., 2013). Moreover, several studies have established that macrophage polarization can also be regulated by environmental cues, like the presence of nutrients, fatty acids, and other metabolites (Weichhart, Hengstschläger, & Linke, 2015).
Macrophages are also an important cellular component of the tumor microenvironment, which are predominantly polarized toward an M2-like phenotype and are generally termed tumor-associated macrophages (TAMs; Allavena & Mantovani, 2012). These cells can function as a source of local and systemic signals to enhance the suppression of antitumor immunity, tumor vascularization (angiogenesis), tissue remodeling, and repair (Mantovani et al., 2013). They also support the intravasation of cancer cells in the primary tumor site and extravasation at distant metastatic sites although the pathways and phenotypes have heterogeneity in different tumors (DeNardo et al., 2011; Mantovani & Sica, 2010; Qian et al., 2011; Squadrito & De Palma, 2011). Several studies have shown that TAMs force cancer cells to gain CSC-like properties through a process called the epithelial–mesenchymal transition (EMT; Ding, Jin, Chen, Shao, & Wu, 2012; Fan et al., 2014; C.-Y. Liu et al., 2013).
EMT is a process in which cells with an epithelial phenotype are transformed into cells with a mesenchymal phenotype (Thiery, Acloque, Huang, & Nieto, 2009). This process is considered a critical step for embryonic development, which involves changes that lead to the loss of cell–cell junctions, the loss of apico-basal polarity, and acquiring migratory and invasive properties (Davies, 1996; Marcucci, Stassi, & De Maria, 2016). In adults, EMT contributes to organ fibrosis, tissue regeneration, wound healing, and tumor progression (Kalluri, 2009; Marcucci et al., 2016).
Tumor cells undergoing EMT are characterized by increased motility and invasiveness, which enable their distribution to distant sites and enable them to make metastases. Furthermore, these tumor cells become resistant to apoptosis and antitumor drugs, that is, they contribute to immunosuppression, and hence act as cancer stem-like cells (CSCs; Kodama et al., 2016; Mani et al., 2008). Another feature of tumor cells undergoing EMT is limited proliferative potential (Mani et al., 2008; Marcucci et al., 2016; Figure 1). However, promotion of the reverse process, mesenchymal–epithelial transition (MET), allows tumor cells to reacquire epithelial traits and functioning in a way that leads to the proliferation and growth of seeded metastatic cells (Marcucci et al., 2016).
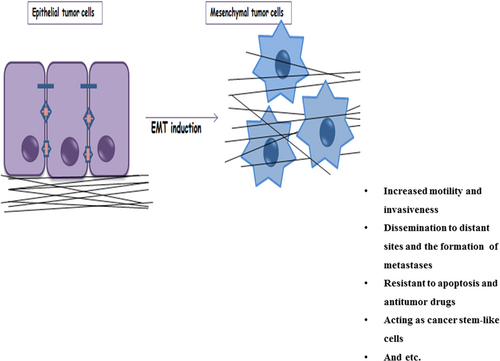
Functional consequences of EMT and the acquisition of mesenchymal traits by tumor cells. Tumor cells undergoing EMT are characterized by increased motility and invasiveness, which enable their dissemination to distant sites and metastases formation. In addition, they become resistant to apoptosis and antitumor drugs, contribute to immunosuppression, and act as cancer stem-like cells. EMT: epithelial–mesenchymal transition [Color figure can be viewed at wileyonlinelibrary.com]
In this regard, a defense strategy against TAM-associated outcomes could be immunizations against molecules overexpressed by TAMs and thereby remodeling the tumor microenvironment that attracts these macrophages and mediating their function. Another level of control of TAM-associated EMT is provided by compounds that target EMT and thus contribute to metastasis suppression. These compounds may be especially promising for tumor treatment with the main epithelial phenotype that can obtain malignant behavior upon EMT, which clearly would be undesirable. For both cases, to acquire maximum control, novel strategies are required to develop efficient diagnostic and/or therapeutic tools. We envisioned that nanotechnology-based strategies could be useful to provide essential breakthroughs in the fight against cancer.
Nanotechnology is the art and science of the engineering and manufacturing of materials at the atomic and molecular scales. In recent years, engineered nanoparticles have started to become the most important components in the field of nanotechnology, especially in material science, photonics, electronics, supramolecular assemblies, and drug delivery. In particular, the medical application of nanotechnologies, usually termed nanomedicine, has been promising in the development of various types of drug-loaded nanocarriers, such as liposomes, nanoparticles, and micelles. Much research is currently focused on the engineering of nanoparticulate systems able to serve as efficient diagnostic and/or therapeutic tools against severe diseases, like cancer (Farokhzad & Langer, 2009; Nicolas, Mura, Brambilla, Mackiewicz, & Couvreur, 2013).
Therefore, the main objective of this review is to focus on the potential involvement of TAMs in various mechanisms of the induction, progression, and maintenance of EMT and to discuss the classification of compounds that may affect these mechanisms. Moreover, it is attempted to highlight some compounds of particular interest and to consider subjects related to their clinical application. Finally, we focused on the application of nanotechnology to studies of TAMs.
2 FACTORS INVOLVED IN EMT REGULATION
EMT in tumor cells can be triggered by stimuli from the tumor microenvironment, including acute inflammation, hypoxia, low pH, obesity, alcohol, nicotine, innate and adaptive immune responses, mechanical stress, altered extracellular matrix (ECM), treatment with antitumor drugs, and so forth (Carstens, Lovisa, & Kalluri, 2014; Marcucci et al., 2016; Nieto, 2013). However, the different responses to identical sticstiks between cell types imply that EMT is not necessarily an “all-or-nothing” transition by which tumor cells lose all epithelial markers and obtain a complete mesenchymal phenotype. Rather, partial EMTs can take place, in which cells share epithelial and mesenchymal features. For example, cells can progressively acquire mesenchymal markers, such as vimentin and N-cadherin, but rarely lose all of their preexisting epithelial markers, specifically E-cadherin (Christiansen & Rajasekaran, 2006; Serrano-Gomez, Maziveyi, & Alahari, 2016; Tam & Weinberg, 2013).
The EMT is also induced by many signaling agents or mediators in both embryos and cancer cells (Andriani et al., 2014; Marcucci, Bellone, Caserta, & Corti, 2014; Scheel et al., 2011). The activity of EMT-promoting mediators is regulated by other mediators that inhibit EMT or EMT-related properties, such as the physiological inhibitors of the WNT pathway, some of which have shown antitumor activity in preclinical studies (Cruciat & Niehrs, 2013; Nieto, 2013). Another interesting example is bone morphogenetic protein (BMP)-7, which acts as an endogenous inhibitor of transforming growth factor-β (TGF-β)–induced EMT. Furthermore, the TGF-β–induced loss of the key epithelial protein, E-cadherin, is reversed by it. Such pathways and agents may function alone or in combination, and the response is dependent on the cell context (Kalluri & Weinberg, 2009; Zeisberg et al., 2003).
Many experiments have demonstrated that a large number of signaling networks have key roles in EMT induction. For example, members of the TGF-β/BMP family, Wnt, Notch, epidermal growth factor, and fibroblast growth factor have been reported to be involved in EMT (Kalluri & Weinberg, 2009). Transcription factors and transcriptional coactivators affect these pathways, by which they coordinate the expression and functional activation of a cohort of EMT-inducing master transcription factors, such as Snail, Twist, and zinc finger E-box-binding homeobox (ZEB)1 and ZEB2 (also known as SLUG; Craene & Berx, 2013; Sánchez-Martín et al., 2003). The role of these factors can be further enhanced by the close cooperation with the epigenetic machinery that provides firm suppression of the epithelial features (Craene & Berx, 2013). For example, the activity of EMT-related transcription factors can be regulated by epigenetic mechanisms that allow the regulation of gene activity and expression without changes in the DNA sequence. These changes could be deacetylation and specific methylation or demethylation patterns, which create the context and enable transcription factors to regulate the expression of EMT-related traits (Cedar & Bergman, 2009; Craene & Berx, 2013; Serrano-Gomez et al., 2016; Tam & Weinberg, 2013). Certain types of histone methylation can lead to the local formation of heterochromatin, which is easily reversible, whereas DNA methylation (e.g., DNA methylation of the promoter region of the E-cadherin gene) causes stable long-term suppression and heritable effects (Cedar & Bergman, 2009; Dumont et al., 2008). However, there are other transcription factors that oppose the activity of EMT-inducing transcription factors, supporting a mutual regulation between these two types of transcription factors (Cieply, Farris, Denvir, Ford, & Frisch, 2013).
Another level of control of EMT is provided by small noncoding RNAs or microRNAs (miRNAs), which have emerged as critical posttranscriptional regulators of EMT (J. Zhang and Ma, 2012). It has been shown that miRNAs act as epigenetic regulators and are able to regulate a variety of cellular and molecular pathways (Banikazemi et al., 2018; Golabchi et al., 2018; Jafari et al., 2018; Masoudi, Mehrabian, & Mirzaei, 2018; Mirzaei, Yazdi, Salehi, & Mirzaei, 2016; Mirzaei, Ferns, Avan, & Mobarhan, 2017; Saeedi Borujeni et al., 2018; Simonian, Mosallayi, & Mirzaei, 2018; Tavakolizadeh et al., 2018). Deregulation of miRNAs is associated with the emergence and development of several diseases such as cancer, stroke, diabetes, and cardiovascular diseases (Gholamin et al., 2016, 2018; Hoseini et al., 2018; Mirzaei, Naseri, et al., 2016; Mirzaei, Sahebkar, et al., 2016; Mirzaei, Gholamin, et al., 2016; Mirzaei, 2017; Mirzaei, Fathullahzadeh, et al., 2018; Mirzaei, Momeni, et al., 2018; Moridikia, Mirzaei, Sahebkar, & Salimian, 2018; Rabieian et al., 2018; Rashidi, Malekzadeh, Goodarzi, Masoudifar, & Mirzaei, 2017; Rashidi, Hoseini, Sahebkar, & Mirzaei, 2017). These molecules exert their effects via targeting various signaling pathways that are involved in many vital biological processes (Fathullahzadeh, Mirzaei, Honardoost, Sahebkar, & Salehi, 2016; Golabchi et al., 2018; Keshavarzi, Darijani, et al., 2017; Keshavarzi, Sorayayi, et al., 2017; Mashreghi et al., 2018; Mirzaei, Khataminfar, et al., 2016; Mirzaei, Sahebkar, Jaafari, Goodarzi, & Mirzaei, 2017; Mohammadi, Goodarzi, Jaafari, Mirzaei, & Mirzaei, 2016; Saadatpour et al., 2016; Salarinia et al., 2016). Several miRNAs induce or suppress EMT through the direct or indirect regulation of EMT-related features or transcription factors expression. However, miRNAs at the mesenchymal side could affect the mesenchymal phenotype. For instance, a tight negative feedback loop is formed by the miRNAs such as miR-200 and miR-34 with several EMT transcription factors (EMT-TFs). Moreover, the epithelial phenotype is further supported by miR-101, which promotes E-cadherin expression. Nonetheless, miR-21 is the most regulated miRNA in solid tumors and a potent inducer of EMT (Coppola et al., 2013; Craene & Berx, 2013; Sekhon, Bucay, Majid, Dahiya, & Saini, 2016).
3 FACTORS INVOLVED IN M2-POLARIZED TAMS
Macrophages are one of the main regulators that are present in the tumor microenvironment. It is known that a large amount of TAMs could be associated with bad prognosis. Moreover, several studies have indicated that TAMs are related to various aspects of tumor metastasis (Bingle, Brown, & Lewis, 2002; Condeelis & Pollard, 2006; Solinas et al., 2010). Several pathological studies have indicated that molecular profiles are related to poor prognosis in lymphomas and breast carcinomas, and that there are a variety of genes characteristic of macrophages such as CD68. Moreover, the genetic ablation of macrophages is associated with the inhibition of metastasis and tumor development in mouse models (Paik et al., 2004). The study of the induction of invasion and TAM transcriptome indicated that TAMs are a unique subpopulation with particular gene expression profiles associated with tissue and organ development (Biswas et al., 2006; Ojalvo, Whittaker, Condeelis, & Pollard, 2010). M2-like phenotype is mostly the phenotype of TAMs (Sica, Schioppa, Mantovani, & Allavena, 2006). The absence of M1-orienting signals (e.g., interferon-γ (IFN-γ) or bacterial components as well as the expression of M2 stimuli) in the tumors could lead to the preferential polarization of M1 to M2 macrophages. Multiple lines of evidence have revealed that immune complexes/TLR ligands, specific growth factors (e.g., macrophage colony-stimulating factor [M-CSF]), and poststimulation with some cytokines (e.g., IL-4 and IL-13), or IL-10 and glucocorticoids could contribute to the differentiation of M2 macrophages from monocytes (Mantovani et al., 2002). It has been shown that there are infiltrations of Th2 lymphocytes in several cancers, and Th2 lymphocytes are known as an important source of IL-4 and IL-13 cytokines (Nevala et al., 2009). Generally, M2 macrophages are capable of secreting IL-12low, IL-10high, IL-1decoyRhigh, IL-1RAhigh, CCL17, and CCL22, a large amount of mannose, scavenger, and galactosetype receptors, wound-healing promotion, poor Ag-presenting capability, scavenging of debris, angiogenesis, and tissue remodeling. Moreover, the existence of M2-polarized myeloid cells and associated myeloid suppressor cells inhibits the immune/inflammatory response via downregulating M1 macrophages and T cell–mediated functions (Mantovani et al., 2002). On the other hand, M1 macrophages differentiated with the stimulation of IFN-γ and microbial stimuli (e.g., LPS) and could be defined by the upregulation of IL-12 and IL-23 and the resulting activation of polarized Th1 response, cytotoxic activity, and good capability as antigen-presenting cells (Mantovani et al., 2002).
The regulation of NF-κB activation through p50 homodimers could be related to M2 differentiation (Saccani et al., 2006). Peroxisome proliferator–activated receptor g is another protein that is involved in M2-like differentiation (Odegaard et al., 2007). Phosphatase SHIP has a key role in balancing macrophage polarization, although some studies proposed that it could act indirectly via the regulation of IL-4 production by basophils (Kuroda et al., 2009). CCL2 and IL-6 are other factors that could contribute to macrophage polarization via sustaining the survival of myeloid cells at the tumor site (Mirzaei, Ferns, et al., 2017).
Recently in a study, Guo et al. (2017) indicated that a large amount of M2 macrophages in hepatocellular carcinoma (HCC) tissues was observed. They showed that the utilization of oxaliplatin treatment could lead to M2 macrophages upregulated interleukin-17 (IL-17), which is known as the main inflammatory cytokine. The upregulated IL-17 by M2 macrophages could be associated with the inhibition of oxaliplatin-induced apoptosis. By knocking down the IL-17 receptor and lysosome-associated membrane protein 2A (a key protein in chaperone-mediated autophagy) in HCC cells, they observed that IL-17 is able to induce chaperone-mediated autophagy, which has a critical role in the suppression of oxaliplatin-induced apoptosis treatment. Moreover, their results revealed that chaperone-mediated autophagy is associated with tolerance to oxaliplatin treatment via decreasing the expression of cyclin D1. Hence, the upregulation of cyclin D1 could induce oxaliplatin-induced apoptosis. The expression of cyclin D1 could be inhibited by IL-17, but it is increased when the IL-17 receptor was knocked down. These findings indicated that M2 macrophages are able to generate large amounts of IL-17 in the HCC microenvironment, which are associated with the inhibition of oxaliplatin-induced tumor cell apoptosis via activating chaperone-mediated autophagy and in turn decreasing the expression of cyclin D1 (Guo et al., 2017).
IL-35 is known as an immunosuppressive cytokine that is an important regulator. This cytokine could affect B cells, T cells, macrophages, and dendritic cells. Zou et al. (2017) indicated that IL-35 is able to induce N2 neutrophil polarization via increasing the production of IL-6 and G-CSF. IL-35 can stimulate neutrophil infiltration into the tumor microenvironment. Their results revealed that macrophages induced by IL-35 could produce proinflammatory cytokines (i.e., IL-1β and IL-6), and IL-1β stimulated γδ T cells to produce IL-17, which led to an increase in the production of G-CSF. Moreover, they showed that the upregulation of G-CSF, IL-6, and IL-35 could be associated with the upregulation of MMP-9 and Bv8 expressions and the downregulation of TRAIL expression in neutrophils. Moreover, the upregulation of G-CSF/IL-6 could induce the improved activation of STAT3 and ERK pathways in neutrophils, which led to an increase in the iNOS expression to suppress T cell activation. These findings proposed that IL-35 could induce tumor progression via functioning as an upstream cytokine to induce cancer-related inflammation and regulate neutrophil polarization (Zou et al., 2017). In another study, J. Zhang et al. (2016) investigated the role of IL-35 in various models, including a keratin 14 (K14)-vascular endothelial growth factor A (VEGF-A)-transgenic (Tg) mouse model, a human keratinocyte cell line (HaCaT), and an imiquimod-induced psoriasis mouse model. Their results indicated that IL-35 is able to inhibit IL-6, CXCL8, and S100A7 expressions, which were highly upregulated by a mixture of five proinflammatory cytokines in HaCaT. Utilization of a plasmid containing a human IL-35 sequence, which was coated by cationic liposomes, revealed powerful immunosuppressive effects on imiquimod-induced psoriasis mouse models and K14-VEGF-A-Tg. Their results showed that our many kinds of proinflammatory cytokines were significantly decreased, whereas IL-10 was remarkably stimulated by IL-35. Moreover, they indicated that IL-35 is able to reduce the total number of macrophages and the ratio of M1/M2 macrophages (J. Zhang et al., 2016).
4 FUNCTION OF M2-POLARIZED TAMS IN EMT INDUCTION
Several studies have recently demonstrated that M2-polarized TAMs play a critical role in EMT induction that supplies tumor cells with malignant functionalities. For instance, Knauf et al. (2011) developed papillary thyroid cancer (PTC) models that are locally invasive and have well-defined foci of poorly differentiated thyroid carcinoma (PDTC). A microarray analysis using RNA has shown that a considerable deregulation of genes is involved in cell adhesion and intracellular junctions, with changes consistent with an EMT. In the same article, it was reported that transforming growth factor β1 (TGF-β1) signaling is the pathway responsible for the specific induction of EMT through an MAPK-dependent process. In accordance with other previous studies, it was also shown that a large number of TAMs, a known source of TGF-β, was present in regions of PDTC compared with PTC (Ryder M., Ghossein R. A., Ricarte-Filho, Knauf, & Fagin, 2008). Furthermore, quantitative reverse transcriptase-PCR for TGF-β has displayed that both TAMs and thyroid cancer cells express TGF-β, with a slightly greater expression in TAMs (Knauf et al., 2011).
These observations were confirmed by Bonde, Tischler, Kumar, Soltermann, and Schwendener (2012), who specifically demonstrated the contributions of intratumoral macrophages to EMT regulation through TGF-β signaling and activation of the β-catenin pathway in intratumoral cancer cells.
Moreover, Fan et al. (2014) used Hepa1–6 cells in the investigation of the effects of TAMs upon the progression and metastasis of HCC. This study reconfirmed that TGF-β1 secreted from TAMs is a potent inducer of EMT and suggested that the depletion of TGF-β1 could block the acquisition of CSC-like attributes by the inhibition of TGF-β1–induced EMT.
C.-Y. Liu et al. (2013) also investigated the potential role of toll-like receptor 4 (TLR4)/interleukin-10 (IL-10) signaling in the EMT of pancreatic cancer. It is surprising to note that the receptor is primarily manifested on macrophages and that the activation of TLR4 on M2-polarized TAMs stimulates a rise in the cytokine (IL-10). This study has shown that M2-polarized TAMs caused a mesenchymal phenotype in cocultured pancreatic cancer cells through TLR4/IL-10 signaling activation. Moreover, it was observed that coculture with pancreatic cancer cells increased TLR4 mRNA and protein expressions in M2-polarized TAMs, suggesting a reciprocal interaction between TAMs and cancer cells (C.-Y. Liu et al., 2013). Although significant progress has not been made toward targeting this pathway, it would be desirable to develop inhibitors of this pathway that may prevent or reverse EMT.
5 SIGNALING PATHWAYS INVOLVED IN EMT INDUCTION BY M2-POLARIZED TAMS AND POTENTIAL INHIBITORS
5.1 TGF-β signaling pathway
Numerous studies have shown that both EMT and CSC generation is associated with TGF-β signaling and the inhibition of TGF-β signaling in CSCs, which can re-establish an epithelial phenotype (Dong, Liu, Jin, & Wang, 2017; Fan et al., 2014; Giannelli, Villa, & Lahn, 2014; Grygielewicz et al., 2016; F. Liu, Kong, Lv, & Gao, 2015; Shipitsin et al., 2007; Y. Yu et al., 2014). In some examples, however, it functions as a tumor suppressor by inhibiting proliferation and inducing apoptosis (Yagi et al., 2002). The enabling of the TGFβ-induced functions is possibly regulated by the rigidity of the tumor ECM (Leight, Wozniak, Chen, Lynch, & Chen, 2012).
The TGF-β superfamily comprises TGFβs, activins, BMPs, and other homodimers and heterodimers of ligands that function through membrane receptors that are serine/threonine kinase, as well as Tyr kinase (Massagué, 2000). TGF-β signaling toward EMT is moderated by both Smad-dependent and Smad-independent pathways, such as p38 MAP kinase (p38 MAPK). Although the mechanism through which Smads manage signaling is limited to TGF-β signaling, the function of p38 MAPK could be potentiated by other pathways, including Ras and Wnt, which collaborate with TGF-β to induce EMT (Fuxe, Vincent, & Garcia de Herreros, 2010; Heldin, Landström, & Moustakas, 2009; Pang et al., 2016; Xu, Lamouille, & Derynck, 2009). In this regard, TGF-β signaling could be described as a pathway that converts stimuli to transcriptional reprogramming, which promotes the inactivation of genes encoding epithelial markers (E-cadherin and other junction proteins) and the activation of genes encoding mesenchymal markers (vimentin and N-cadherin; Vincent et al., 2009; Xu et al., 2009). As a result, tumor cells undergoing TGF-β–induced EMT obtain the capacity to disseminate throughout the organism and migrate away from the primary tumor.
5.2 Smad-dependent pathways in TGF-β–induced EMT
TGF-β signals via TGF-β type I and type II serine-threonine kinase receptors. In response to the TGF-β ligand, the type II receptor kinases phosphorylate the type I receptors, which then leads to the activation of cellular responses to TGF-β. TGF-β–induced activation of the receptor complex leads to the recruiting and activating of receptor-regulated Smads (R-Smads), including Smad2 and Smad3, through direct phosphorylation of the two distal serines of the C-terminal SSXS motif by the type I receptor kinases (Derynck & Zhang, 2003). These activated Smads then form a heterotrimeric complex with the common partner Smad (co-Smad; Smad4 in mammalian) and translocate into the nucleus, where they cooperate with and associate with DNA binding transcription factors to activate or suppress target gene transcription (Ikushima & Miyazono, 2010; Figure 2).
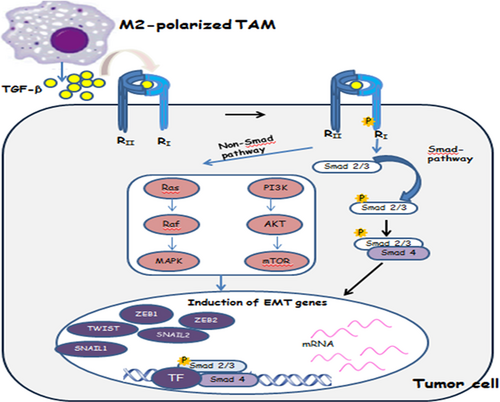
SMAD and non-SMAD pathways in TGF-β–induced EMT. In the SMAD pathway, Smad2 and 3 are activated and form complexes with Smad4, which then regulate the transcription of target genes through interactions with other DNA binding transcription factors. In the induction of EMT, the activated Smads mediate transcriptional regulation through transcription factors, which results in the repression of epithelial marker gene expression and the activation of mesenchymal gene expression. In the non-SMAD pathway, PI3 kinase signaling is induced by TGF-β, leading to the activation of Akt-mTOR signaling and consequently to increased translation. Activation of the RAF–RAS–MAPK pathway can also induce EMT, and it has been shown to potentiate PI3K–AKT–mediated EMT induction. EMT: epithelial–mesenchymal transition; MAPK: mitogen-activated protein kinase; mTOR: mammalian target of rapamycin; PI3K: phosphoinositide 3-kinase; R: receptor; TAM: tumor-associated macrophages; TF: transcription factor; TGF-β: transforming growth factor-β; ZEB1: zinc finger E-box-binding homeobox 1 [Color figure can be viewed at wileyonlinelibrary.com]
5.3 Smad-independent pathways in TGF-β–induced EMT
In addition to Smads, which are critical signal transducers in TGF-β signaling, direct interaction of TGF-β with the type II and/or type I receptors is also known to prompt signaling cascades through pathways that are mainly considered as important pathways that affect tyrosine kinase (Xu et al., 2009). The non-Smad signaling pathways slow down activation by TGF-β, and in some cases it may result from Smad-mediated transcription responses and activation with rapid kinetics (5–15 min); however, in other cases, it suggests independence from transcription and follows similar kinetics as Smad signaling (Derynck & Zhang, 2003; Nawshad, LaGamba, Polad, & Hay, 2005). Among the non-Smad signaling responses, activation of Erk MAP kinase signaling, Rho-like GTPases, and the PI3 kinase/Ak/mammalian TOR complex 1 (mTORC1) pathway has been linked to TGF-β–induced EMT via the regulation of distinct processes like cytoskeleton rearrangement, cell growth, survival, migration, or invasion (Figure 2). Erk MAP kinase signaling associates with the TβRI receptor and is phosphorylated at Tyr and Ser by TβRI in response to TGFβ. Rho-like GTPases are involved in cell motility and in the invasive behavior in cancer-associated EMT. Furthermore, the PI3 kinase/Akt-mTORC1 pathway increases translation and cell size and activates AKT, which can also depress the translation of specific mRNAs (Lamouille, Xu, & Derynck, 2014).
The treatment of cells with molecules that selectively or particularly block the ability of one or several of these pathways has been shown to dramatically inhibit EMT and the downstream of transcriptional responses by TGF-β, which suggested strongly that the cooperation of Smad-independent signaling totalizes Smad signaling in the enhancement of EMT response.
Moreover, it has been documented that TGF-β inhibitors are the most extensively investigated agents because TGF-β is one of the most frequently upregulated signal transduction cascades in human cancers; thus, there is a strong rational for targeting TGF-β signaling in tumors. Moreover, TGF-β is a multifunctional cytokine, and its inhibition results in effects that could be unrelated to EMT inhibition, like the enhancement of adaptive antitumor immune responses (J. Liu et al., 2012; Zhong et al., 2010). Signal transduction modulators ensure the provision of more effective, less-toxic medical therapies for cancer patients. PI3K/AKT/mTOR inhibitors, RAS/RAF/MAPK inhibitors, monoclonal antibodies (mAbs), and tyrosine kinase inhibitors are some examples of this class of compounds that have been shown to inhibit EMT in preclinical studies. In some cases, these inhibitors have been examined in clinical trials and have been approved for the treatment of certain cancers (Table 1).
| Classes of inhibitors | Examples of agents and development status | References |
|---|---|---|
| PI3K/AKT/mTOR | PI3K inhibitors: Idelalisib is approved for CLL; PX-866 is up to Phase II for CRPC; XL-147 is up to Phase II for advanced malignancies. Several compounds such as GDC-0941, RX-0201, PBI-05204, and GSK-2141795 are now in clinical trials for malignant cancers. | Chakrabarty, Sanchez, Kuba, Rinehart, and Arteaga (2012); www.clinicaltrials.gov |
| AKT inhibitors: GSK690693 is now in clinical trials for relapsed or refractory hematologic malignancies; Perifosine is now in clinical trials for metastatic cancer. | W. Chen, Wu, Zhang, Wang, and Shi (2013) and Pal, Reckamp, Yu, and Figlin (2010); www.clinicaltrials.gov | |
| mTOR inhibitors: Everolimus is approved for some cancer indications; Temsirolimus is approved for RCC. Rapamycin and rapalogs are now in clinical trials for cancer treatment. | Chiarini, Evangelisti, McCubrey, and Martelli (2015); www.clinicaltrials.gov | |
| Dual PI3K/mTOR inhibitors: GDC0980, BGT226, and BEZ235 are up to Phase II for cancer. NVPBEZ235 has been developed for advanced solid tumors. | Chang et al. (2013) and Lin et al. (2014); www.clinicaltrials.gov | |
| RAS/RAF/MAPK | RAS inhibitors: Tipifarnib is up to Phases I, II, and III for AML, lymphoma, breast, glioma, and melanoma. | Arvizo et al. (2013) and Mulholland et al. (2012) |
| RAF inhibitors: Vemurafenib and dabrafenib (BRAF inhibitors) are approved for late-stage melanoma, and sorafenib (an RAF inhibitor) is approved for RCC and HCC. | www.clinicaltrials.gov | |
| MAPK inhibitors: Trametinib (an MEK inhibitor) is approved for melanoma; rdEA119 is up to Phase I for advanced cancer. | www.clinicaltrials.gov | |
| Rho-like GTPases | Several compounds of this class are now in clinical trials for the treatment of tumors. | Shen et al. (2015); www.clinicaltrials.gov |
| TGF-β–TGF-β receptor | mAbs and tyrosine kinase inhibitors are up to Phase II for cancer treatment. | Giannelli et al. (2014) and Rodon et al. (2014); www.clinicaltrials.gov |
- Note. CLL: chronic lymphocytic leukemia; CRPC: castration-resistant prostate cancer; EMT: epithelial–mesenchymal transition; HCC: hepatocellular carcinoma; mAb: monoclonal antibody; MAPK: mitogen-activated protein kinase; MEK: MAPK/ERK kinase; mTOR: mammalian target of rapamycin; PI3K: phosphoinositide 3-kinase; RCC: renal cell carcinoma; TGF-β: transforming growth factor-β.
For instance, one of the most promising inhibitors of TGF-β is one small molecule named TGF-β RTK inhibitor (LY2157299) that has entered clinical development and is also known as galunisertib. In vitro studies have shown that this compound blocks the invasion and migration of HCC cells, extends their expression of E-cadherin, and decreases the secretion of E-cadherin in the supernatant (Fransvea, Angelotti, Antonaci, & Giannelli, 2008).
The mTOR signaling pathway is another important pathway that could be involved in macrophage polarization. It has been shown that this signaling pathway acts as a central regulator of a variety of biological processes such as cell metabolism, growth, proliferation, and survival. It is known that important cellular processes (i.e., angiogenesis, insulin resistance, immune cell activation, tumor development, and adipogenesis) need an active mTOR pathway. Deregulation of the mTOR pathway is associated with the initiation and progression of several diseases, such as cancer and type 2 diabetes. Hence, mTOR inhibitors such as rapamycin and its analogs could be used as one of the important therapeutic options in the treatment of several diseases (e.g., solid tumors, rheumatoid arthritis, and during organ transplantation). The mTOR kinase plays critical roles in controlling cell proliferation and growth. The mTOR kinase plays an important part in two multiprotein complexes: mTORC1, which is able to regulate protein synthesis, and mTORC2, which is able to control cytoskeleton reorganization (Düvel et al., 2010; Zarogoulidis et al., 2014).
Multiple lines of evidence have revealed that the activation/inhibition of mTORC1 is associated with inflammation as well as the activation of mTOR via affecting macrophages and other cells of the immune system, which could lead to controlling the main regulators involved in inflammation (i.e., IL-10, TGF-β, PD-L1, and NFκ light-chain enhancer of activated B cells (NF J B) activity; Byles et al., 2013). It is known that glucose metabolism via the AKT/mTORC1 signaling pathway could lead to the sustained IL-4–mediated M2 activation of macrophages (Covarrubias, Aksoylar, & Horng, 2015). These findings proposed that the activation of M1 and M2 macrophages could be mediated by mTORC1 expression, which could be context dependent (Covarrubias et al., 2015). Moreover, the loss of tuberous sclerosis 1 (TSC1), which negatively regulates mTORC1, could lead to the increase of M1 and decrease of M2 activation (Byles et al., 2013).
mTORC2 is another multiprotein that could be involved in macrophage polarization via regulating macrophagic growth factors (e.g., M-CSF; Festuccia, Pouliot, Bakan, Sabatini, & Laplante, 2014; S. C.-C. Huang et al., 2016). Several studies have confirmed that macrophages and dendritic cells are able to increase their inflammatory response in the absence of mTORC2 (Moynihan et al., 2016). The lack of Rictor, which is known as an important component of mTORC2, led to the increase of the M1-like phenotype (Moynihan et al., 2016). S. C.-C. Huang et al. (2016) indicated that increased glucose use is important for IL-4–stimulated macrophages, and this happens via the activation of the mTORC2 pathway. They showed that M-CSF is able to affect mTORC2 in a pathway that involved PI3K and AKT, resulting in stimulation of the transcription factor interferon regulatory factor 4 (IRF4) expressions. Finally, IRF4 could increase glucose flux via glycolysis (S. C.-C. Huang et al., 2016).
mTOR inhibitors are the most investigated examples that have displayed potent suppression ability on aggressive malignancies in preclinical studies. In this regard, significant research have been made toward the therapeutic application of these compounds. However, there are no ongoing clinical trials with it for the prevention of cancer as well as EMT, although they were approved for use for the treatment of some types of cancer, including advanced renal cell carcinoma (Chiarini et al., 2015).
5.4 Application of nanotechnology in TAM studies
Nanotechnology is a multidisciplinary technology, which covers a wide and diverse array of devices derived from engineering, biology, physics, and chemistry. Some cancer-related examples of nanotechnologies are injectable drug delivery nanovectors like liposomes for cancer therapy, biologically targeted therapies, nanosized magnetic resonance imaging (MRI) contrast agents for intraoperative imaging in the context of neuro-oncological interventions, and novel nanoparticle-based methods for high-specificity detection of DNA and protein. Moreover, it has shown promise for the early detection of transforming cell populations via in vivo imaging or ex vivo analysis. Furthermore, it will allow the appropriate combination of agents chosen (based on accurate biological information on the tumor), then the targeting of these agents (while avoiding biological barriers) to the early cancer lesions to contain or eliminate them without collateral effects on healthy tissue, and followed by real-time monitoring of the treatment effects (Ferrari, 2005).
Nanotechnology has also been reported to be involved in the field of TAM research and may achieve valuable advances in the development of anticancer therapeutic strategies with high efficacy and few side effects. It has been shown that various types of agents such as peptides, proteins, and carbohydrates could be used for targeting several ligands (Table 2).
| Type of ligand | Ligand | Imaging agent/active part | NanoCarrier | Cancer(s) | References |
|---|---|---|---|---|---|
| Peptide | Lyp-1 | Fluorescein and Doxorubicin | Liposomes | Lymphatic metastatic tumors | Z. Yan et al. (2012) |
| Y-shaped peptide | Antiapoptotic peptide and iron oxide | Carbon nanotube | Breast cancer | L. Yan et al. (2014) | |
| M2pep | siRNA | Nanoparticle | Lung tumor | Conde et al. (2015) | |
| Protein | Legumain | Hydrazinocurcumin, Doxorubicin | Liposomes | Breast cancer | X. Zhang et al. (2013) |
| Antibody | Anti-mouse CD206 antibody | DyLight680 succinimidyl ester | – | Hepatoma | Z. Huang et al. (2012) |
| Monoclonal antibody | – | Liposomes | – | Etzerodt et al. (2012) | |
| Nanobodies | 99mTc | – | Breast cancer | Movahedi et al. (2012) | |
| Carbohydrate | Mannose | 64Cu and a fluorescent dye | Liposomes | Lung cancer | Locke, Mayo, Yoo, Williams, and Berr (2012) |
| Mannose | Doxorubicin | Nanoparticle | Lung adenocarcinomas | Fritz et al. (2014) | |
| Mannose | siRNA | Micelles | Breast cancer | S. S. Yu et al. (2013) | |
| Galactosylated cationic dextran | Oligonucleotide | Nanoparticle | Hepatoma | Z. Huang et al. (2012) | |
| Carboxydextran | Iron oxide for MRI | Nanoparticle | Breast cancer | Daldrup-Link et al. (2011) | |
| Dextran | Fluorochrome VT680 | Nanoparticle | Colon carcinoma, lung adenocarcinoma, and soft tissue sarcoma | Leimgruber et al. (2009) |
- Note. MRI: magnetic resonance imaging; siRNA: small interfering RNA.
It has been shown that scavenger receptors such as lectin (CD206/mannose receptors) are upregulated selectively on TAMs. CD206 receptor is a mannose receptor that could bind to mannose and fucose residues and then begin its endocytosis via a clathrin-mediated pathway. These properties led to the use of carbohydrate moieties (e.g., dextrans, mannose, and galactose) for coated nanoparticles containing drug and nucleotide for targeting TAMs by CD206 receptors (Ortega et al., 2015). S. S. Yu et al. (2013) used the synthesized mannose copolymer to develop a micellar system for targeting siRNA to TAMs. Their results indicated that mannose functionalization is able to improve the macrophage uptake of micelles and lead to knockdown of the target gene. Another study used mannosylated nanoparticles for targeting siRNA to TAMs. Their results revealed that nanoparticles targeted selectively in ovarian tumor and lung tumor, undergoing rapid internalization by the delivered TAMs to inhibit cancer metastasis without having any side effects on kidney and liver functions (Ortega et al., 2015).
Ryan et al. developed mannosylated polymer nanoparticles that bypass the endosomal uptake to deliver therapeutic siRNA to TAMs, to reprogram them, and adopt an immunogenic, antitumor phenotype. They have also shown that the delivery of siRNA to tumor, in particular TAMs, might be designed specifically and effectively using nanoparticles (Ortega et al., 2015). In this regard, Kenji et al. have proposed silicon nanocarriers that specifically deliver therapeutic agents to tumor-associated macrophages as well as to endothelial cells within the stroma of pancreatic tumors (Yokoi et al., 2013).
Peptides are one of the main ligands that could be targeted by various nanocarriers. Peptides are known as small amino acid sequences that have linear or cyclic structures. Peptides have small molecular sizes and are easy to synthesize, and thus these properties could lead to environmental stability. Hence, peptides could be introduced as effective ligands for targeting by nanocarriers (Conde et al., 2015). Cieslewicz et al. (2013) provided a peptide sequence M2pep (YEQDPWGVKWWY). This peptide had high affinity to TAMs in a mixed-cell population. Therefore, it can be used as a new targeting vector for the intracellular delivery of an apoptotic peptide. It is known that M2pep could be used to decorate a nanoparticle system for siRNA delivery to TAMs, which led to the inhibition of VEGF expression and related tumor burden (Conde et al., 2015).
Antibody is another type of agent that could be applied for targeting various ligands to TAMs. Antibodies are known as Y-shaped proteins that can recognize specific regions on a target antigen. The binding of antibody on a nanoparticle surface could assist in the delivery of nanoparticles to specific cells. Sun et al. developed a near-infrared fluorescence-responsive probe by fusing anti-mouse CD206 antibody with DyLight680 succinimidyl ester. They showed that this probe, generating pertinent in vivo images in a 4T1 tumor-bearing animal model, specifically tags onto TAMs (Sun et al., 2015).
Although extensive studies have been carried out toward creative solutions of nanoparticles for the most difficult problems of TAMs (Conde et al., 2015; Song, Liu, Shi, Zhang, & Chen, 2015; X. Zhang et al., 2013; Zhu, Niu, O’Mary, & Cui, 2013), there are other interesting examples that address the function of TAMs in nanotherapeutics delivery. For instance, a recent study has reported that TAMs act as a local reservoir for therapeutic nanoparticles involving platinum (IV) that accumulates significant transporters in which platinum (IV) gradually gets released to the neighboring tumor cells (Miller et al., 2015). In addition, Alizadeh, Zhang, Hwang, Schluep, and Badie (2010) have shown a delivery technique based on TAMs that may phagocyte the nanoparticles and improve their transport into malignant brain tumors. This method may provide a valuable drug delivery technology to find a way around obstacles like the blood–brain barrier. Another area of research on TAMs using nanotechnology is the production of nanomaterials that are specific for immune cell subsets and can be used as imaging replacements for nanotherapeutics. In this regard, new in vivo imaging clinical tools for TAMs may provide prognostic information, display differences in TAM concentrations within a single tumor or across metastases, direct biopsies, determine tumor borders both during the presurgical planning phase and during surgical resection, and quantitate responses to treatment (Kircher, Mahmood, King, Weissleder, & Josephson, 2003; Zimmer et al., 1997).
In addition to cancer, macrophage-targeting nanoparticles could be used for atherosclerosis, myocardial infarction, and stroke imaging because macrophage infiltrates are common in these diseases (Nahrendorf et al., 2008; Weissleder, Nahrendorf, & Pittet, 2014). These experiences indicate that promising approaches based on nanomaterials can be developed to manage cancer, metastasis, and some of the principal challenges associated with them.
6 CONCLUSION
Although significant progress has been made toward the therapeutic application of antitumor compounds, there are still several challenges that need to be addressed. Most studies have confirmed that tumor growth and metastasis are triggered by a small population of cancer stem cells, which are defined functionally by their ability to resist apoptosis and antitumor drugs and to contribute to immunosuppression. Although currently available therapeutic agents can reduce the mass of metastatic tumors, these effects are usually unstable and often do not extend the life of patients considerably. One possibility for the failure of these treatments is that existing therapies fail to kill cancer stem cells effectively. On the contrary, EMT has been described over the past decade as a cell-biological program that is required during the acquisition of stemness features by carcinoma cells.
Although some studies have shown the role of TAMs and their released cytokines (e.g., IL-1, TNF-α, IL-10) in all aspects of tumor cell invasion and metastasis and reinforced the association between inflammation and EMT, further large and well-designed studies are still necessary to carefully determine the effect of different signaling pathways triggered by the tumor microenvironment. Therefore, it seems pivotal to target tumor cells and their microenvironment, besides considering the inhibitory strategy of EMT to further improve successful tumor therapy. However, it must be possible to particularly identify and attack signaling pathways in CSCs without negatively affecting healthy stem cells (targeted therapy). Therefore, because nanotechnology is opening the doors to exploiting the targeting and imaging methods, essential breakthroughs in the fight against cancer will hopefully be possible. In this study, issues that appeared the most crucial for the near future were discussed.
ETHICAL APPROVAL
This study does not contain any studies with human participants performed by any of the authors.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.