Berberine attenuates pulmonary arterial hypertension via protein phosphatase 2A signaling pathway both in vivo and in vitro
Abstract
Excessive proliferation, migration, and antiapoptosis of pulmonary artery (PA) smooth muscle cells (PASMCs) underlies the development of pulmonary vascular remodeling. The innervation of the PA is predominantly sympathetic, and increased levels of circulating catecholamines have been detected in pulmonary arterial hypertension (PAH), suggesting that neurotransmitters released by sympathetic overactivation may play an essential role in PAH. However, the responsible mechanism remains unclear. Here, to investigate the effects of norepinephrine (NE) on PASMCs and the related mechanism, we used 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide, the proliferating cell nuclear antigen and the cell counting kit-8 assay to evaluate the proliferation of PASMCs, Boyden chamber migration, and wound-healing assays to assess migration and western blot analysis to investigate protein expression. We demonstrated that the phosphorylation level of the protein phosphatase 2A (PP2A) catalytic subunit (Y307) was higher in PAH patients and PAH models than in controls, both in vivo and in vitro. In addition, NE induced the proliferation and migration of PASMCs, which was attenuated by berberine (BBR), a Chinese herbal medicine, and/or PP2A overexpression. PP2A inhibition worsened NE-induced PAH and could not be reversed by BBR. Thus, PP2A is critical in driving PAH, and BBR may alleviate PAH via PP2A signaling pathways, thereby offering a potential therapeutic option for PAH.
Abbreviations
-
- BBR
-
- berberine
-
- CCK-8
-
- cell counting kit-8
-
- ERK1/2
-
- extracellular signal-regulated protein kinases 1 and 2
-
- GAPDH
-
- glyceraldehyde 3-phosphate dehydrogenase
-
- GFP
-
- green fluorescent protein
-
- MTT
-
- 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
-
- PADN
-
- percutaneous pulmonary artery denervation
-
- PAH
-
- pulmonary arterial hypertension
-
- PASMC
-
- pulmonary artery smooth muscle cells
-
- PCNA
-
- proliferating cell nuclear antigen
-
- PP2Ac
-
- protein phosphatase 2A catalytic subunit
-
- RVHI
-
- right ventricle hypertrophy index
-
- SD rat
-
- Sprague–Dawley rat
-
- VEGFR
-
- vascular endothelial growth factor receptor
-
- α1-AR
-
- α1-adrenoceptor
-
- α-SMA
-
- α-smooth muscle actin
1 INTRODUCTION
Pulmonary arterial hypertension (PAH) is an occlusive disease mainly characterized by uncontrolled proliferation, migration, and apoptosis resistance of the pulmonary artery (PA) smooth muscle cells (PASMCs), leading to a persistent increase in pulmonary vascular resistance, increased afterload in the right ventricle (RV) and, ultimately, right-heart failure and death (Rabinovitch, 2012; Seeger & Pullamsetti, 2013). Although significant progress has been made in the past decade in the treatment of PAH, current approaches are limited to pharmacological vasodilation and symptomatic relief (Gomberg-Maitland et al., 2013; Humbert et al., 2010; H. Liu, Chen, et al., 2016), resulting in unacceptable mortality (Barst, Chung, Zamanian, Turner, & McGoon, 2013; Weatherald et al., 2017). Thus, the further identification of mechanisms of PA remodeling that may serve as new therapeutic targets is urgently needed.
Similar to the vasculature and innervation of other organs, the vascular bed of the lung is regulated by the nervous system, and the innervation of the PA is predominantly sympathetic (Kummer, 2011; Shirai et al., 2014). Hypoxia has been shown to cause sympathetic overactivation (Velez-Roa, 2004), and prolonged stimulation of α1-adrenoceptors (α1-ARs) induces hypertrophy and hyperplasia of arterial smooth muscle cells (Faber, Szymeczek, Salvi, & Zhang, 2006; Vecchione, 2002; H. Zhang & Faber, 2001), which are major features of PAH. In addition, in patients with primary and secondary pulmonary hypertension (PH), venous norepinephrine (NE) levels are higher than in healthy controls due to chemoreflex-dependent sympathetic overactivation (Ciarka, Doan, Velez-Roa, Naeije, & van de Borne, 2010; Velez-Roa, 2004; Wensel et al., 2009), suggesting that catecholamines may contribute significantly to pulmonary vascular remodeling. Furthermore, we previously showed that percutaneous pulmonary artery denervation (PADN) abolished the increase in pulmonary arterial pressure in a dog model of PAH (S. L. Chen, Zhang, Zhou, et al., 2013; Zhou et al., 2015) and in PAH patients (S. L. Chen, Zhang, Xu, et al., 2013). However, published data have not fully demonstrated the mechanisms by which catecholamines contributes to PAH (Galiè & Manes, 2013).
Protein phosphatase 2A (PP2A), a major serine–threonine phosphatase involved in the regulation of many cellular processes such as cell proliferation and death, cell mobility, cytoskeleton dynamics, and various signaling pathways (Perrotti & Neviani, 2013; Shi, 2009), is a heterotrimeric complex that consists of a scaffolding subunit (A), a regulatory subunit (B), and a catalytic subunit (C; Xu et al., 2006). The phosphorylation of multiple residues on the C subunit C terminus is critical for modulating PP2A activity (Sangodkar et al., 2016). Phosphorylation of Y307 was shown to inhibit the methylation of L309 by leucine carboxyl methyltransferase-1 (LCMT-1), limiting the specific B subunit binding to the core enzyme, which increased T304 phosphorylation and inhibited PP2A activity (Sangodkar et al., 2016; Shi, 2009). PP2A catalytic subunit (PP2Ac) expression is significantly lower in pancreatic cancer tissue than in noncancerous tissue (W. Li, Chen, Gong, et al., 2011; W. Li, Chen, Zong, et al., 2011), and its overexpression induces apoptosis and represses pancreatic cancer cell growth (Tao et al., 2015), suggesting a tumor suppressor role for PP2A. Therefore, PP2A may be a potential target for PAH treatment since PAH and tumors share similar mechanisms.
Here, we investigated whether PP2A is involved in the NE-induced proliferation and migration of PASMCs and whether berberine (BBR)—a Chinese herbal medicine that has been shown to not only reverse the phosphorylation of PP2A in Alzheimer’s disease (AD; X. Liu, Zhou, et al., 2016) but also act as an AR antagonist—can alleviate PAH via PP2A. Thus, we hope to provide new insight into the treatment of PAH.
2 MATERIALS AND METHODS
2.1 Reagents
Antibodies against PP2A, Akt, phospho-Akt473, extracellular signal-regulated protein kinases 1 and 2 (ERK1/2), phospho-ERK1/2, P38, phospho-P38, proliferating cell nuclear antigen (PCNA), green fluorescent protein (GFP), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and β-actin were purchased from Cell Signaling Technology, Inc. (Beverly, MA). Phospho-PP2Ac (Y307) and α-smooth muscle actin (α-SMA) was purchased from Abcam (Cambridge, UK). Secondary antibodies were purchased from BioWorld Technology, Inc. (Tulare County, CA) and applied at a 1:10,000 dilution. Norepinephrine, BBR, and the vascular endothelial growth factor receptor (VEGFR) inhibitor SU5416 were purchased from Sigma (St. Louis, MO).
2.2 PASMC isolation and culture
Explant-derived PASMCs were obtained from peripheral small pulmonary arteries of Sprague–Dawley (SD) rats (200–250 g). PA sections were cut to expose the luminal surface. The endothelium was removed by gentle scraping with a scalpel blade, and the intima was peeled away from the underlying adventitial layer. The medial explants were sliced into ~1–2 mm2 segments and allowed to adhere for 2 hr at 37°C and 5% CO2. Then, the cells were cultured in Dulbecco’s modified essential medium (DMEM; Gibco BRL, Rockville, MD) containing 4.5 mmol/L of d-glucose supplemented with 20% fetal bovine serum (FBS; Gibco BRL), 100 U/ml of penicillin, and 100 μg/ml of streptomycin at 37°C in 5% CO2 and 95% air. PASMCs were identified by α-SMA. Experiments were performed with cells between passages 3 and 8. All PASMCs were starved for 24 hr before treatment to rule out the effects of FBS.
2.3 Assessment of migration and proliferation of PASMCs
PASMC migration was assessed using the Boyden chamber migration assay and wound-healing assay, and PASMC proliferation was evaluated using the MTT cell proliferation and cytotoxicity assay kit (Beyotime, Haimen, China) and cell counting kit-8 kit (Medchem Express, New Jersey, NJ), according to the manufacturer’s protocol, and PCNA expression.
2.4 Small interfering RNA (siRNA) transfection and overexpression
All siRNAs were obtained from Dharmacon (Lafayette, CO) and transfected into PASMCs using the Lipofectamine RNAiMAX Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. The AD-PPP2CA-GFP vector (Genechem, Shanghai, China) was used to overexpress PP2Ac.
2.5 Preparation of the PAH model
All animal procedures were approved by the Institutional Animal Care and Use Committee of Nanjing Medical University (Nanjing, China). Male SD rats weighing 200–250 g at 6 weeks of age were obtained from the Animal Center of Nanjing Medical University and randomly divided into three groups: The control group (n = 6), the PAH group (n = 7), and the treatment group (n = 7). The rats in the second and third groups were injected subcutaneously with SU5416 (20 mg per kg body weight) under isoflurane anesthesia and exposed to chronic hypoxia (10% oxygen) for 28 days (hypoxia + SU5416); the treatment group also received intragastric administration of BBR (100 mg·kg−1·day−1) for 28 days.
2.6 Western blot analysis
The study was approved by the Third Affiliated Hospital of Nanjing Medical University. Informed consent was obtained from all volunteers before sample collection. Samples from human (donor and PAH patients) and rat lung (normoxic, SU5416/hypoxia) were first cut into pieces and homogenized in cold phosphate buffer saline (PBS). After centrifugation and removing the PBS, tissue homogenates and cells were lysed in RIPA buffer with a protease inhibitor complex and phosphatase inhibitors (Roche, Basel, Switzerland), incubated on ice for 30 min, and centrifuged at 12,000g at 4°C for 20 min. The protein concentration in the supernatant was determined by spectrophotometry (BCA assay; Pierce, Rockford, IL) with bovine serum albumin as the standard. Total protein samples (50–100 μg) were separated on 10% sodium dodecyl sulfate poly-acrylamide (SDS-PAGE) gels and transferred to PVDF membranes. Membranes were incubated for 1 hr with 5% nonfat dry milk in TBST buffer (20 M of Tris–HCl, pH 7.6, 150 mM of NaCl, and 0.05% Tween-20) to block nonspecific binding. Membranes were subsequently incubated with specific primary antibodies under gentle shaking at 4°C overnight, followed by incubation with secondary antibodies for 1 hr. The proteins were finally visualized with chemiluminescence and a Syngene Bio Imaging Device (Syngene, Cambridge, UK), and the immunoreactive band density was analyzed using the ImageJ software (National Institutes of Health, Bethesda, MD).
2.7 Histological analysis
Lungs were removed, fixed in a 4% paraformaldehyde solution and embedded in paraffin. The left ventricle along the anterior longitudinal mid-line was cut into 5-μm-thick sections for hematoxylin and eosin (H&E; Sigma) and Verhoeff’s Van Gieson (EVG; Sigma) staining as previously described (Zhou et al., 2015).
2.8 Evaluation of cardiac function using echocardiography
The cardiac function of SD rats was assessed using the Vevo2100 system (Fujifilm VisualSonics, Inc., Toronto, ON, Canada) with a high-frequency (30 MHz) MS-400 transducer. The RV internal dimension in diastole (RVID, d), pulmonary artery acceleration time (PAT), pulmonary ejection time (PET), left ventricle ejection fraction (LVEF), fraction shortening (FS), and peak E/A ratio (E/A) were measured. The FS percentage was calculated as ([LVID, d-LVID, s]/LVID, d) × 100, and the LVEF percentage was calculated as ([LV vol, d-LV vol, s]/ LV vol, d) × 100.
2.9 Measurement of right ventricular systolic pressure (RVSP)
The RVSP was measured by right-heart catheterization according to M. Wang, Wang, et al. (2013). Rats were anesthetized with 35 mg/kg of pentobarbital sodium by intraperitoneal injection before catheterization.
2.10 Assessment of PA remodeling and right ventricular hypertrophy
The wall thickness and medial wall thickness percentage were used to assess PA remodeling as previously described (Zhou et al., 2015). The pulmonary trunk and aorta were separated from the excised heart, and the RV wall was separated from the LV wall and ventricular septum. The weights of the RV, LV, and ventricular septum were determined, and the ratio of the RV weight to LV plus septum weight (RV/[LV + S]) was calculated to determine RV hypertrophy (RVH).
2.11 Statistical analysis
All continuous variables are expressed as the means ± standard error of mean (SEM). These data were compared using Student’s paired or unpaired t tests to evaluate the statistical significance of the differences between the two groups when appropriate. One-way analysis of variance (ANOVA) was taken to compare differences between multiple groups. Statistical analyses were performed using SPSS software, version 17.0 (SPSS, Inc., Chicago, IL). Statistical significance was defined as p < 0.05.
3 RESULTS
3.1 PP2A phosphorylation is upregulated in PAH
To investigate the role of PP2A in NE-related PAH, PASMCs were stimulated with various concentrations of NE at multiple time points. Here, the phosphorylation level of PP2Ac (Y307) but not total PP2Ac increased with NE concentration (Figure 1a), the highest of which was 10−5 mol/L, as a higher concentration would be cytotoxic. The phosphorylation level of PP2Ac also showed a time-dependent increase, peaking at 12 hr (Figure 1b). Since hypoxia is a key factor in PAH and leads to the increase in NE, we also examined the effects of hypoxia on PP2Ac, which were found to be similar to those of NE (Figure 1c). Finally, we isolated pulmonary small vessels from the lung tissue of PAH patients and healthy donors and determined that the phosphorylation level of PP2Ac was higher in PAH patients than in healthy donors (Figure 1d). In both the experimental PAH model and PAH patients, we observed a high level of PP2Ac phosphorylation, indicating that PP2A may play a role in PAH.
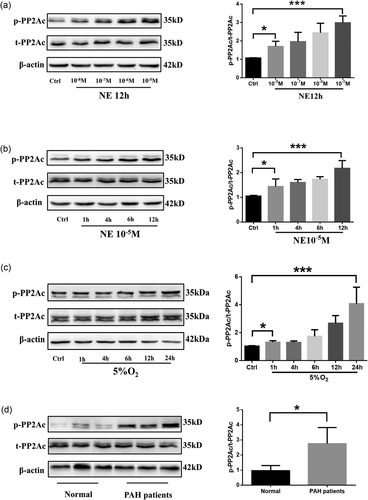
PP2A phosphorylation is upregulated in PAH. (a) Western blot analysis of phosphorylation level of PP2A stimulated with various concentrations of NE; n = 5. (b) Western blot analysis of phosphorylation level of PP2A stimulated with NE at various time points; n = 3. (c) Western blot analysis of phosphorylation level of PP2A treated with hypoxia at various time points; n = 5. (d) Western blot analysis of phosphorylation level of PP2A in lung tissues from healthy and PAH donors; n = 3 and *p < 0.05. All values are represented as the mean ± SEM. Comparisons of parameters were performed with the unpaired Student’s t test or one-way ANOVA. ANOVA: analysis of variance; NE: norepinephrine; PAH: pulmonary arterial hypertension; PP2A: protein phosphatase 2A; PP2Ac: protein phosphatase 2A catalytic subunit
3.2 PP2A plays an important role in PASMC proliferation and migration
Next, we sought to identify the role of PP2A in the development of NE-induced PAH. First, we tested the effects of NE on PAMSCs. Here, PASMC proliferation and migration were promoted by NE treatment. Because PP2A phosphorylation leads to its inactivity, we overexpressed PP2A (Figure 2a). We showed that the proproliferative and promigrative effects of NE were inhibited by PP2A overexpression (Figures 2b,c,d,h), indicating an antiproliferative role for PP2A in PAH. Next, we examined the possible role of PP2A in PAH by investigating the expression and phosphorylation level of some essential molecules in PAH. The phosphorylation level of Akt, ERK1/2, and P38 was upregulated by NE treatment; however, these effects were notably diminished by the overexpression of PP2A (Figure 2e,f,g,i).
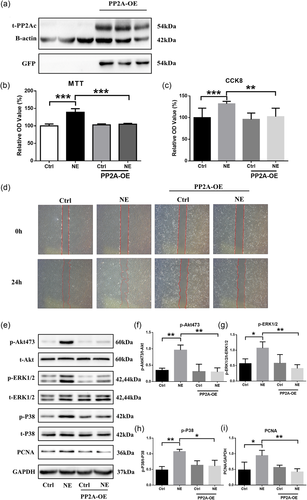
PP2A plays an important role in PASMC proliferation and migration. (a) Western blot analysis of protein expression levels of t-PP2A transfected with AD-PPP2CA-GFP vector; n = 3. (b, c, h) Cell viability and proliferation assessed by MTT, CCK-8, and PCNA; n = 3. (d) Cell migration assumed by wound-healing test; n = 3. (e–g, i) Western blot analysis of phosphorylation levels of Akt, ERK1/2, and P38; n = 3; *p < 0.05, **p < 0.01, and ***p < 0.001; All values are represented as the mean ± SEM. Comparisons of parameters were performed with the unpaired Student’s t test or one-way ANOVA. ANOVA: analysis of variance; CCK-8: cell counting kit-8; ERK1/2: extracellular signal-regulated protein kinases 1 and 2; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; GFP: green fluorescent protein; MTT: 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide; NE: norepinephrine; PASMC: pulmonary artery smooth muscle cells; PCNA: proliferating cell nuclear antigen; PP2A: protein phosphatase 2A catalytic subunit; PP2A-OA: PP2A overexpression [Color figure can be viewed at wileyonlinelibrary.com]
3.3 Effects of BBR on PASMC proliferation and migration
Berberine, an extract isolated from Chinese herbal medicine, was reported to reverse the phosphorylation of PP2A (Y307) in AD (X. Liu, Zhou, et al., 2016) and therefore may be effective in the treatment of PAH. Thus, we first tested whether BBR could reverse the phosphorylation of PP2A (Y307) induced by NE and found that BBR reversed PP2A (Y307) phosphorylation at 12 hr of NE stimulation, while 1 hr showed no obvious effects (Figure 3a). We also found a similar effect of BBR on hypoxia-induced PAH (Figure 3f). BBR was also shown to reduce the proliferation and migration of PASMCs induced by NE (Figure 3b–e). Interestingly, unlike long-term (12 hr) treatment, short-term BBR treatment (1 hr) had no significant effects on the proliferation or migration of PASMCs (data not shown), indicating that the effects of BBR may not be immediate.
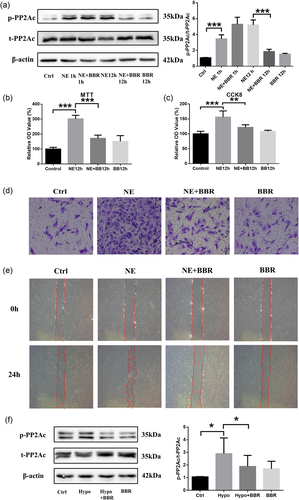
Effects of BBR on PASMC proliferation and migration. (a) Western blot analysis of phosphorylation level of PP2A treated with NE and/or BBR. n = 3. (b, c) Cell viability and proliferation assessed by MTT, CCK-8, and PCNA; n = 3. (d, e) Cell migration assumed by wound-healing test and Boyden chamber migration assay; n = 3. (f) Western blot analysis of phosphorylation level of PP2A treated with hypoxia and/or BBR; n = 3; *p < 0.05, **p < 0.01, and ***p < 0.001. All values are represented as the mean ± SEM. Comparisons of parameters were performed with the unpaired Student’s t test or one-way ANOVA. ANOVA: analysis of variance; BBR: berberine; CCK-8: cell counting kit-8; MTT: 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide; NE: norepinephrine; PASMC: pulmonary artery smooth muscle cells; PCNA: proliferating cell nuclear antigen; PP2A: protein phosphatase 2A [Color figure can be viewed at wileyonlinelibrary.com]
3.4 BBR attenuates PAH via PP2A signaling pathways in vitro
To test whether BBR attenuated PAH via the PP2A pathway, we first used PP2Ac siRNA to inhibit the activity of PP2A and then tested the effects of NE. PASMCs treated with NE and PP2A siRNA displayed a greater proliferation and migration ability than those only treated with NE, and these effects could not be suppressed by BBR (Figure 4a–d). We also examined the expression and phosphorylation of key molecules and found that BBR did not inhibit the phosphorylation level of Akt, ERK1/2, or P38 in NE-stimulated cells treated with PP2Ac siRNA, suggesting a key role for PP2A on the effects of BBR (Figure 4e–i).
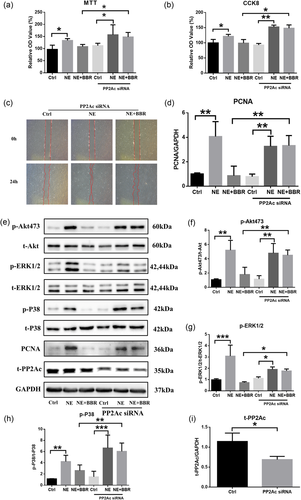
BBR attenuates PAH via PP2A signaling pathways in vitro. (a, b, d) Cell viability and proliferation assessed by MTT, CCK-8, and PCNA; n = 3. (c) Cell migration assumed by wound-healing test; n = 3. (e, f, g, h, i) Western blot analysis of phosphorylation levels of Akt, ERK1/2, P38, and PP2A; n = 3; *p < 0.05, **p < 0.01, and ***p < 0.001. All values are represented as the mean ± SEM. Comparisons of parameters were performed with the unpaired Student’s t test or one-way ANOVA. ANOVA: analysis of variance; BBR: berberine; CCK-8: cell counting kit-8; ERK1/2: extracellular signal-regulated protein kinases 1 and 2; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; MTT: 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide; NE: norepinephrine; PAH: pulmonary arterial hypertension; PCNA: PCNA: proliferating cell nuclear antigen; PP2A: protein phosphatase 2A; PP2Ac: protein phosphatase 2A catalytic subunit; siRNA: small interfering RNA [Color figure can be viewed at wileyonlinelibrary.com]
3.5 BBR attenuates PAH via PP2A signaling pathways in vivo
To investigate the effects of BBR on experimental animal models, we randomly divided SD rats into three groups and ensured that the baseline hemodynamic variables of these three groups were similar. After 28 days of different treatments, we characterized these rats in detail, including their hemodynamic and biometric parameters, RV hypertrophy, and pulmonary arterial vascular remodeling. After 28 days of exposure to hypoxia, the PAH group did not show worse cardiac function than the control group and the BBR treatment group (Figure 5c) but did show a large RVID (Figure 5d). Furthermore, the RVSP of the PAH group was also significantly greater than that of the other groups (Figure 5a,b). Finally, the RV hypertrophy indexindex (RVHI), calculated by RV/(left ventricle + septal), in the BBR treatment group was significantly lower than in the PAH group (Figure 5e).
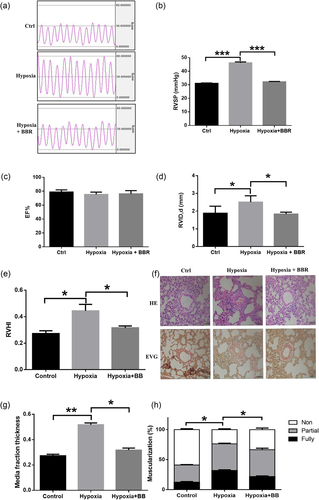
BBR attenuates PAH in vivo. (a, b) Measurement of RVSP of three groups. (c) Measurement of left ventricle ejection fraction of three groups. (d) Measurement of right ventricle internal dimension in diastole of three groups. (e) RVHI calculated by right ventricle to left ventricle + septum (RV/LV + S) weight ratios of three groups. (f) H&E and Verhoeff’s Van Gieson (EVG) staining of pulmonary vessels in rats. Scale bar = 50 μm. (g) Assessment of medial thickness in rats. Morphometric analysis was used to categorize vessels as small (<50 μm), medium (51–100 μm), or large (>100 μm). (h) Percentage of muscularization of rats (Ctrl: normoxia, n = 6; hypoxia, n = 7; hypoxia + BBR, n = 7); *p < 0.05, **p < 0.01, and ***p < 0.001. All values are represented as the mean ± SEM. Comparisons of parameters were performed with one-way ANOVA. ANOVA: analysis of variance; BBR: berberine; EF: ejection fraction; H&E: hematoxylin and eosin; LV: left ventricle; PAH: pulmonary arterial hypertension; PCNA: proliferating cell nuclear antigen; PP2Ac: RV: right ventricle; RVHI: right ventricle hypertrophy index; RVID: right ventricle internal dimension; RVSP: right ventricular systolic pressure; S: septum [Color figure can be viewed at wileyonlinelibrary.com]
In this study, the muscularization of the PAH group was more severe than that of the control group, whereas the muscularization of the BBR treatment group was not as severely affected as that of the PAH group (Figure 5f). Importantly, the medial wall thickness percentage in the PAH group was significantly higher than in the BBR treatment group (Figure 5g), and the rates of no muscularization and full muscularization in the BBR treatment group were significantly different from the respective rates in the PAH group. However, there was no significant difference in the rate of partial muscularization between these two groups (Figure 5h).
We finally investigated whether PP2A was involved in the effects of BBR treatment in vivo. Hypoxia-induced increase phosphorylation level of these key proteins in the lungs were significantly attenuated by BBR treatment compared with vehicle controls, which confirmed the effects of BBR in vivo (Figure 6a–e).
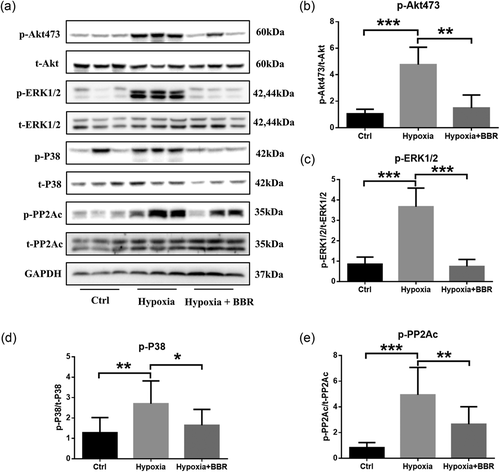
BBR attenuates PAH via PP2A in vivo. (a–e) Western blot analysis of phosphorylation level of Akt, ERK1/2, P38, and PP2A; n = 3; *p < 0.05, **p < 0.01, and ***p < 0.001. All values are represented as the mean ± SEM. Comparisons of parameters were performed with one-way ANOVA. ANOVA: analysis of variance; BBR: berberine; ERK1/2: extracellular signal-regulated protein kinases 1 and 2; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; PAH: pulmonary arterial hypertension; PP2A: protein phosphatase 2A
4 DISCUSSION
Biochemical and clinical evidence indicates that muscularization of small pulmonary vessels plays an essential role in the pathogenesis of PAH (Rabinovitch, 2012), and imbalance between PASMC proliferation, migration, and apoptosis may contribute to it. The current study provides solid evidence that phosphorylation of PP2Ac (Y307) plays an important role in the progression of PAH and that reconstitution of PP2A activity may provide a potentially effective drug target for the treatment of PAH. This notion is based on the findings that (a) the phosphorylation of PP2A was upregulated in PAH patients and two experimental PAH models; (b) phosphorylation of PP2A was involved in the proliferation and migration of PASMCs induced by NE or hypoxia, while PP2A overexpression reversed this effect; (c) BBR could alleviate PAH both in vivo and in vitro; and (d) genetic ablation of PP2A reproduced the PAH features relieved by BBR in vitro.
Previous studies revealed that the innervation of PAs is mainly sympathetic (Kummer, 2011; Shirai et al., 2014) and that sympathetic system (SN) overactivation may be intimately related to the severity and prognosis of PAH (Ciarka et al., 2010; Velez-Roa, 2004). First, hypoxia, which has been recognized as a major cause of PAH, leads to overactivation of SN and prolonged stimulation of α-adrenergic receptors (Faber et al., 2006; H. Zhang & Faber, 2001), thereby switching the phenotype of PASMCs from contractile with low migration–proliferation abilities to a proliferative phenotype. Second, NE is the primary transmitter released by SN activation, and its growth-promoting effects lead to the uncontrolled proliferation of PASMCs (Faber et al., 2007). In this study, we found that a high concentration of NE (10,000 nM) induced PASMC proliferation and migration, as R. Liu et al. (2017) previously reported. Additionally, a low concentration of NE (100 nM) was shown to partially enhance these effects, potentially due to low NE sensitivity after prolonged NE stimulation and/or β-adrenoceptor (β-AR) activation. Recently, β3-AR agonists were shown to significantly reduce the pulmonary vessel resistance index and improve RV function in an experimental PAH model (García-Álvarez et al., 2016). Nebivolol, a selective β1-antagonist and β-2,3-agonist, provided a significant improvement focusing on endothelial dysfunction and pulmonary vascular remodeling in PAH patients and experimental animal models (Perros et al., 2015). Our recent study also showed that the lower level of NE caused by PADN was beneficial in PAH (S. L. Chen, Zhang, Zhou, et al., 2013). Together, these studies reveal that NE is strongly associated with PAH.
PP2A is a heterotrimeric complex that plays a key role in the regulation of the cell cycle and DNA damage via serine–threonine phosphorylation signaling pathways (Perrotti & Neviani, 2013; Sangodkar et al., 2016; Shi, 2009). A variety of research has shown that PP2A has a strong relationship with various cancers (Perrotti & Neviani, 2013), whereas little evidence has revealed a correlation between PP2A and PAH (Kovacs et al., 2016; Sankhe et al., 2017). Thus, we sought to investigate the link between PP2A and PAH in NE-related models. Previous findings suggested the essential role of PP2A activity in regulating the proliferation and apoptosis of vascular smooth muscle cells (VSMCs; Y. Y. Chen et al., 2014; Ueda, Lu, Baur, Aronovitz, & Karas, 2013; Zrelli, Matsuka, Araki, Zarrouk, & Miyazaki, 2011). The phosphorylation of multiple residues on the C subunit C terminus is critical for modulating PP2A activity. Here, we found that NE and hypoxia did not alter the expression of PP2A, indicating that the decrease in PP2A activity was not due to a change in PP2A synthesis. Therefore, alterations in its posttranslational modification (e.g., phosphorylation) were considered. Once the Y307 was phosphorylated, the activity of PP2A was inhibited. A main finding of this study was that the phosphorylation level of PP2Ac(Y307) showed a time- and dose-dependent elevation induced by NE. Moreover, we also found that overexpression of PP2A reversed the proliferation and migration induced by NE and that inhibition of PP2A enhanced it, suggesting an essential role for PP2A in NE-related PAH. Furthermore, we investigated the possible mechanism of PP2A by examining the expression and phosphorylation level of important molecules involved in PAH. Recent studies have reported the key role of Akt in PAH (Tang et al., 2015). The Akt-S473–mTORC1 signaling pathway is associated with PASMCs in hypoxic PH. ERK1/2 and P38, as vital members of the MAPK family, promote proliferation and have antiapoptotic effects, which have also been observed in experimental PAH models and patients with PAH (R. Liu et al., 2017; Nasim et al., 2012). PP2A possesses antiproliferation activity that depends on its ability to negatively regulate the PI3K–Akt pathway via Akt dephosphorylation (Perrotti & Neviani, 2013). Inactivation of PP2A would also lead to ERK activation (W. H. Liu, Chen, Cheng, Lin, & Chang, 2013; Silverstein, Barrow, Davis, & Mumby, 2002). Here, we showed that the phosphorylation level of Akt, ERK1/2, and P38 were increased by NE and downregulated by PP2A overexpression, demonstrating that PP2A may partly reverse NE-related PAH via these signaling pathways. All of these data support the centrality of PP2A in NE-related PAH. Imatinib has been reported to increase PP2A activity (Neviani et al., 2005) and suppress the proliferation of PASMCs (Perros et al., 2008). However, the use of imatinib as a therapeutic option for PAH has been abolished due to noticeable side effects and an unfavorable benefit–risk ratio (Humbert et al., 2014). Phosphorylation of B subunits might also have a crucial role in the regulation of PP2A activity (Perrotti & Neviani, 2013). We focus on the function of the C subunits of PP2A and omitting other subunits, this is a limitation of this study. Thus, more work is required to determine the mechanism of PP2A.
BBR, a plant alkaloid isolated from traditional Chinese medicinal herbs, has long been used for dysentery and infectious diarrhea (Pirillo & Catapano, 2015), and recent research has demonstrated other effects of BBR in the cardiovascular system. BBR is reported to inhibit cardiomyocyte hypertrophy and restore nitric oxide synthase activity and nitric oxide levels via the peroxisome proliferator-activated receptor pathway (Q. Wang, Zuo, et al., 2013). In addition, BBR reduced the apoptosis of cardiomyocytes induced by hypoxia by modulating several processes and decreased VSMC proliferation and migration by interfering in several cellular pathways, such as the Akt and ERK signaling pathways (Cho et al., 2005; Lee et al., 2006). Li and Zhang show that CPU86017(p-chlorobenzyltetrahydroberberine chloride) could reverse the PA remodeling via suppressing the endothelin-1 (ET-1)–reactive oxidative species pathway of endothelial cell and attenuating the accumulation of extracellular matrix (N. Li, Dai, & Dai, 2008; T. T. Zhang, Cui, Dai, & Su, 2005). However they did not explore the mechanism of BBR on PASMCs.
In our study, we found that BBR was also effective in alleviating NE- and hypoxia-induced PAH, potentially due to its role as an agonist of PP2A. A previous study reported that BBR modulated the activity of PP2A in AD, as evidenced by the decreased phosphorylation of PP2Ac at Y307 (X. Liu, Zhou, et al., 2016), which also restored PP2A activity in tau-transfected HEK293 cells (Yu et al., 2011). Our previous data also showed the possible connection between PP2A and NE (data not shown); therefore, we hypothesized that BBR may antagonize the effects of NE on PP2A. In this study, we found that BBR attenuated experimental PAH both in vivo and in vitro. Moreover, we observed that BBR was unable to reverse the proliferation and migration of PASMCs and the phosphorylation level of ERK, P38, and Akt, which was induced by NE when the activity of PP2A was inhibited, indicating an essential role for PP2A phosphorylation in BBR treatment.
Although BBR may be effective for the treatment of PAH patients, there are still some problems to be solved. First, the BBR dose for patients may be too high due to its poor aqueous solubility and dissolution (Y. Zhang, Cui, Gao, & Jiang, 2013). Second, BBR has some side effects, so further exploration, especially of the molecular biology and preclinical evidence for the efficacy of BBR treatment is required.
In conclusion, our study demonstrated an important role of PP2A in PAH and a possible mechanism of BBR in the treatment of PAH. Although there may still be some problems associated with BBR treatment, BBR may be a promising drug for the treatment of PAH. We hope to provide a new strategy for the therapeutic intervention of PAH with this finding.
ACKNOWLEDGEMENT
This study was supported by National Natural Science Foundation of China (No. 91639303).
CONFLICTS OF INTEREST
The authors declare no conflicts of interests, financial, or otherwise.