Small molecule inhibitor RepSox prevented ovariectomy-induced osteoporosis by suppressing osteoclast differentiation and bone resorption
Abstract
Osteoporosis (OP) is a serious metabolic disease that, due to the increased number or function of osteoclasts, results in increased bone brittleness and, therefore, fragile fracture. Some recent studies report the importance of the transforming growth factor β (TGFβ) pathway in bone homeostasis. RepSox is a small molecule inhibitor of TGFβRI that has a wide range of potential application in clinical medicine, except OP. The aim of our study is to evaluate the effects of RepSox on the differentiation and bone resorption of osteoclasts in vitro and in vivo in an ovariectomy (OVX)-induced OP model. An initial analysis showed TGFβRI messenger RNA expression in both bone samples and bone cells. In the in vitro study, RepSox inhibited the receptor activator of nuclear factor κB ligand (RANKL)-induced osteoclast differentiation and bone resorption activity. Real-time polymerase chain reaction (PCR) analysis showed that RepSox suppressed osteoclastic marker gene expression in both dose-dependent and time-dependent manners. In addition, RepSox did not affect osteoblast differentiation, migration or osteoblastic-specific gene expression in vitro. Furthermore, western blot analysis indicated the underlying mechanisms of the RepSox suppression of osteoclastogenesis via the Smad3 and c-Jun N-terminal kinase/activator protein-1 (JNK/AP-1) signaling pathways. Finally, our animal experiments revealed that RepSox prevented OVX-induced bone loss in vivo. Together, our data suggest that RepSox regulates osteoclast differentiation, bone resorption, and OVX-induced OP via the suppression of the Smad3 and JNK/AP-1 pathways.
1 INTRODUCTION
Osteoporosis (OP) is a kind of metabolic bone disease that is characterized by decreased bone mass and deteriorated bone microstructure, resulting in increased bone brittleness and, therefore, fragile fracture (Lems & Raterman, 2017). Although traditional drugs, such as bisphosphonate (Jin et al., 2017), calcitonin (Liu et al., 2017), estrogen (Sharifi & Lewiecki, 2014), and selective estrogen receptor modulators (Genant, 2011), shown promising therapeutic results for the treatment of OP, there are still some side effects involved, including gastrointestinal complications (Kotian, Boloor, & Sreenivasan, 2016), musculoskeletal pain (Reyes, Hitz, Prieto-Alhambra, & Abrahamsen, 2016), and medication-related osteonecrosis of the jaw (Patel et al., 2017). Therefore, further identifying more effective anti-OP compounds with fewer side effects has become one of the main focuses of current research.
Osteoblasts and osteoclasts are the two most important cell types in bone homeostasis for maintaining the balance of bone mass. Osteoclasts are the only cells in the body that are capable of bone resorption (Soysa, Alles, Aoki, & Ohya, 2012). An increased number or function of osteoclasts can lead to excessive bone loss and thus cause OP. Therefore, osteoclasts are one of the key cellular targets for new anti-OP drugs (Edwards & Weivoda, 2012).
Transforming growth factor β (TGFβ) is abundant in the bone matrix and is produced by osteoblasts and fibroblasts, as well as osteoclasts. TGFβ is regarded as one of the well-known factors that stimulate bone formation around bony tissues (Itonaga et al., 2004). TGFβ injection stimulates bone formation in vivo (Marcelli, Yates, & Mundy, 1990). However, in the in vitro experiments, the effect of TGFβ on bone formation mainly depends on the osteoblast life cycle. Briefly, in the early stages, TGFβ recruits osteoblast progenitors, stimulating their proliferation and differentiation (bone matrix production), expanding the pool of committed osteoblasts, and thus increasing bone formation; but in the later phases, TGFβ blocks osteoblast differentiation and mineralization (Janssens, ten Dijke, Janssens, & Van Hul, 2005), possibly by repressing the transcriptional activity of Runx2 through Smad3 (Alliston, Choy, Ducy, Karsenty, & Derynck, 2001).
Interestingly, some correlations between the TGFβ pathway and bone mineral density have been found. It was reported that TGFβ has a direct stimulatory effect on osteoclastogenesis (Sells Galvin, Gatlin, Horn, & Fuson, 1999). Furthermore, neutralizing TGFβ with a specific antibody (1D11) increased osteoblast numbers while simultaneously decreasing active osteoclasts in the marrow, resulting in a profound increase in bone volume and quality in vivo (Edwards et al., 2010). Therefore, we suspect that inhibition of the TGFβ signaling pathway may play a preventive role in bone loss. RepSox is a small molecule that can selectively inhibit TGFβRI (Gellibert et al., 2004). Literature reports that RepSox could attenuate skin fibrosis (Ide et al., 2017), improve the developmental potential of rhesus monkey-pig interspecies somatic cell nuclear transfer embryos (Zhu et al., 2017), and advance the development of leukemic stem/progenitor cell (LPC)-targeted therapies (Jajosky et al., 2014). However, the effects of RepSox on bone remains unclear. Given the importance of TGFβ on bone homeostasis and the wide potential application of RepSox in clinical medicine, we aim to evaluate the effects of RepSox on the proliferation, differentiation, and bone resorption of osteoclasts in vitro and in ovariectomy (OVX)-induced OP in vivo.
2 MATERIALS AND METHODS
2.1 Media and reagents
RepSox, Triton X-100, 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI), and a tartrate-resistant acid phosphatase (TRAP) staining kit were purchased from Sigma-Aldrich (St. Louis, MO). Alpha modification of Eagle s medium (α-MEM), fetal bovine serum (FBS), and penicillin-streptomycin solution were purchased from Gibco-BRL (Gaithersburg, MD). A cell counting kit-8 (CCK-8) was obtained from Dojindo Molecular Technology (Tokyo, Japan). Recombinant mouse macrophage colony-stimulating factor (M-CSF) protein and receptor activator of nuclear factor κB ligand (RANKL) were obtained from R&D Systems (Minneapolis, MN), reconstituted in sterile phosphate buffer saline containing 0.1% bovine serum albumin (BSA), and stored at ‒20℃. The specific primary antibodies and secondary antibodies used in the experiments were obtained from Abcam (Cambridge, UK).
2.2 Cell culture
Bone marrow macrophages (BMMs) were isolated from the whole bone marrow of six-week-old female C57BL/6 mice as described previously (Li et al., 2015). In brief, cells were separated from femoral and tibial bone marrow and cultured in a T75 flask with α-MEM containing 10% FBS, 1% penicillin/streptomycin, and 30 ng/ml M-CSF. MC3T3-E1 cells were incubated in α-MEM with 10% FBS in a humidified atmosphere of 5% CO2 at 37℃. The medium was changed every 2 days.
2.3 Clinical bone tissue specimen assay
Ethical approval for this investigation was obtained from the Research Ethics Committee of the Fifth Affiliated Hospital of Wenzhou Medical University, and an informed consent was obtained from all patients in the study. The bone tissue specimens were isolated from patients with osteoarthritis and OP during an operation, as described previously (Jiang, Zhang, Jiang, Chen, & Dai, 2008). The patients’ clinical information is listed in Supporting Information Table S1. Part of the bone tissue was ground into powder, and total RNA was extracted using a Qiagen RNeasy Mini kit (Qiagen, Valencia, CA) and then subjected to complementary DNA (cDNA) synthesis. Real-time polymerase chain reaction (PCR) was performed using a SYBR Premix Ex Tag kit (TaKaRa Biotechnology, Dalian, China) and an ABI 7500 Sequencing Detection System (Applied Biosystems, Foster City, CA).
2.4 Cell viability assay
The cytotoxic effect of RepSox on the BMMs or MC3T3-E1 cells was assessed using CCK-8 assays, according to the manufacturer's protocol. The BMMs were seeded in 96-well plates at a density of 8 × 103 cells/well in triplicate and cultured in complete α-MEM supplemented with 30 ng/ml M-CSF; after 24 hr, the cells were treated with different concentrations of RepSox for another 48 or 96 hr. MC3T3-E1 cells were cultured in 96-well plates in complete α-MEM at a density of 2 × 103 cells/well with increasing concentrations of RepSox for 48 or 96 hr. Ten microliters of CCK-8 buffer was added to each well, the cells were incubated at 37℃ for 2 hr, and the absorbance was measured at 450 nm on an ELX800 microplate reader (Bio-Tek, Vermont). Cell viability was calculated relative to that of the control cells from the optical density (OD) using the following formula: cell viability = (experimental group OD − zeroing OD)/(control group OD − zeroing OD).
2.5 In vitro osteoclastogenesis assay
The BMMs were plated into a 96-well plate at a density of 8 × 103 cells/well (in triplicate) in complete α-MEM and incubated for 24 hr. Next, 30 ng/ml M-CSF, 100 ng/ml RANKL, and different concentrations of RepSox (0, 125, 250 or 500 nM) were added to each well. The cell culture medium was replaced every 2 days until mature osteoclasts had formed. The cells were washed twice with PBS, fixed with 4% paraformaldehyde for 20 min, and stained for TRAP using the staining kit. TRAP-positive cells with more than three nuclei were regarded as osteoclasts. The number and the area of osteoclasts (percentage of the well) per well were measured using ImageJ software.
2.6 In vitro bone absorption assay
The BMMs were seeded at a density of 2.4 × 104 cells/cm2 onto bovine bone slices (in triplicate) and incubated with 30 ng/ml M-CSF and 100 ng/ml RANKL until mature osteoclasts had formed, followed by treatment with 0, 125, 250, or 500 nM RepSox for 48 hr. Adherent cells were completely removed from the slices. Resorption pits were imaged using a scanning electron microscope (FEI Quanta 250), and the bone resorption area was quantified using ImageJ software.
2.7 RNA extraction and quantitative PCR assay
Quantitative PCR was used to calculate the relative specific gene expression during osteoclast and osteoblast formation. Briefly, the BMMs were seeded in 12-well plates at a density of 105 cells/well and cultured in complete α-MEM supplemented with 30 ng/ml M-CSF for 24 hr. After that, the BMMs were treated with 100 ng/ml RANKL and 500 nM RepSox for 0, 1, 3, or 5 days or with different concentrations of RepSox (0, 250 or 500 nM) for 5 days. MC3T3-E1 cells were seeded in 12-well plates and cultured in complete α-MEM. When the cells were confluent, they were treated with osteogenic medium (α-MEM supplemented with 10% FBS, 50 μg/ml ascorbic acid, 10 mM glycerophosphate, 100 nM dexamethasone and 1% penicillin/streptomycin) and different concentrations of RepSox for 7, 14, or 21 days. Total RNA was extracted using the Qiagen RNeasy Mini kit (Qiagen) and followed by cDNA synthesis using reverse transcriptase (TaKaRa Biotechnology). Real-time PCR was performed using a SYBR Premix Ex Tag kit (TaKaRa, Biotechnology) and an ABI 7500 Sequencing Detection System (Applied Biosystems) according to the manufacturers’ protocols. The cycling conditions used were 40 cycles of denaturation at 95℃ for 15 s and amplification at 60℃ for 1 min. GAPDH was used as an internal control gene, and all reactions were run in triplicate. The mouse primer sequences are listed in Table 1, and the human primer sequences are listed in Table 2.
| Gene | Sequence (5′-3′) | |
|---|---|---|
| TGFβRI | ForwardReverse | CCTCGAGACAGGCCATTTGTGCCAGCTGACTGCTTTTCTG |
| NFATc-1 | ForwardReverse | TCCACCCACTTCTGACTTCCCTTCGCCCACTGATACGAG |
| DC-STAMP | ForwardReverse | GCTGTATCGGCTCATCTCCTAAGGCAGAATCATGGACGAC |
| c-Fos | ForwardReverse | GTTCGTGAAACACACCAGGCGGCCTTGACTCACATGCTCT |
| Cathepsin K | ForwardReverse | TCCGCAATCCTTACCGAATAAACTTGAACACCCACATCCTG |
| TRAP | ForwardReverse | CCATTGTTAGCCACATACGGCACTCAGCACATAGCCCACA |
| ATP6V0A3 | ForwardReverse | AATCATGGACGACTCCTTGGGGCCACCTCTTCACTCCGGAA |
| ATP6V0D2 | ForwardReverse | AAGCCTTTGTTTGACGCTGTTTCGATGCCTCTGTGAGATG |
| ALP | ForwardReverse | GCTGATCATTCCCACGTTTTACCATATAGGATGGCCGTGA |
| BMP-2 | ForwardReverse | GATCTGTACCGCAGGCACTCTTCCCACTCATCTCTGGAAGTT |
| Runx2 | ForwardReverse | AGATGACATCCCCATCCATCGTGAGGGATGAAATGCTTGG |
| OCN | ForwardReverse | GGCGTCCTGGAAGCCAATGTGGACCAGGAGGACCAGGAAGTCCACGT |
| Col-1a1 | ForwardReverse | TGATCACTCCCACGTTTTCACTGGGCCTGGTAGTTGTTGT |
| ANGPT1 | ForwardReverse | AACCGGATTCAACATGGGCAGAGCGTTGGTGTTGTACTGC |
| Osx | ForwardReverse | CTTCCCAATCCTATTTGCCGTTTCGGCCAGGTTACTAACACCAATCT |
| GAPDH | ForwardReverse | ACCCAGAAGACTGTGGATGGCACATTGGGGGTAGGAACAC |
| Gene | Sequence (5′-3′) | |
|---|---|---|
| TGFβRI |
|
|
| NFATc-1 |
|
|
| DC-STAMP |
|
|
| c-Fos |
|
|
| Cathepsin K |
|
|
| TRAP |
|
|
| Runx2 |
|
|
| Osx |
|
|
| ALP |
|
|
| BMP-2 |
|
|
| OCN |
|
|
| OPN |
|
|
| GAPDH |
|
AGAAGGCTGGGGCTCATTTGAGGGGCCATCCACAGTCTTC |
2.8 Western blotting
The BMMs were seeded in 6-well plates and cultured in complete α-MEM supplemented with 30 ng/ml M-CSF. After the cells reached 90% confluence, they were harvested by pretreatment with or without 500 nM RepSox for 1 hr and then stimulated with 100 ng/ml RANKL for 0, 10 or 30 min. Total protein was extracted from cultured cells by using radio immunoprecipitation assay (RIPA) lysis buffer. Protein concentrations were determined using a BCA protein assay kit (Thermo Fisher Scientific). Thirty micrograms of each protein lysate was resolved using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 10% gels and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore). Next, the membranes were blocked with 5% nonfat dried skimmed milk powder dissolved in TBST (TBS with 1% Tween-20) for 2 hr and then washed three times with TBST. The membranes were incubated with primary antibodies at 4℃ overnight and secondary antibodies for 1 hr at room temperature. Antibody reactivity was detected by exposure in an Odyssey image scanner (Li-COR. Inc.). Quantitative analysis of the band intensity was done using ImageJ software.
2.9 Luciferase reporter gene assay
The BMMs stably expressed the AP-1 luciferase reporter gene, as described previously (Weber & Markillie, 2003). Briefly, the BMMs were seeded into 12-well plates and cultured with medium for 24 hr. Then, cells were pretreated with or without different concentrations of RepSox for 1 hr followed by stimulation with 100 ng/mL RANKL for 24 hr. Luciferase activity was measured using the Promega Luciferase Assay System (Promega, Madison, WI) and normalized to that of the control group.
2.10 Osteoclastogenesis rescue assay
For the rescue assay, the BMMs were seeded into a 96-well plate at a density of 8 × 103 cells/well and cultured with complete α-MEM medium containing 30 ng/ml M-CSF. After 24 hr, 100 ng/ml RANKL and 250 nM RepSox with or without various concentrations of anisomycin (a specific JNK activator) were added to each well. After 5–7 days, mature osteoclasts had formed. The cells were fixed and stained with a TRAP staining kit, and the number of TRAP-positive cells were counted.
2.11 Immunofluorescence microscopy analysis
The BMMs were seeded in 6-well plates containing coverslips and cultured for 24 hr. Then, the cells were harvested by pretreatment with 500 nM RepSox for 1 hr followed by stimulation with 100 ng/ml RANKL for 0, 10 or 30 min. After the treatment, the cells were washed with PBS, fixed with 4% paraformaldehyde for 20 min at room temperature, permeabilized with 0.5% Triton X-100 in PBS for 10 min and blocked with 5% BSA in PBS for 30 min at room temperature. After blocking, the cells were incubated with a rabbit monoclonal antibody to p-Smad3 (1:200) overnight at 4℃. After further washing three times with PBS, the cells were incubated with Alexa Fluor 488-goat anti-rabbit IgG (1:200) for 1 hr. The nuclei were stained with DAPI. All images were captured on a Nikon ECLIPSE Ti microscope (Nikon, Tokyo, Japan).
2.12 OVX-induced OP model
All experiments and animal care were performed in accordance with the guidelines and procedures authorized by the Animal Experimental Ethical Committee of Wenzhou Medical University. An OVX-induced OP model was used to evaluate the effect of RepSox on OP in vivo, as described previously (Guo et al., 2017). Briefly, 32 healthy 8-week-old female C57BL/6 mice were randomly divided into four groups: sham, vehicle, low, and high. The mice in the vehicle, low, and high groups underwent an OVX, and the sham group mice received only an abdominal incision. After 1 week, the mice in the low and high groups were injected intraperitoneally with RepSox at a dose of 2 and 5 mg/kg, respectively, twice per week for 4 weeks. The mice in the sham and vehicle groups received only an injection of the same volume of PBS. The mice in each group were weighed before injection. At the end of the experiment, the mice were killed and both the femurs and lumbar vertebrae were excised and fixed in 4% paraformaldehyde for histological and microcomputed tomography (micro-CT) analysis. Biochemical analysis of serum was used in our study.
2.13 Micro-CT scanning
The fixed femurs and lumbar vertebrae were analyzed using a high-resolution micro-CT (Skyscan 1072) instrument according to the manufacturer's protocols. The trabecular bone volume per total volume (BV/TV), mean trabecular thickness (Tb.Th), mean trabecular number (Tb.N), and mean trabecular separation (Tb.Sp) were measured, as reported previously (Ouyang et al., 2014).
2.14 Histological and histomorphometric analysis
The fixed femurs were decalcified in 10% EDTA for 3 weeks and then embedded in paraffin. Histological sections were prepared for TRAP and hematoxylin and eosin (H&E) staining. The samples were observed and photographed under a high-quality microscope. The mean number of TRAP-positive multinucleated osteoclasts normalized with bone area (OcN/BA) and the ratio of osteoclast area to bone area (OcA/BA) were calculated in each sample.
2.15 Analysis of serum markers
Procollagen type 1 N-terminal peptide (P1NP), a serum marker of bone formation, and the bone resorption marker C-terminal collagen cross-links (CTX-1) were measured in the serum of mice using an ELISA kit (Westang Biological Technology Co., Ltd., Shanghai, China) according to the protocols.
2.16 Alkaline phosphatase and Alizarin Red staining
MC3T3-E1 cells were seeded in 12-well plates and cultured in complete α-MEM. When the cells were confluent, the culture medium was replaced by osteogenic medium with different concentrations of RepSox, as described previously (Kim, Song, & Hwang, 2014). The medium was changed every 2 days. Seven and 21 days later, the cells were washed three times with PBS, fixed with 4% paraformaldehyde for 20 min, and alkaline phosphatase (ALP) or Alizarin Red staining was performed using a staining kit (Beyotime Biotechnology, Shanghai, China) according to the manufacturers’ instructions.
2.17 Scratch-wound healing assay
MC3T3-E1 cells were seeded in 6-well plates and cultured to confluence, scraped in a straight line with a p200 pipet tip, and treated with RepSox at concentrations of 0, 250, 500, and 1,000 nM for 12 and 24 hr. The wound area was observed under an optical microscope.
2.18 Statistical analysis
The data are presented as the mean ± standard deviation (SD), and each experiment was carried out at least three times. The results were analyzed using SPSS 21.0 software (IBM Corp.). Analysis of variance with Dunnett’s post hoc test were used for comparisons between the groups. The results with p < 0.05 indicated statistical significance among the groups.
3 RESULTS
3.1 Expression of TGFβRI in bone samples and bone cells
The expression of TGFβRI in bone specimens was initially evaluated. Compared with the osteoarthritis samples, the OP bone samples expressed a higher level of TGFβRI messenger RNA (mRNA; Figure 1a). In addition, the osteoclast- and osteoblast-specific genes were also increased (Figure 1b,c). Next, we determined the expression of TGFβRI in osteoclast and osteoblast cells. During osteoclast differentiation, the expression of TGFβRI and osteoclastogenesis marker genes increased at 1, 3, and 5 days compared with that at day 0 (Figure 1d). In the process of osteoblast differentiation, TGFβRI was constantly expressed during osteogenesis, and the expression of osteoblastogensis marker genes was increased at 7, 14, and 21 days compared to at 0 days (Figure 1e). These data show that TGFβRI was expressed both in osteoblasts and osteoclasts, and TGFβRI expression was enhanced in OP and during osteoclast differentiation.
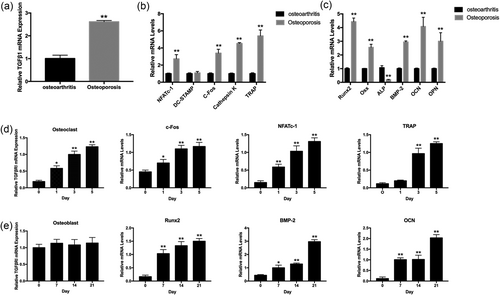
TGFβRI mRNA expression in vitro and vivo. (a) Relative TGFβRI mRNA expression in osteoarthritis and OP patients. (b) Osteoclast-related and (c) osteoblast-related mRNA expression in osteoarthritis and OP patients. (d) Relative expression of TGFβRI mRNA and osteoclastogenesis marker genes in osteoclasts at 0, 1, 3, and 5 days. (e) Relative expression of TGFβRI mRNA and osteoblastogensis marker genes in osteoblasts at 0, 7, 14, and 21 days. (*p < 0.05, **p < 0.01). mRNA: messenger RNA; OP: osteoporosis
3.2 RepSox inhibits RANKL-induced osteoclast differentiation
The chemical structure of RepSox is shown in Figure 2a. To investigate the effect of RepSox on osteoclast formation, we treated primary BMMs with M-CSF and RANKL in the absence or presence of various concentrations of RepSox. As shown in Figure 2b, BMMs differentiated into mature, TRAP-positive, multinucleated osteoclasts in the control group. In contrast, the formation of TRAP-positive osteoclasts was significantly inhibited by the presence of RepSox at various concentrations (Figure 2c). The number of osteoclasts (TRAP-positive cells with more than three nuclei) was 223.60 ± 12.77 per well in the control group, 157.40 ± 9.32 per well after treatment with 125 nM RepSox, 84.40 ± 8.67 per well after treatment with 250 nM RepSox and 21.55 ± 3.16 per well after treatment with 500 nM RepSox (p < 0.01). To exclude the possibility that the inhibitory effect of RepSox on osteoclast formation was due to a cytotoxic effect, a cell viability assay was performed. We demonstrated that cell viability was not affected by RepSox at concentrations lower than 500 nM, which significantly inhibited osteoclastogenesis (Figure 2d). The calculated IC50 for RepSox in BMMs was 183.2 μM at 48 hr and 175.15 μM at 96 hr. Together, these results demonstrate that RepSox suppressed osteoclast differentiation in a dose-dependent manner without toxicity to BMMs.
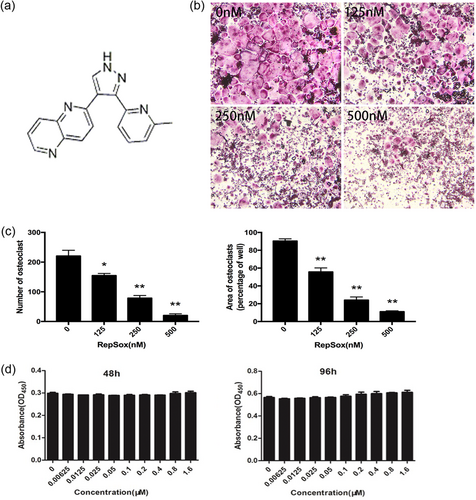
RepSox inhibits RANKL-induced osteoclast formation without toxicity. (a) RepSox chemical structure. (b) BMMs were treated with 30 ng/ml M-CSF, 100 ng/ml RANKL and various concentrations of RepSox, cultured for 5–7 days and then subjected to TRAP staining. (c) The numbers and areas of TRAP-positive osteoclasts were measured. (d) BMMs were treated with different concentrations of RepSox, 30 ng/ml M-CSF, and 100 ng/ml RANKL for 48 hr and 96 hr, and cell viability was then measured using a CCK-8 assay. (*p < 0.05, **p < 0.01). BMMs: bone marrow macrophages; CCK-8: cell counting kit-8; M-CSF: macrophage colony-stimulating factor; RANKL: receptor activator of nuclear factor κB ligand; TRAP: tartrate-resistant acid phosphatase [Color figure can be viewed at wileyonlinelibrary.com]
3.3 RepSox inhibits osteoclastic bone resorption activity in vitro
Since RepSox suppresses osteoclast differentiation efficiently, we determined whether RepSox also inhibits osteoclastic bone resorption. Consequently, mature osteoclasts were used to explore the effect of RepSox on bone resorption. Mature osteoclasts treated with different concentrations of RepSox for 48 hr did not affect the viability of cells (Figure 3b). As shown in Figure 3a,c, large bone resorption pits were observed in the control group. However, after treatment with different concentrations RepSox, the resorption area was decreased to 50% in the 125 nM group, to 20% in the 250 nM group, and very few resorption pits were observed in the 500 nM group (p < 0.01). These data indicated a potential inhibitory effect of RepSox on osteoclastic bone resorption.
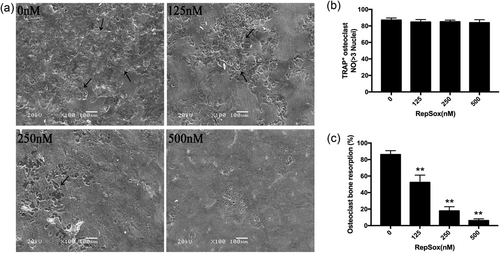
RepSox inhibits osteoclastic bone resorption activity in vitro. (a) After mature osteoclasts were treated with different concentrations of RepSox for 48 hr, the bone resorption pits were observed by scanning electron microscopy. (b) The numbers of TRAP-positive osteoclasts on bovine bone discs after treatment with various concentrations of RepSox for 48 hr. (c) Resorption pit areas were measured using ImageJ software. (*p< 0.05, **p < 0.01). TRAP: tartrate-resistant acid phosphatase
3.4 RepSox suppresses RANKL-induced osteoclast-specific gene expression
Because RepSox inhibited osteoclast formation and bone resorption, we further explored the underlying mechanisms. Therefore, the expression of osteoclastic-specific genes was examined. In response to RANKL stimulation, TGFβRI and osteoclast-related genes, including NFATc-1, c-Fos, Cathepsin K, TRAP, DC-STAMP, ATP6V0D2 and ATP6V0A3, were upregulated in the control group. In contrast, a general suppressive effect on gene expression was observed in the RepSox-treated groups. RepSox suppressed the osteoclastic marker gene expression in both dose-dependent (Figure 4a) and time-dependent (Figure 4b) manners. Together, these data further demonstrate that RepSox suppresses osteoclast formation in vitro.
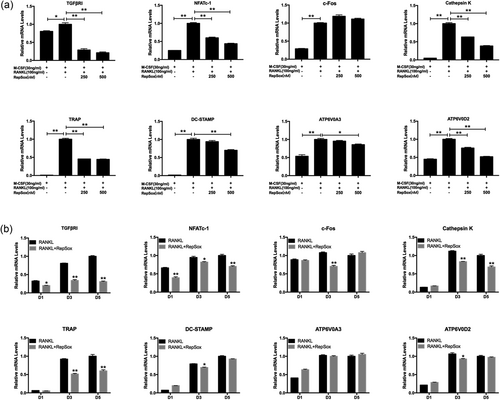
RepSox suppresses RANKL-induced osteoclast-specific gene expression. (a) BMMs were cultured with 30 ng/ml M-CSF, 100 ng/ml RANKL and different concentrations of RepSox for 5 days, or (b) with or without 500 nM RepSox for 1 day (D1), 3 day (D3) or 5 days (D5), and osteoclast-specific gene expression was measured by real-time PCR. The results were normalized to GAPDH expression. (*p < 0.05, **p < 0.01). M-CSF: macrophage colony-stimulating factor; PCR: polymerase chain reaction; RANKL: receptor activator of nuclear factor κB ligand
3.5 RepSox does not affect osteoblast differentiation, migration or osteoblastic-specific gene expression in vitro
Although RepSox impaired osteoclast formation, it is important to consider if RepSox has any effect on osteoblasts. Therefore, MC3T3-E1 cells were used for osteoblastic differentiation and mineralization research. Cell viability was significantly different in the 96-hr treatment group (p < 0.05), but there was no significant difference in the 48-hr treatment group (Figure 5a). ALP staining clearly showed that there was not only no significant difference but also a slight promotion between the control and RepSox-treated groups (Figure 5b). In addition, Alizarin Red staining also demonstrated that the total mineralized area was similar between the control group and the RepSox-treated groups (Figure 5c). In addition, RepSox did not affect osteoblasts migration (Figure 5d). We further explored the expression of osteoblastic-specific genes, including ALP, BMP-2, Runx2, OCN, Col-1a1, ANGPT1 and Osx. The osteoblast marker mRNA expression was not affected by RepSox at concentrations of 62.5, 125, 250, 500 and 1,000 nM at 7 days (Figure 5e). These results indicated that RepSox does not affect osteoblast differentiation or mineralization in vitro.
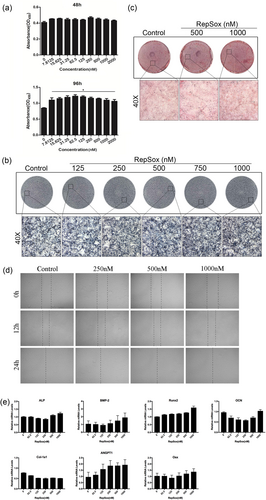
RepSox does not affect osteoblast differentiation, migration or gene expression in vitro. (a) MC3T3-E1 cells were cultured in 96-well plates at a density of 2 × 103 cells/well with increasing concentrations of RepSox for 48 hr or 96 hr, and cell viability was measured using CCK-8 assays. (b) MC3T3-E1 cells were seeded in 12-well plates and cultured in osteogenic medium with different concentrations of RepSox for 7 days or (c) 21 days; ALP or Alizarin Red staining was performed, respectively. (d) Wound healing assays were used to detect the migratory potential in MC3T3-E1 cells after treatment with RepSox for 12 and 24 hr at a concentration of 0, 250, 500, or 1,000 nM. Images were taken using a fluorescence microscope. (e) MC3T3-E1 cells were cultured in osteogenic medium with different concentrations of RepSox for 7 days, and osteoblast-specific gene expression was analyzed by real-time PCR. (*p < 0.05). ALP: alkaline phosphatase; CCK-8: cell counting kit-8; PCR: polymerase chain reaction [Color figure can be viewed at wileyonlinelibrary.com]
3.6 RepSox suppresses the Smad3 and JNK/AP-1 signaling pathways during osteoclastogenesis
To further explore the potential mechanisms on how RepSox affects osteoclast formation, critical signaling pathways involved in osteoclast differentiation were investigated. Mitogen-activated protein kinase (MAPK) signaling pathways, including the extracellular signal-regulated kinase (ERK), JNK and p38 pathways, are some of the key pathways during osteoclast formation; thus, we investigated MAPK activation. In our study, we initially observed that phosphorylation of Smad3 was increased after stimulation with RANKL for 10 min, but RepSox reduced this Smad3 phosphorylation. In addition, the phosphorylation of ERK, JNK, and p38 was increased after stimulation with RANKL for 10 min, and RepSox reduced the phosphorylation of JNK but not ERK or p38 activation (Figure 6a). Quantitative analysis confirmed an inhibitory effect of RepSox on Smad3 and JNK phosphorylation (Figure 6b). Then, the levels of c-Fos, a protein downstream of JNK, were also decreased by RepSox treatment (Figure 6c,d). Next, by using AP-1 luciferase reporter gene assays, the transcriptional activity of AP-1 was obviously increased when treated with RANKL. However, RepSox suppressed AP-1 luciferase activity in a dose-dependent manner (Figure 6e). Furthermore, a rescue assay showed that osteoclast formation was inhibited in the presence of only RepSox; however, after anisomycin was added to the cells, impaired osteoclastogenesis was rescued, and mature osteoclasts were observed (Figure 6f,g). These results suggest that RepSox inhibits c-Jun N-terminal kinase/activator protein-1 (JNK/AP-1) during osteoclast differentiation.
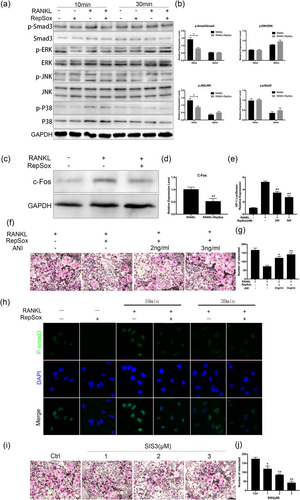
RepSox suppresses Smad3 and JNK/AP-1 signaling pathways during osteoclastogenesis. (a) BMMs were pretreated with or without 500 nM RepSox for 1 hr and stimulated with 100 ng/ml RANKL for 0, 10 and 30 min; the cell lysates were analyzed by western blotting. (b) Smad3, ERK, JNK and p38 band intensities were quantified and the phosphorylated bands are expressed as a percentage corresponding to their total protein. (c) BMMs were seeded in 6-well plates and pretreated with or without 500 nM RepSox for 1 hr and stimulated with 100 ng/ml RANKL for 10 min. Cells were lysed for western blotting with specific antibodies against c-Fos and GAPDH. (d) Fold changes in c-Fos protein levels were normalized to the expression of GAPDH. (e) Stably transfected BMMs with an AP-1 luciferase reporter construct were seeded in 12-well plates and cultured with medium for 24 hr. Then, cells were pretreated with or without different concentrations of RepSox for 1 hr followed by stimulation of 100 ng/ml RANKL for 24 hr. AP-1 luciferase activity was measured. (f) BMMs were stimulated with 30 ng/ml M-CSF, 100 ng/ml RANKL and 250 nM RepSox, with or without indicated concentrations of anisomycin. After mature osteoclasts formed at 5–7 days, the cells were fixed and stained with TRAP staining. (g) TRAP-positive multinuclear cells were counted. (h) Representative confocal laser immunofluorescence photomicrographs showing the location of Smad3 in BMMs from different groups. (i) BMMs were treated with 30 ng/ml M-CSF, 100 ng/ml RANKL and various concentrations of SIS3 (a Smad3 inhibitor), cultured for 5–7 days and then subjected to TRAP staining. (j) The numbers of TRAP-positive osteoclasts were measured. (*p < 0.05, **p < 0.01). BMMs: bone marrow macrophages; ERK: extracellular signal-regulated kinase; JNK/AP-1: c-Jun N-terminal kinase/activator protein-1; M-CSF: macrophage colony-stimulating factor; RANKL: receptor activator of nuclear factor κB ligand; TRAP: tartrate-resistant acid phosphatase [Color figure can be viewed at wileyonlinelibrary.com]
Subsequently, we analyzed the level of Smad3 protein using immunofluorescence staining. The intensity of Smad3 in BMMs was increased when treated with 100 ng/ml RANKL for 10 min, but treatment with RepSox clearly decreased RANKL-induced Smad3 expression (Figure 6h), which was consistent with the western blotting results. To further confirm that RepSox suppressed the osteoclast formation by affecting Smad3 activation, we further used the specific inhibitor of Smad3 (SIS3) to validate this. As shown in Figure 6i, osteoclast formation was significantly restrained when the cells were treated with SIS3. Statistical analysis showed that when the BMMs were treated with SIS3 at 1, 2, and 3 μM concentrations, the number of osteoclasts was significantly lower than that of the control group (Figure 6j). These data suggest that RepSox may affect osteoclast formation by suppression of the Smad3 and JNK/AP-1 signaling pathways.
3.7 RepSox prevents OVX-induced bone loss in vivo
Given that RepSox suppression affected osteoclast formation and function but not osteoblast differentiation in vitro, we hypothesized that RepSox could prevent osteoclastic osteolysis in vivo and may become a new therapeutic drug for osteolytic diseases. The use of RepSox, at least as a TGFβRI inhibitor, for other diseases, such as prostate cancer, may have a protective effect on bone mass (Wan et al., 2012). Thus, the effect of RepSox on OVX-induced OP was measured, and the histomorphology of all mice was analyzed. Initially, three-dimensional reconstructed images of femurs, lumbar vertebrae, and cancellous bones were analyzed (Figure 7a). After OVX, excessive femur bone loss was observed in the vehicle group by micro-CT, and the BV/TV (1.82 ± 0.43%) was significantly reduced compared with the sham group (4.36 ± 0.34%) (p < 0.01; Figure 7c), which demonstrated that our OVX mouse model was successful. When treated with RepSox, further bone loss was prevented. In the low-dose group, in which RepSox was injected at 2 mg/kg twice a week for 4 weeks, the BV/TV was 2.41 ± 0.40%, approximately 20% higher than that in the vehicle group. A more significant difference was observed in the high-dose group, in which RepSox was injected at 5 mg/kg twice a week for 4 weeks, the BV/TV was increased approximately 70% compared with the vehicle group. In addition, other indices such as Tb.N was increased, and Tb.Sp was reduced. A similar effect can also be observed in the lumbar tissues, as shown in Figure 7b. In addition, RepSox did not affect the weight or internal organs of the mice in any group (Supporting Information Figure S1 and S2). These data together indicate a protective effect of RepSox against bone loss.
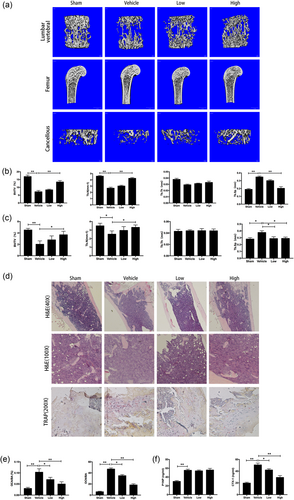
RepSox inhibited OVX-induced OP in vivo. (a) The femur and lumbar vertebrae were analyzed by micro-CT, and three-dimensional reconstructed images from each group are shown. (b) The trabecular bone volume per total volume (BV/TV), mean trabecular number (Tb.N), mean trabecular thickness (Tb.Th), mean trabecular separation (Tb.Sp) of the lumbar vertebrae and (c) femurs in each group were measured. (d) The femur sections were fixed, decalcified, dehydrated, and sectioned. Sections were stained with H&E and TRAP staining. (e) Ratio of osteoclast area to bone area (OcA/BA) and the number of TRAP-positive multinucleated osteoclasts normalized to the bone area (OcN/BA) were analyzed. (f) Serum levels of P1NP and CTX-1 are shown. All experiments were performed at least three times. (*p < 0.05, **p < 0.01). CTX-1: C-terminal collagen cross-links; H&E: hematoxylin and eosin; micro-CT: microcomputed tomography; OP: osteoporosis; OVX: ovariectomy; P1NP: procollagen type 1 N-terminal peptide; TRAP: tartrate-resistant acid phosphatase [Color figure can be viewed at wileyonlinelibrary.com]
To further confirm the effect of RepSox on preventing bone loss, histological analysis was performed. As shown in Figure 7d, in contrast to the massive amount of TRAP-positive multiple osteoclasts lined along the eroded bone surface in the vehicle group, the number of osteoclasts was reduced in the RepSox treatment groups. The OcN/BA was 45.53 ± 3.14% in the vehicle group and 20.21 ± 2.83% in the high-dose group; meanwhile, the OcA/BA was 0.12 ± 0.03% in the vehicle group and 0.06 ± 0.02% in the high-dose group (Figure 7e), which is also consistent with the results of the inhibitory effect of RepSox on osteoclastic bone resorption in vitro. Serum CTX-1, a marker of bone resorption, was lower in RepSox treated mice, whereas there was no difference in P1NP, a marker of bone formation (Figure 7f). In summary, these data demonstrated that RepSox inhibited osteoclast formation, function, and osteoclastic osteolysis both in vitro and in vivo.
4 DISCUSSION
Disruption of the dynamic balance between osteoclasts and osteoblasts with increased osteoclast formation and resorption is one of the main causes of OP (Amirhosseini et al., 2018). In bone metabolism systems, TGFβ is secreted by bone cells, such as osteoblasts, osteoclasts, and chondrocytes. Many studies have demonstrated the critical role of TGFβ as a master switch regulating the spatiotemporal coupling of bone resorption and formation. There are three TGFβ receptor isoforms related to bone metabolism in mammals. Among these isoforms, TGFβRI is known as the major isoform existing in bones, and it is considered to be the most important cytokine related to adult bone homeostasis. TGFβ has a strong effect on the physiology of osteoblasts, but its roles in osteoclasts are ambivalent. On one hand, in the presence of M-CSF, TGFβ can directly induce mononuclear phagocyte osteoclast precursors to differentiate into osteoclastic cells in the absence of RANKL, but these cells manifest as small multinucleated cells compared with the numerous TRAP-positive osteoclasts induced by RANKL (Itonaga et al., 2004). On the other hand, TGFβ also inhibits the formation and stimulates the apoptosis of osteoclasts in bone marrow cultures (Chenu, Pfeilschifter, Mundy, & Roodman, 1988; Hughes et al., 1996). In addition, a study found that TGFβ has no effect on the function of mature osteoclast bone resorption (Hattersley & Chambers, 1991). TGFβRII knockout in mice results in osteopenia due to reduced osteoblast numbers but not osteoclast numbers or activity (Weivoda et al., 2016). All paradoxical results about the roles of TGFβ in osteoclasts mainly exist because the analysis depends on the environment, which can bring about the potential for indirect or confounding influences.
In our experiment, we selected a small molecule TGFβRI/ALK5 inhibitor (RepSox) to evaluate its effect on osteoclasts and osteoblasts. Interestingly, our study found an off-target effect of RepSox. That is, RepSox inhibited osteoclast formation and osteoclastic bone resorption via the Smad3 and JNK/AP-1 pathways in vitro and protected OP-related bone loss in vivo but had no impairing effects on osteoblast proliferation, migration, differentiation, or osteoblastic-specific gene expression in vitro. A possible explanation for this phenomenon is that TGFβ has a strong effect on osteoblasts; high concentrations of RepSox may actually impact osteoblast activity by inhibiting TGFβRI, but at a concentration of 1,000 nM, RepSox inhibits osteoclasts with no effect on osteoblasts, according to our data. In other words, lower concentrations RepSox are not enough to produce an inhibitory effect on osteoblasts but they do have an effect on reducing osteoclast formation and preventing OP bone loss. In addition, we hypothesize that lower concentrations RepSox may actually influence osteoblast cell activity, but other essential pathways eliminate this effect; thus we could not observe the inhibitory phenomenon in the ALP or Alizarin Red staining results. Because TGFβ and bone morphogenetic protein (BMP) pathways have signaling crosstalk, attenuating TGFβRI may repress the expression of I-Smad, resulting in enhanced BMP signaling and, accordingly, accelerating osteoblastic differentiation (Maeda, Hayashi, Komiya, Imamura, & Miyazono, 2004). All in all, the exact effect of RepSox on osteoblasts in vitro and on bone formation in vivo needs further investigation. Basically, RepSox has a strong inhibitory effect on osteoclast formation and osteoclastic bone resorption in vitro and could prevent OP bone loss in vivo.
The TGFβ pathway is an important intracellular signaling transduction pathway. Activation of this signaling pathway begins with the TGFβ ligand binding to its receptors and phosphorylation of the receptors. Then, the phosphorylated TGFβR acts directly on the Smad substrates, and finally, activated Smad together with other nuclear factors passes the signal from cell membrane to the nucleus, which results in activation or inhibition of target gene transcription (Akhurst & Padgett, 2015). Previously, the biological functions of the TGFβ/Smad pathway were mainly thought to be inflammation (Gittes, 2016), immune regulation (R. Lin, Sampson, Li, & Zhu, 2015), tissue repair (Hozzein et al., 2015), and embryonic development (Muñoz-Espín et al., 2013). In recent years, it has been found that TGFβ plays an important role in regulating osteoclast differentiation and function. SB431542, a TGFβRI inhibitor, can inhibit RAW264.7 cell differentiation into osteoclast-like cells by suppression of TGFβ/TβR1/Smad3 (Yu et al., 2018), which is similar to our findings. Smad3, the main downstream signaling molecule of TGFβ, is an important effector of the TGFβ signaling pathway and participates in osteoclast formation and function. In a coculture of giant cell tumor stromal cells and RAW264.7 cells, TGFβ significantly increased multinucleated TRAP-positive cell formation, but SIS3 reversed this effect (Lou et al., 2013). Similar to our experiment, RepSox suppressed osteoclast formation by inhibiting Smad3 phosphorylation in BMMs, while SIS3 decreased osteoclast formation from BMMs. Our data further demonstrated that the TGFβ/Smad pathway plays an important role in osteoclastogenesis.
Most studies have demonstrated that the MAPK pathways (ERK, JNK and p38) are very important to osteoclast formation (J. Lin, Lee, Choi, & Lee, 2015; Ouyang et al., 2014; Zhu et al., 2016). Inhibition of MAPK activity could reduce osteoclast formation and bone destruction. JNK is a member of the MAPK family, and some research has demonstrated the importance of JNK signaling in osteoclastogenesis (Liu et al., 2014; Min, Kang, Jung, Jang, & Min, 2018). JNK can be stimulated by RANKL; subsequently, activated JNK phosphorylates downstream factors c-Fos and c-Jun, and these two factors combine to form AP-1, which is an essential translation factor during osteoclast formation. In our study with RepSox treatment, JNK phosphorylation and c-Fos expression were inhibited, and AP-1 transcriptional activity was suppressed. In addition, JNK also activates NFATc1, and NFATc1 is a key transcriptional factor that regulates the expression of several genes associated with osteoclast differentiation and function (Park et al., 2016). Our data show that RepSox suppresses NFATc1 mRNA expression in both dose- and time-dependent manners, and the western blot assays attest that RepSox can suppress JNK pathways. This finding is supported by the discovery that SB431542 and the Smad3 inhibitor SIS3 blocked Emdogain-stimulated expression of the transcription factor NFATc1 during in vitro osteoclastogenesis (Gruber et al., 2014).
However, our study has several limitations. We only evaluated the effect of RepSox on osteoclasts and osteoblasts, and further evaluation of other bone cells such as osteocytes is required. Apart from this, we only measured the effect of RepSox on MAPK pathways; the effect on other pathways, including the PI3K-AKT and IκBα pathways, remains unclear. Overall, our results show that TGFβRI may play an important role in osteoclasts. RepSox inhibits osteoclast formation and function both in vitro and in vivo, suggesting that RepSox has potential therapeutic value for the treatment of osteoclast-related osteolytic diseases.
ACKNOWLEDGMENTS
This study was financially supported by the Zhejiang Science and Technology Department Public Welfare Technology Application Research Project (Project Number: 2016KYA200) and the General Project of Zhejiang Natural Science Foundation (Project Number: Y17C100002). Importantly, L. Mei wants to thank W. Sang in particular for patience, care, and support over the past years.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.