Analysis of pro- and anti-inflammatory cytokine gene variants and serum cytokine levels as prognostic markers in breast cancer
Abstract
The aim of current study was to evaluate the genetic variation in all the genes encoding pro- and anti-inflammatory cytokines in association with breast cancer development in patients from Malwa region of Punjab. The importance of the levels of interleukin (IL)-17, tumor necrosis factor, interferon γ, IL-10, IL-6, IL-4, and IL-2 with respect to clinicopathological data, prognosis, and disease-free survival was also determined in these patients. Two hundred and fifty female breast cancer patients and 250 age-matched controls were screened for variations in cytokine-encoding genes using global screening array microchip and PCR-RFLP. The level of cytokines was estimated in 150 patients and 60 age-matched controls using BD™ Cytometric Bead Array (CBA) Human Th1/Th2/Th17 cytokine kit by BD Accuri flow cytometer. The difference in cytokine levels was evaluated by Mann–Whitney test. No significant variation in the genes encoding various cytokines was found between patients and controls. Out of the seven cytokines evaluated, the levels of IL-6 and IL-17a were found to be significantly high in patients in comparison with controls ( p = 0.001 and 0.02, respectively). The elevated levels of these cytokines are also associated significantly with poor outcome. We did not find any specific variation in the genes encoding various cytokines between patients and controls. However, there was a significant difference in the serum levels of IL-6 and IL-17a between patients and controls, and the elevated levels of these two cytokines associated significantly with poor outcome in breast cancer patients and, therefore, can be used as prognostic markers.
1 INTRODUCTION
Breast cancer is the most frequently diagnosed cancer and is the foremost cause of death among women worldwide. Although new treatments for breast cancer are being developed, this cancer accounts for 14% of total cancer deaths worldwide (Jemal et al., 2011). Significant efforts have been made to identify prognostic biomarkers so that the high-risk patients are identified at the earliest using novel diagnostic and therapeutic approaches (Lin, Gan, Han, Yao, & Min, 2015). Inflammation has a very strong association with different types of cancer. Tumor cells are highly proliferative in nature, and this proliferation is facilitated by many secretory factors, especially the inflammatory molecules released by tumor cells themselves or by other cells in the tumor microenvironment.
The strong association between inflammation and cancer is reflected by the altered levels of pro- and anti-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), transforming growth factor-β (TGF-β), interleukin (IL)-1, IL-2, IL-6, IL-10, IL-17, and so forth, in tumor microenvironment where these promote tumorigenesis by the alteration of various hallmarks of cancer and multiple signaling pathways including apoptosis, proliferation, metastasis, angiogenesis, and metabolism (Kumari, Dwarakanath, Das, & Bhatt, 2016; Landskron, De la Fuente, Thuwajit, Thuwajit, & Hermoso, 2014). IL-6 acts as a pro as well as an anti-inflammatory cytokine and is secreted by macrophages and T cells to stimulate the immune response. Fibroblast from breast tissue has been reported to secrete IL-6, which is involved in the growth and invasiveness of MCF-7 cell lines (Baumgarten & Frasor, 2012). Sullivan et al. (2009) reported that the overexpression of IL-6 in MCF-7 cells induces the epithelial to mesenchymal transition and increases their invasiveness. Ding et al. (2018) found that patients with high levels of IL-6 showed shortened overall survival than those with normal IL-6 levels.
IL-17, a proinflammatory cytokine, is secreted by CD4 and CD8 cells. Increased serum levels of IL-17 have been found to be associated with worse prognosis in patients with colorectal and gastric cancers (Gonda et al., 2018). Kaewkandan et al. (2018) analyzed axillary lymph nodes (ALN) of women with large and locally advanced breast cancer and found that metastatic ALN exhibited high levels of IL-10. In addition, they also observed low levels of IL-2 and interferon γ (IFN-γ) in breast cancer patients with metastatic ALN. The role of IL-4 was demonstrated in a synergistic breast cancer mouse model and interaction between IL-4 and IL-4 receptor was found to be associated with metastatic breast cancer by activating mitogen-activated protein kinase pathway (Venmar, Carter, Hwang, Dozier, & Fingleton, 2014). In addition, variation in genes encoding various cytokines has been investigated widely in association with predisposition and the prognosis of breast and cervical cancers, by altered expression levels of cytokines (N. Liu, Song, & Shi, 2015; Wang et al., 2012).
As per the cancer registry of Guru Gobind Singh Medical College and Hospital (main referral center in this region), breast cancer is one of the most common types of cancer in Malwa region of Punjab. In spite of this, no empirical studies on breast cancer have been taken up in this area till date. Therefore, the current study was carried out with an aim to investigate the variation in the cytokine-encoding genes by global screening array (GSA) and also to evaluate the serum levels of various cytokines including IL-17, TNF, IFN-γ, IL-10, IL-6, IL-4, and IL-2 in association with clinicopathological data, prognosis, and disease-free survival among breast cancer patients from Malwa region of Punjab. To the best of our knowledge, this is the first study from India evaluating the association of both variation in cytokine-encoding genes and their levels in association with disease progression in breast cancer patients.
2 MATERIALS AND METHODS
Two hundred and fifty newly diagnosed female breast cancer patients evaluated at Guru Gobind Singh Medical College and Hospital, Faridkot, Punjab, were included in the study. This is the main referral hospital for cancer patients in this region. This study was approved by the ethical committee of the study hospital and the Central University of Punjab. The diagnosis was made by fine-needle aspiration cytology, mammography, and histopathology. Patients with major cardiac, renal, hepatic, skeletal, and neurological disorders and other cancers were excluded from this study. Patients with infections were also excluded from the study. Two hundred and fifty individuals belonging to the same ethnic group were recruited from the same demographic area as a control group. The controls had no clinical evidence of cancer and other disorders and none of their first-degree relatives were affected by any other type of cancer. Information on demographic features and risk factors was collected using a structured questionnaire.
Blood samples from patients were collected in EDTA vacutainers and vacutainers with clot activator after the confirmation of the disease and after obtaining written informed consent. DNA was isolated using phenol–chloroform method. Qualitative and quantitative analyses were performed using a NanoDrop ND-1000 UV-Vis Spectrophotometer (Thermo Fisher Scientific India Pvt. Ltd, Mumbai, India) and agarose gel electrophoresis (0.8% agarose–1× TBE gels stained with ethidium bromide), respectively. Genotyping was performed on Illumina Infinium HD assay platform using GSA microchip (Illumina, Inc., Powai, Mumbai India) with 200 ng of genomic DNA according to the manufacturer’s instructions. GSA microchip contains more than 700,000 up-to-date markers, optimized for human genome-wide backbone for unparalleled genomic coverage, including clinically relevant content and all PharmGKB markers. Subsequent sample processing and array hybridization were performed according to the manufacturer’s instructions (Illumina, Inc.). Genome Studio (Illumina, Inc.) was used for data preprocessing and analyses. Genotypes were called within Genome Studio with the Gen Call algorithm of Genotyping Module v1.0. The final sample call rate was 99.99%. The data were subsequently exported to R/Bioconductor to calculate χ2 and odds ratio. Annotation was performed using ClinVar, 1000 Genomes, ExAC, Cosmic, and dbSNP databases. A p ≤ 5 × 10−8 was considered statistically significant.
2.1 Cytokine analysis
Serum was separated by subjecting the blood samples to centrifugation. The serum was stored at −80oC till estimation. Serum samples of 150 breast cancer patients and 60 controls were analyzed for cytokine levels. Cytokine levels in the serum samples were detected by BD Accuri flow cytometer (Becton Dickinson Holdings Pte Ltd, Singapore) using commercially available kit BD™ Cytometric Bead Array (CBA) Human Th1/Th2/Th17 cytokine kit (BD Biosciences, USA) following manufacturer’s instructions. In brief, cytokine standards were prepared using a vial of lyophilized Human Th1/Th2/Th17 and assay diluent by the method of serial dilutions. Capture bead was added into each tube that is samples, standards, and negative control and was incubated for 30 min at room temperature in the absence of light. The flow cytometer was calibrated using cytometer setup beads and the assay was performed.
2.2 Statistical analysis
Data were calculated as mean ± SD. The distribution of cytokines was not normal. It was positively skewed. Therefore, p values were calculated using rank sum test using Sigma Plot 11.0 software (Starcom Information Technology Ltd, Bangalore, India). The Mann–Whitney U test was performed for the comparison of mean values of IL-17, TNF, IFN-γ, IL-10, IL-6, IL-4, and IL-2 among cases and controls. The Mann–Whitney test was also performed to evaluate the association of cytokines with receptor status and poor outcome among breast cancer patients.
2.3 Follow-up
The follow-up of the patients was conducted telephonically or personally during their follow-up visits to the hospital at an interval of 3, 6, 12, 15, 18, and 21 months to check death, survival, and metastasis.
3 RESULTS
Two hundred and fifty female breast cancer patients and age-matched controls were included in the study. The mean age of patients at the presentation of the disease was 53.62 ± 12.31 years. The youngest patient presenting breast cancer was 23 years old. More than half of the patients were residing in the urban area. Pregnancy was not observed in any patients at the time of diagnosis. Obesity was observed in 44.8% of patients; 12.3% of patients had first-degree relative suffering from breast or any other type of cancer; 46.4% patients had an exposure to pesticides, as the majority of the patients belonged to the farming community; 55% of patients suffered from left breast tumorigenesis; 63% of patients underwent modified radical mastectomy; and lumpectomy was performed in 5% of patients. The predominant histopathological subtype of breast cancer was infiltrating ductal carcinoma followed by infiltrating lobular carcinoma, in situ lobular, and ductal carcinoma. Histopathological reports were available for only 93 patients. Among these, 37.6% were positive for estrogen receptor (ER) or progesterone receptor, whereas triple-negative breast cancer, which is the most aggressive type of breast cancer, was observed in 17.5% of cases. Disease-free survival was observed in 72% of patients and overall survival in 74% of patients (Table 1).
| Personal data | Item | Number | % |
|---|---|---|---|
| Gender | Female | 250 | 100 |
| Menopausal status | Premenopausal | 85 | 34 |
| Postmenopausal | 155 | 62 | |
| Hysterectomy | 10 | 4 | |
| Parity | Parent | 242 | 96.8 |
| Nulliparous | 8 | 3.2 | |
| Weight | Obese | 112 | 44.8 |
| Normal | 118 | 47.2 | |
| Underweight | 20 | 8 | |
| Family history | Breast cancer | 11 | 4.5 |
| Other | 19 | 7.8 | |
| Disease-free survival | 180 | 72 |
We are evaluating all the gene variants for their possible association with breast cancer development and drug response using GSA microchip and PCR-RFLP. In this context, the genes encoding all the pro- and anti-inflammatory cytokines were also screened for their association with breast cancer development in comparison with the healthy controls. However, we did not find any significant variation in the genes encoding the pro- and anti-inflammatory cytokines including TNF-α, TGF-β, IL-1, IL-17, TNF-β, IFN-γ, IL-10, IL-6, IL-4, IL-2, and so forth.
The mean levels of cytokines IL-17, TNF, IFN-γ, IL-10, IL-6, IL-4, and IL-2 were found to be 7.40 ± 11.69, 2.78 ± 9.18, 0.94 ± 3.03, 1.64 ± 3.11, 15.3 ± 40.36, 1.61 ± 4.31, and 1.48 ± 7.07, respectively. However, a significant difference in the levels of IL-6 and IL-17a was observed between patients and controls (p = 0.001 and 0.02, respectively; Table 2). No significant difference was observed in the levels of other cytokines between patients and controls. Evaluating the association of these cytokines with receptor-positive breast cancer, a significant association of IL-6 elevated levels was observed with ER-positive breast cancer (p = 0.001). Elevated levels of IL-17a associated significantly with triple-negative breast cancer (p = 0.02). As for the outcome, both the proinflammatory cytokines IL-6 and IL-17a associated significantly with poor outcome, including death, metastasis, and recurrence (Table 3).
| Cytokine | Patients | Controls | p Value |
|---|---|---|---|
| IL-6 | 15.33 ± 40.36 | 3.01 ± 3.12 | 0.001 |
| IL-17a | 7.40 ± 11.69 | 1.91 ± 2.95 | 0.02 |
| TNF (pg/ml) | 2.82 ± 9.24 | 1.05 ± 2.21 | 0.80 |
| IFN-γ (pg/ml) | 0.96 ± 3.05 | 0.22 ± 0.55 | 0.71 |
| IL-10 (pg/ml) | 1.64 ± 3.11 | 0.52 ± 1.20 | 0.53 |
| IL-2 (pg/ml) | 1.50 ± 7.07 | 0.72 ± 2.04 | 0.94 |
| IL-4 (pg/ml) | 1.69 ± 4.31 | 0.20 ± 0.42 | 0.81 |
- Note. IL: interleukin; IFN-γ: interferon γ; TNF: tumor necrosis factor.
| Parameter | IL-6 | IL-17a | p Value |
|---|---|---|---|
| Death (26%) | 41.54 ± 72.33 | 17.19 ± 15.17 | <0.05 |
| Metastasis (20%) | 20.35 ± 25.88 | 16.03 ± 17.89 | <0.05 |
| Recurrence (8%) | 26.5 ± 117.44 | 14.5 ± 4.45 | <0.05 |
| Disease-free survival (72%) | 6.62 ± 12.96 | 3.85 ± 7.58 | Reference |
- Note. IL: interleukin.
4 DISCUSSION
Cytokines, the low-molecular-weight proteins, act as mediators for the cell to cell communication. They are synthesized by immune and stromal cells and are involved in the regulation of proliferation, cell survival, differentiation, immune cell activation, cell migration, and apoptosis. Cytokines modulate antitumor response but during chronic inflammation these proteins have been reported to induce cell transformation and malignancy depending on the balance of pro- and anti-inflammatory cytokines, their concentration, receptor expression content, and also activation state of cells in the surrounding (Landskron et al., 2014).
Genetic variations in pro- and anti-inflammatory cytokines have been investigated in many cancers (T. Z. Liu et al., 2017; Mandal, Abebe, & Chaudhary, 2014). A number of variants have been reported in IL-6 and IL-17a influencing the levels of these cytokines (Wang et al., 2012; Zhao et al., 2015). Previous studies have reported the association of specific gene polymorphisms with susceptibility to cancer. In addition, some of these variants were also associated with elevated levels of specific cytokines. The −174G/C variant in IL-6 gene is reported to be associated with increased incidence of prostate, ovarian, and bladder cancers (T. Z. Liu et al., 2017; Mandal et al., 2014; Zhai, Yang, Gao, & Ding, 2017). A meta-analysis reported the association of +874 polymorphism of IFN-γ with an increased risk of cervical cancer among Asian ethnic group (T. Z. Liu et al., 2017). Wang et al. (2012) found a significant association of rs2275913 (IL-17a) with breast cancer in Chinese Han women. In the current study, although all the cytokine genes were screened, we could not find a significant association with variation in any of the cytokine-encoding genes in association with the disease development.
Evidence suggests that various cytokines (especially proinflammatory) are associated with poor prognosis in breast cancer (Hong, Angelo, & Kurzrock, 2007). Current approaches for prognosticating breast tumor burden and prognosis based on stratifying patient risk group are gaining a lot of interest. The noninvasive and inexpensive analysis of tumor markers in serum is gaining a lot of attention for monitoring disease progression and treatment efficacy. Although, gene expression profiling holds the promise of providing new avenue for the prognosis and prediction of breast cancer outcomes, one critical limitation of gene expression–based prognostication is that it does not allow uninterrupted monitoring of patients and assessment during and/or after treatment and surgery (Noman et al., 2017). Therefore, the current study was carried out with an aim to investigate the levels of various cytokines including IL-17, TNF, IFN-γ, IL-10, IL-6, IL-4, and IL-2 in breast cancer patients in comparison with healthy controls and correlate it with disease outcome in the patients from Malwa region of Punjab, where breast cancer is widely feared.
Out of these cytokines, only IL-6 and IL-17a were found to be significantly elevated in the patients in comparison with healthy controls. The patients were followed up after 3, 6, 9, 12, 15, and 21 months for metastasis, recurrence, and death. Elevated levels of IL-6 associated significantly with poor outcome including death, recurrence, and metastasis. Increased expression of IL-6 is implicated in the proliferation and differentiation of malignant cells. IL-6 levels have been reported to be high in serum and tumor tissue of wide range of cancers including multiple myeloma, lung cancer, endometrial cancer, colorectal cancer, renal cell carcinoma, cervical cancer, ovarian cancer, prostate cancer, and breast cancer (Guo, Xu, Lu, Duan, & Zhang, 2012; Kumari et al., 2016). Elevated levels of IL-6 have also been associated with aggressive tumor growth and response to therapies in different types of cancer.
Current study reports a significant association of elevated IL-6 levels with poor outcome in breast cancer patient from Malwa region of Punjab, where breast cancer is widely feared due to its steeply rising cancer graph. This is in confirmation with the previous study that reported high levels of circulating IL-6 to be associated with poor prognosis and shorter survival, whereas lower levels of IL-6 were found to be associated with good outcome and better response to therapies in a Bangladeshi population (Noman et al., 2017). A meta-analysis carried out by Lin et al. (2015) determined the prognostic role of IL-6 in the context of survival in breast cancer patients and reported a correlation between IL-6 expression and survival. Further, IL-6 has also been shown to be a potent promoter of growth, survival, and migration of breast cancer cells (Bachelot et al., 2003). Patients who underwent several cycles of chemotherapy were reported to show a decline in IL-6 levels during a follow-up study carried out by Lin et al. (2015). IL-6 has been found to be important in various tumor behaviors including the development of malignancies, cell migration, invasion, proliferation, apoptosis, angiogenesis, and differentiation of tumor cells (Guo et al., 2012; Keller, Wanagat, & Ershler, 1996; Suchi et al., 2011). IL-6 directly supports tumorigenesis through the alteration of intrinsic and extrinsic activities and also modulates stromal cells that promote tumorigenesis indirectly (Fisher, Appenheimer, & Evans, 2014).
Canonical and trans-signaling pathway of IL-6 promotes tumor progression and metastasis (Grivennikov & Karin, 2008; Johnson, O’Keefe, & Grandis, 2018; Kwak, Dantuma, Merchant, Bushnev, & Sugaya, 2010; Lederle et al., 2011). In the canonical pathway, IL-6 ligand binds to membrane-bound receptor of IL-6 and subsequently ligates to glycoprotein 130 (gp130) and activates Janus kinase and signal transducer and activator of transcription (JAK-STAT) pathway. In the case of trans-signaling pathway, a soluble IL-6 receptor binds to IL-6 that further ligates to gp130 to stimulate STAT3 activation. IL-6 functions via JAK-STAT and phosphoinositide 3-kinase (PI3K) pathways. JAK-STAT pathway promotes cell proliferation by excessive production of cyclin E and E2F. Phosphorylated STAT dimer binds at IFN-γ-associated sequences element and initiates a change in a number of apoptosis-regulatory genes including Bcl-xL, MCL-1, XIAP, c-myc, and Fas and in turn hampers apoptotic pathway (Darnell, 1997). PI3K pathway promotes hypermethylation of P53 promoter and inhibits cell cycle arrest and apoptosis (Figure 1). IL-6 regulates the survival, self-renewal, and invasion of breast tumor stem cells and, therefore, directly contributes to cancer recurrence and metastasis. The findings of the current study are in accordance with previous studies (Lin et al., 2015). Shibayama et al. (2014) found that elevated levels of IL-6 are associated with shorter survival and poor prognosis. IL-6 levels were also reported to be increased in recurrent metastatic lesions in comparison with primary metastasis (Guo et al., 2012). Elevated IL-6 levels also associated significantly with ER-positive breast cancer in the current study.
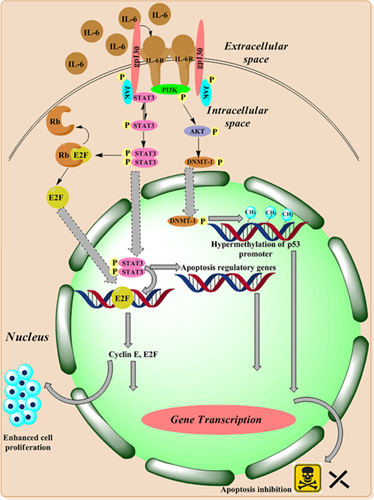
Involvement of IL-6 in tumor progression. IL-6 binds to IL-6 receptor and gp130 activates downstream signaling pathway and thereby promoting antiapoptosis, cell proliferation, and inhibits p53. DNMT: DNA methyltransferase; gp130: glycoprotein 130; IL: interleukin; JAK: Janus kinase; PI3K: phosphoinositide 3-kinase; STAT: signal transducer and activator of transcription [Color figure can be viewed at wileyonlinelibrary.com]
Previous studies have established that suppressor of cell signaling (SOCS) and protein inhibitor of activated STAT (PIAS) may also be compromised in breast cancer to promote cell survival. A study carried out by Jiang et al. (2017) evaluated the expression of SOCS and their influence on IL-6-induced activation of the JAK-STAT pathway in breast cancer myeloid–derived suppressor (MDSC) cell lines. They found that more MDSC were recruited in breast cancer tissues (in which SOCS inhibition was detected) expressing high levels of IL-6, which also enhanced the suppressor effects on T-cell immunity in vitro. The authors, therefore, summarized that IL-6 induced SOCS-3 dysfunction and long-term activation of JAK-STAT pathway in breast cancer MDSC cell lines (Jiang et al., 2017). The authors further proposed that blocking IL-6 pathway might be a promising therapeutic strategy for eliminating and inhibiting MDSCs to improve prognosis. In addition, the constitutional activation of the IL-6-mediated JAK-STAT pathway through hypermethylation of SOCS-1 has also been reported in a human gastric cell line (To et al., 2004). Recent studies have indicated that PIAS proteins can also dysregulate the activity of STAT3 (Yuan, Zhang, & Niu, 2015). However, in the current study, although IL-6 levels were found to be high in breast cancer patients showing a poor prognosis, we did not evaluate the status SOCS and PIAS genes. It might be that these proteins were dysfunctional in those patients. However, this needs to be established by carrying out future research in this direction.
In addition to IL-6, IL-17a levels were also found to be significantly elevated in breast cancer patients and associated with poor outcome in the current study. Cochaud et al. (2013) reported a significant association of elevated levels of IL-17a with reduced disease-free survival and triple-negative breast cancer. We also found a significant association of IL-17a with triple-negative breast cancer, which is in accordance with previous studies. Elevated IL-17a level is a prognostic factor that negatively influences the disease-free survival (Chen et al., 2013). The IL-17a levels and IL-17a-producing cells (CD8+) increase significantly in various malignancies, including breast cancer (Jung, Kim, Cho, & Kim, 2009; Zhu et al., 2008). IL-17a acts on different cell types and regulates the production of granulocyte–macrophage colony-stimulating factor, IL-1, TNF-α, chemokines, activation of nitric oxide synthase 2, and leukocyte recruitment (Park et al., 2005). Benevides et al. (2013) reported that elevated IL-17a levels correlated positively with tumor aggressiveness, poor prognosis, and disease-free survival. IL-17a promotes higher expression of genes that promote excessive cell proliferation and tumor survival (Benevides et al., 2015). IL-17a has been shown to be responsible for tumor progression through angiogenesis (Tartour et al., 1999). It is involved in metastatic breast cancer by recruiting neutrophils at the site of tumor. Neutrophils secrete factors like elastase, cathepsin G, matrix metalloproteinase (MMP)-8 and MMP-9, which are responsible for the degradation of extracellular matrix and, hence, facilitate invasion (Dumitru et al., 2011). Neutrophils isolated from the tumor site and spleen had higher expression of markers CXCL1, TNF-α, MMP-9, and vascular endothelial growth factor (VEGF) and associated with disease progression (Nozawa, Chiu, & Hanahan, 2006). IL-17a is also involved in the release of IL-6 in the tumor microenvironment and thereby activates STAT3 pathway and, hence, excessive proliferation (Du, Xu, Fang, & Qi, 2012; Fossiez et al., 1998; Ma, Huang, & Kong, 2018; Wang et al., 2009). IL-17a inhibits apoptosis by the activation of the nuclear factor-κB pathway leading to the expression of antiapoptotic genes Bcl-2 and MCL-1 (Lederle et al., 2011; Figure 2). Therefore, IL-17a is emerging as a marker for tumor aggressiveness, poor prognosis, and disease-free survival. The drawback of current study is that we could not assess the levels of IL-6 and IL-17a after the patients underwent surgery and chemotherapy.
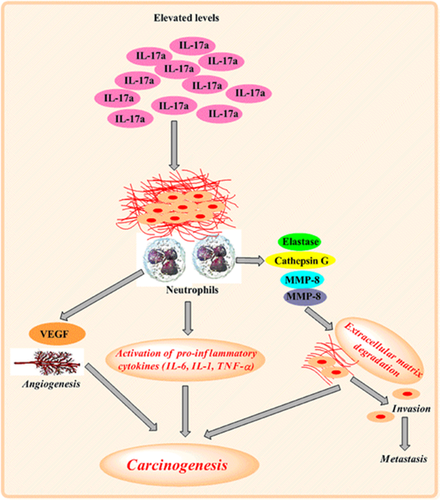
Role of IL-17a in disease progression. IL-17a binds to the transmembrane receptor, recruits neutrophils to the site of tumor, which in turn secrete extracellular degradation factors, leading to distant metastasis. Secretion of proinflammatory cytokines by recruited neutrophils leads to carcinogenesis and increased expression of VEGF that causes angiogenesis at the tumor site. IL: interleukin; MMP: matrix metalloproteinase; TNFα: tumor necrosis factor-α; VEGF: vascular endothelial growth factor [Color figure can be viewed at wileyonlinelibrary.com]
The strength of the study is that we evaluated variations in all the pro- and anti-inflammatory cytokine genes to establish an association with the development of the disease in patients from Malwa region of Punjab and, moreover, the patients were enrolled at the time of diagnosis. Therefore, all the patients were untreated at the time of serum sample collection for estimating the levels of the cytokines. To the best of our knowledge, this is the first study from India evaluating the association of cytokines with breast cancer development and prognosis.
In conclusion, the variation in genes encoding various cytokines is not a risk factor for the development of breast cancer in Malwa region of Punjab. However, IL-6 and lL-17a levels have emerged as important prognostic markers in breast cancer from Malwa region of Punjab. Evidence suggests that blocking IL-6 and IL-17a may provide therapeutic gain in those cancers, which are associated with higher levels of these cytokines. Although many cytokines have shown effective therapeutic antitumor activity in the preclinical setting, translation to clinical practice is only limited to IFN-γ and IL-2, which have been approved for oncologic indications (Colombo & Trinchieri, 2002). IL-2, INF-γ, and INF-β have been used in advanced breast cancer treatment. Occasionally, IFN-γ, IL-6, and IL-12 have also been used (Landskron et al., 2014). However, further studies are warranted for the validation of IL-6 and IL-17a as prognostic tools in a larger patient cohort belonging to various ethnic groups, as well as in a longer follow-up study.
ACKNOWLEDGMENTS
Financial assistance provided by Central University of Punjab, Bathinda (CUPB) is acknowledged with thanks.
CONFLICTS OF INTEREST
There are no conflicts of interest.