Aerobic exercise, but not metformin, prevents reduction of muscular performance by AMPk activation in mice on doxorubicin chemotherapy
Abstract
Doxorubicin (DOX) is a chemotherapy agent widely used in clinical practice, and it is very efficient in tumor suppression, but the use of DOX is limited by a strong association with the development of severe muscle atrophy and cardiotoxicity effects. Reversion or neutralization of the muscular atrophy can lead to a better prognosis. Recent studies have proposed that the negative effect of DOX on skeletal muscle is linked to its inhibition of AMP-activated protein kinase (AMPk), a key mediator of cellular metabolism. On the basis of this, our goal was to evaluate if aerobic exercise or metformin treatment, activators of AMPk, would be able to attenuate the deleterious effects on skeletal muscle induced by the DOX treatment. C57BL6 mice received either saline (control) or DOX (2.5 mg/kg body weight) intraperitoneally, twice a week. The animals on DOX were further divided into groups that received adjuvant treatment in the form of moderate aerobic physical exercise (DOX+T) or metformin gavage (300 mg/body weight/day). Body weight, metabolism, distance run, muscle fiber cross-sectional area (CSA), and protein synthesis and degradation were assessed. We demonstrated that aerobic training, but not metformin, associated with DOX increased the maximal aerobic capacity without changing muscle mass or fiber CSA, rescuing the muscle fatigue observed with DOX treatment alone. This improvement was associated with AMPk activation, thus surpassing the negative effects of DOX on muscle performance and bioenergetics. In conclusion, aerobic exercise increases AMPk activation and improved the skeletal muscle function, reducing the side effects of DOX.
Abbreviations
-
- AMPk
-
- AMP-activated protein kinase
-
- CSA
-
- cross-sectional area
-
- CT
-
- control
-
- DOX
-
- doxorubicin
-
- GC
-
- gastrocnemius complex
-
- MET
-
- metformin
-
- MPS
-
- muscle protein synthesis
-
- MS
-
- maximal speed run
-
- ROS
-
- reactive oxygen species
-
- T
-
- aerobic training
1 INTRODUCTION
Doxorubicin (DOX) is a chemotherapeutic agent of the anthracycline family widely used as a pharmacological strategy for the treatment of cancer. Currently, cancer is one of the leading causes of death and morbidity in the world (Ferlay et al., 2015; Siegel, Miller, & Jemal, 2015). DOX is widely used, but at less than optimal dosages due to severe side effects (Benjamin, Wiernik, & Bachur, 1974; Biondo et al., 2016; de Lima et al., 2016; Fahim et al., 2011; Lopes Meisel, Dirnagl, Carvalho, Bastos Mde, 2008; Shi, Moon, Dawood, McManus, & Liu, 2011). One of the main side effects targeted for research in the literature is cardiotoxicity (McGowan et al., 2017; Wang, Song, & Zou, 2012). However, the effects on skeletal muscle contribute to the deterioration in the quality of life and life expectancy of patients and are associated with the physical inability and poorer prognosis (Bredahl & Hydock, 2017; Bye et al., 2017; De Beer, Finkle, Voest, Van Heijst, & Schiereck, 1992; Gorselink et al., 2006; Lowe, Watanabe, Baracos, & Courneya, 2009; van Norren et al., 2009). Thus, the characterization of adjuvant treatments that may mitigate the adverse effects of the chemotherapy treatment is of considerable clinical importance.
A reduction in the AMP-activated protein kinase (AMPk) activity has recently been implicated in the negative side effects of DOX treatment. Moreover, our recent findings showed that DOX treatment induced a significant impairment in glucose uptake by AMPk inhibition (de Lima et al., 2016). Therefore, interventions that increase the AMPk activity may be able to mitigate the negative effects of DOX treatment.
Along these lines, recent studies have pointed to beneficial effects of physical exercise and metformin on the attenuation of cardiotoxicity in combination with DOX (Biondo et al., 2016; de Lima et al., 2016; Gratia et al., 2012; Wang et al., 2012). Both physical exercise and metformin are AMPk activators. AMPk is a heterotrimer protein, composed of a catalytic α subunit and regulatory β and γ subunits (Steinberg & Kemp, 2009), and acts as a sensor of the cellular energy level. It is mainly stimulated by high AMP/ATP and/or ADP/ATP ratios (Oakhill et al., 2011). In muscle, contraction is an important factor for AMPk activation (Carling & Hardie, 1989; Jorgensen et al., 2004). Thus, metabolic changes caused by exercise promote increased AMPk activity, whether by acute (Chen et al., 2003; Fujii et al., 2000) or chronic exercise (Jorgensen et al., 2004; Nielsen et al., 2003). Metformin is an antidiabetic drug (biguanide) that indirectly increases AMPk activation by inhibiting complex I of the mitochondrial respiratory chain (Owen, Doran, & Halestrap, 2000).
The potential additive effect on the inhibition of breast tumor growth is observed when there is an association between physical exercise and DOX (Jones et al., 2005). Since changes in the skeletal muscle of patients due to chemotherapy may cause impairment in patients’ quality of life, a growing number of studies have recently sought to investigate the mechanisms responsible for increased catabolism and muscle fatigue (Gilliam & St Clair, 2011; Gilliam et al., 2009, Gilliam et al., 2012). The physical exercise, in turn, has already been shown to be able to reduce the expression of proteins related to proteolysis, such as FoxO1, MuRF-1, BNIP3, and myostatin (Kavazis, Smuder, & Powers, 2014), as well as attenuate muscle dysfunction and fatigue (Bredahl, Pfannenstiel, Quinn, Hayward, & Hydock, 2016).
Some studies investigating metformin have shown that when associated with DOX chemotherapy, it results in greater therapeutic efficacy, such as the observed antitumorigenic effects and reduced rate of tumor remission (Blandino et al., 2012; El-Ashmawy, Khedr, El-Bahrawy, & Abo Mansour, 2017; Hirsch, Iliopoulos, Tsichlis, & Struhl, 2009; Iliopoulos, Hirsch, & Struhl, 2011). However, its effects on lean mass are still obscure. It was only recently that the effect of DOX on diabetic skeletal muscle and its impact on inflammation and metabolism were investigated. Although this model of diabetes does not result in the exacerbation of muscle mass impairment, little is known about the effects of metformin on the processes of muscle catabolism induced by this chemotherapeutic drug (Supriya et al., 2016).
Growing evidence indicates the toxic effect of DOX on skeletal muscle able to generate the disruption in glucose and protein metabolism lead to muscle atrophy. However, the mechanisms and possible treatments for this condition caused by DOX chemotherapy are still unclear. On the basis of this, the objective of our study was to evaluate whether the use of pharmacological strategies (treatment with metformin) and nonpharmacological (aerobic training), which leads to AMPk activation, would be able to attenuate or reverse the loss of lean mass induced by DOX with the focus on balance between degradation and protein synthesis.
2 MATERIALS AND METHODS
2.1 Animals
C57BL/6 male mice aged 8–10 weeks were used. The animals were kept in a room with a light–dark cycle of 12–12 hr and at a temperature of 22 ± 2°C, with a normal diet (Nuvital ration of Nuvilab, Colombo, PR, Brazil) and with food and water ad libitum. All the procedures of this study followed the ethical principles of animal experimentation and were submitted to the Committee of Ethics in Animal Experimentation of the University of São Paulo.
Part of these animals received DOX chloridrate (Eurofarma Laboratory, Campinas, Brazil), 2.5 mg/kg body weight, twice a week (DOX), whereas the control group (CT) received the same volume of saline solution. Animals that received DOX were still further divided into groups of animals that performed treadmill exercise (DOX+T) or received metformin (Sigma-Aldrich, St. Louis, MO), 300 mg/kg of body weight daily by gavage (DOX+MET).
After 6 weeks, mice were fasted for 6 hr and euthanized for the collection of blood and tissue samples. Gastrocnemius (GC) muscle and adipose tissue cushions were weighed and stored for the further analysis of messenger RNA and protein expression.
2.2 Exercise protocol
All groups performed 1 week of treadmill adaptation, in which they ran at 10 m/min for 5 days, before beginning the treatment protocols. From 6 weeks, the DOX+T group was submitted to an aerobic training protocol, 60 min at 60% of the maximal speed run (MS), 5 days/week. The MS consisted of a warm-up (5 min at 10 m/min) phase and from the sixth minute, the treadmill speed increased 3 m/min until the animals had run mechanical alterations, featuring exhaustion (n = 13–14).
2.3 Serum analysis
Fasting blood glucose was assessed using a colorimetric assay. Serum insulin (Millipore Corp., Bedford, MA), adiponectin (DuoSet ELISA, R&D Systems, Minneapolis, MN), testosterone, and corticosterone (Assay Designs, Inc., Ann Arbor, MI) were quantified using the enzyme-linked immunosorbent assay.
2.4 Cross-sectional area analysis
This method was adapted from Baehr et al. (2016). Briefly, frozen muscles were cut 10 μm thick from the medial portion of the GC using a Leica CM 3050S cryostat (Leica Microsystems, Nussloch, Germany). To determine fiber cross-sectional area (CSA; μm2), sections were stained with anti-laminin (1:1,000; Sigma-Aldrich) and visualized using the 3,3′-Diaminobenzidine (DAB) detection method according to the manual. Laminin images were acquired using a bright field Nikon E1000 microscope (Melville, NY). Gastrocnemius muscle images at 200× total magnification were captured using a Nikon DMX1200 digital camera (Nikon Corporation, Melville, NY). For each muscle, four nonoverlapping regions were used, and at least 200 fibers were analyzed per animal. Images were quantified using Image Pro Plus analysis software (Image Pro Plus software, NIH). The same four regions were analyzed across all mice. All analyses were conducted by a single observer blinded to the mouse’s identity. Data are reported as means ± standard error of the mean based on individual animal mean values (n = 5 – 6).
2.5 Enzyme assay
For determination of citrate synthase activity, soleus muscles were lysed using complete protease inhibitor cocktail (Roche) and CelLyticTM MT Cell Lysis Reagent (cat no. C3228; Sigma-Aldrich; n = 6 – 7). Citrate synthase activity was assessed using 8 μg protein with the Citrate Synthase Assay Kit (cat no. CS0720; Sigma-Aldrich).
2.6 Puromycin assay
Protein synthesis was measured using the surface sensing of translation method, as described by Baehr et al. (2016). After dissolving the puromycin (cat. no. 5540222; EMD Millipore, Billerica, MA) in sterile saline, animals received an intraperitoneal (i.p.) injection (0.02 μmol/g body weight) 30 min before starting muscle collections. Thirty minutes after i.p. injection, the gastrocnemius muscle was excised, flash frozen in liquid N2, and stored at –80°C. Gastrocnemius was powdered and homogenized in sucrose lysis buffer (50 mM Tris pH 7.5, 250 mM sucrose, 1 mM EDTA [ethylenediaminetetraacetic acid], 1 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N',N'-tetraacetic acid, 1% Triton X 100, and 50 mM NaF). After a 10 min centrifugation at 8,000g, protein concentrations were determined using the Bradford method (Bio-Rad®, Hercules, CA). Twenty micrograms of protein was separated on 10% acrylamide gels by sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and transferred to the nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ). The membrane was stained with Ponceau S and imaged to see total protein. Membranes were washed in 1× Tris-buffered saline with 0.1% Tween-20 (TBST) three times (5 min each wash), then blocked in 3% nonfat dairy milk for 1 hr. After a wash, the membranes were then probed with a primary antibody against puromycin (cat. no. MAEBE343; EMD Millipore, Temeluca, CA) overnight at 4°C. After a wash, membranes were incubated with peroxidase-conjugated secondary antibody at the concentration of 1:10,000 for 1 hr at room temperature, washed, and incubated in the Western ECL substrate detection system for 5 min before image acquisition (Clarity TM; Bio-Rad). Image acquisition and band quantification were performed using the AI600 (GE) System and Image StudioTM software version 5×.
2.7 Western blotting
The gastrocnemius muscles were carefully homogenized in the extraction buffer containing protease and phosphatase inhibitors. After proper centrifugations, the proteins were determined by the Bradford assay (Bio-Rad®, Hercules, CA). Aliquots of each sample with the same concentration of total protein (20–40 µg) were diluted in Laemmli buffer. The samples were then subjected to electrophoresis on SDS-PAGE, transferred to polyvinylidene difluoride membrane, which was incubated first with primary antibodies at a 1:1,000 dilution against phospho-AMPKα Thr172 (cat. no. 2531), AMPKα (cat. no. 2532), pS6 Thr389 (cat. 9205), 4EBP1 Thr37/46 (cat. no. 2855), 4EBP1 (cat. no. 4644), Beclin (cat. no. 3495), LC3β (cat. no. 12741), ATG7 (cat. no. 8558)—all obtained from Cell Signaling Technology (Danvers, MA)—Puromycin (cat. no. MAEBE343; EMD Millipore, Temeluca, CA), and PGC-1α (cat. no. AB54481; Abcam, Cambridge, MA) and then incubated with an anti-IgG antibody conjugated with a peroxidase-conjugated secondary antibody at a 1:10,000 dilution for 1 hr at room temperature then washed three times per 5 min in TBST. After the incubations and washes, these membranes were incubated with the peroxidase substrate (ECL kit; Bio-Rad®) and quantified by optical densitometry. Ponceau S staining was used as a loading control (Gilda & Gomes, 2013; n = 4–6).
2.8 Proteasome activity
The activities of 20S and 26S proteasome were performed using muscle powder from whole gastrocnemius muscle, as previously described by Baehr et al. (2016). The chymotrypsin-like (β5) activity assays were carried out in a total volume of 100 μl in 96-well opaque plates. The 26S ATP-dependent assays were performed in a homogenization buffer with the addition of 100 μM ATP. The 20S ATP-independent assays were carried out in the assay buffer containing 25 mM 4-(2-hydroxyethyl)-1piperazineethanesulfonic acid (HEPES), 0.5 mM EDTA, and 0.001% SDS (pH 7.5). Proteasome activities were determined using 100 μM of Z-Leu-Leu-Glu-7-AMC (Peptide Institute), Boc-Leu-Ser-Thr-Arg-7-AMC (Bachem), or succinyl-Leu-Leu-Val-Tyr-7-amido-4-methylcoumarin (LLVY-AMC; Bachem) for the β5 subunit. Each assay was conducted in the absence and presence of the proteasome inhibitor bortezomib at a final concentration of 2 mM. Released AMC was measured by a Synergy™ H1 hybrid Multi-Mode Microplate Reader (Biotek Highland Park, Winooski, VT) at an excitation wavelength of 390 nm and an emission wavelength of 460 nm. Fluorescence was measured at 15 min intervals for 2 hr. The activity of the 20S and 26S proteasome was measured by calculating the difference between fluorescence units recorded with or without the inhibitor in the reaction medium. (n = 4 – 6).
2.9 Quantitative real-time polymerase chain reaction
The expression of skeletal muscle genes was assessed by quantitative real-time polymerase chain reaction (qRT-PCR) with SYBR Green marker. Total RNA from the gastrocnemius muscle was extracted with Trizol reagent (Invitrogen Life Technologies, Carlsbad, CA). The complementary DNA (cDNA) was synthesized from 2 μg of total RNA extracted using reverse transcriptase with the High-Capacity cDNA kit (Applied Biosystems, Warrington, UK). RNA was measured by a spectrophotometer at 260 nm, and purity was determined by the ratio 260/280 nm. Gene expression was evaluated by the RT-PCR using a Rotor Gene (Qiagen) and SYBR Green as the fluorescent dye. Quantification was performed using the comparative Ct method. Primer sequences are shown in Supporting Information Table 1 (n = 6–7).
2.10 Statistical methods
The data are presented as mean ± standard deviation and analyzed by one-way analysis of variance, followed by the the Bonferroni post test. Analyses were performed using GraphPad Prism 6.0 software. Differences were considered significant when p < 0.05.
3 RESULTS
3.1 Body weight and food intake
All groups treated with DOX had decreased body weight (Figure 1a), but without change in food intake (Figure 1b), indicating that neither metformin nor aerobic training are able to restore the decreased body weight.
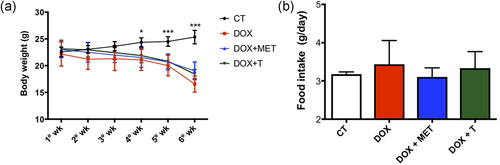
Doxorubicin (DOX) treatment decreases body weight. (a) Body weight during treatment (g); (b) food intake during treatment (g). *p < 0.05, ***p < 0.001. n = 7 − 4. CT, control; MET, metformin; T, aerobic training [Color figure can be viewed at wileyonlinelibrary.com]
The loss of body weight is due to loss of both fat and lean body mass. The chemotherapy treatment caused extensive reduction of adipose tissue and skeletal muscle. The pharmacological treatment with metformin or exercise did not recovery the weight loss of fat pad (subcutaneous, epididymal, retroperitoneal, and mesenteric) and gastrocnemius muscle caused by DOX (Table 1). There was no change in brown adipose tissue weight (Table 1).
| CT | DOX | DOX+MET | DOX+T | |
|---|---|---|---|---|
| Gastrocnemius (mg) | 303.4 ± 49.42 | 209.7 ± 56.53*** | 202.8 ± 39.56*** | 207.5 ± 35.40*** |
| Subcutaneous adipose tissue (mg) | 207.1 ± 48.03 | 89.25 ± 53.79* | 65.92 ± 41.78* | 89.17 ± 34.77* |
| Epididymal adipose tissue (mg) | 306.1 ± 104.6 | 48 ± 34.46* | 86 ± 118.7* | 37.86 ± 19.68* |
| Retroperitoneal adipose tissue (mg) | 78.15 ± 30.86 | 8.833 ± 12.1* | 3.417 ± 3.94* | 3.667 ± 5.92* |
| Mesenteric adipose tissue (mg) | 426.70 ± 89.98 | 234.60 ± 186.5* | 209.40 ± 74.1* | 235.30 ± 79.36* |
| Brown adipose tissue (mg) | 92.29 ± 37.01 | 81.13 ± 23.2 | 57 ± 37.54 | 68.43 ± 33.61 |
- Note. Values represent the mean ± standrad deviation of the data obtained from analysis of 8–10 animals per group. CT, control; DOX, doxorubicin; MET, metformin; T, aerobic training.
- * p < 0.05.
- ** p < 0.01.
- *** p < 0.001 versus CT.
3.2 Effects on systemic parameters
On the basis of the reductions in adipose tissue weight caused by DOX, we evaluated the concentration of adiponectin in serum. No changes were observed (Table 2). Also, both adipose tissue and skeletal muscle play a key role in insulin-mediated glucose uptake. The chemotherapy decreased basal glycemia, without change in insulin concentration (Table 2). Adjuvant treatments did not alter this condition. The balance between anabolic and catabolic hormones is important in skeletal muscle homeostasis. Therefore, we investigated whether the treatments would cause changes in the circulating concentration of testosterone and corticosterone. Corticosterone was increased in the DOX group and both treatments groups (metformin and exercise) were able to reduce them (Figure 2a). The testosterone concentration was not altered (Figure 2b).
| CT | DOX | DOX+MET | DOX+T | |
|---|---|---|---|---|
| Glucose (mg/dl) | 129.2 ± 14.89 | 100.2 ± 22.14** | 97.54 ± 20.96*** | 102.3 ± 17.72** |
| Insulin (ng/ml) | 0.63 ± 0.20 | 0.34 ± 0.20 | 0.51 ± 0.22 | 0.60 ± 0.28 |
| Serum adiponectin (ng/ml) | 1,493 ± 1,084 | 2,193± 1,185 | 1,330 ± 877.6 | 1,226 ± 567.7 |
- Note. Values represent the means ± standard deviation of the data obtained from analysis of 8–12 animals per group. CT, control; DOX, doxorubicin; MET, metformin; T, aerobic training.
- ** p < 0.01.
- *** p < 0.001 versus CT.
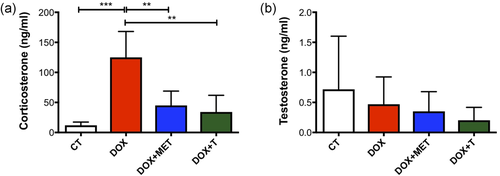
Aerobic physical exercise and metformin reduce the effect of doxorubicin on circulating corticosterone. (a) Serum corticosterone (n = 4 – 5); (b) serum testosterone (n = 9–10). **p < 0.01, ***p < 0.001. CT, control; DOX, doxorubicin; MET, metformin; T, aerobic training [Color figure can be viewed at wileyonlinelibrary.com]
3.3 Aerobic running capacity
As expected, the final maximum velocity after 6 weeks of aerobic training increased in the DOX+T group, whereas in the group treated just with DOX, there was a decrease in this physical capacity (Figure 3a). These differences were not observed in the DOX+MET group. Furthermore, there was no change in the citrate synthase activity for any group (Figure 3b).
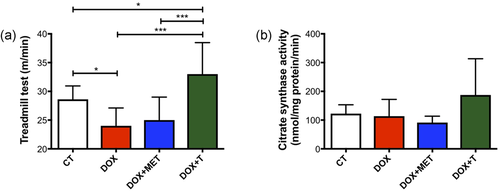
Aerobic exercise prevents the reduction of maximum velocity in treadmill test caused by doxorubicin. (a) Final maximum speed at a treadmill test (m/min); (b) citrate synthase activity in gastrocnemius muscle (mmol/mg/protein/min); *p < 0.05; ***p < 0.001. CT, control; DOX, doxorubicin; MET, metformin; T, aerobic training [Color figure can be viewed at wileyonlinelibrary.com]
3.4 Exercise and metformin effects on CSA in skeletal muscle
Exercise and metformin did not recover the loss of CSA in gastrocnemius muscle (Figure 4a–c). As shown in Figure 4a–c, chemotherapy alone causes severe reduction of fiber area.
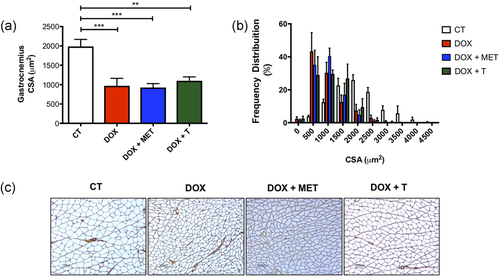
Effects on skeletal muscle caused by doxorubicin. (a) Cross-sectional area (μm2); (b) frequency distribution of mean fiber cross-sectional area; (c) representative histological images. **p < 0.01; ***p < 0.001. CT, control; DOX, doxorubicin; MET, metformin; T, aerobic training [Color figure can be viewed at wileyonlinelibrary.com]
3.5 Effects on proteins involved in muscle metabolism
Although PGC1 alpha levels are unaffected by DOX treatment alone, a combination of DOX treatment with either metformin or exercise training significantly reduces PGC-1 alpha levels (Figure 5a). However, another important protein involved in skeletal metabolism was altered. Physical exercise and metformin are both AMPk activators. On the basis of this, we investigated the effect of the interventions. The AMPKα phT172/total AMPkα expression was increased in the DOX+T group (Figure 5b). There was a tendency to increase this protein in the DOX+MET group (p = 0.069). Adiponectin is one of the possible activators of AMPk. In this sense, we evaluated the receptor expression of this protein in the skeletal muscle. There was a reduction of gene expression of ADIPOR1 in the DOX+T group compared with the CT group (Figure 5c) without alterations in ADIPOR2 gene expression (Figure 5d).
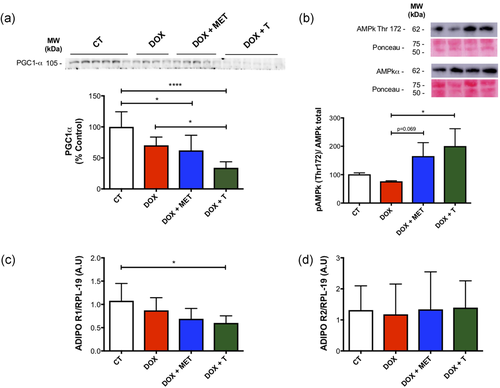
Metabolic changes caused by treatments. (a) PGC-1α expression in gastrocnemius muscle. (b) Representative image of western blot analysis of total AMPkα and AMPKα Thr172 expression in gastrocnemius muscle and its quantification. (c) AdipoR1 gene expression in gastrocnemius muscle; (d) AdipoR2 gene expression in gastrocnemius muscle *p < 0.5., ****p < 0.0001. AMPkα, AMP-activated protein kinase; CT, control; DOX, doxorubicin; MET, metformin; T, aerobic training [Color figure can be viewed at wileyonlinelibrary.com]
3.6 Effects on protein synthesis and its signaling
On the basis of our results demonstrating reduction of muscle mass, we investigated whether protein synthesis could be involved in this process. We observed that protein synthesis is decreased with DOX treatments (DOX group and DOX+MET), and aerobic exercise rescue this effect (Figure 6a). Then, we investigated possible targets in the protein synthesis signaling pathway that could be involved in this process. We did not observe change in pS6k (Thr389) or p4EBP1(Thr37/46)/4EBP1 ratio (Figure 6b,c).
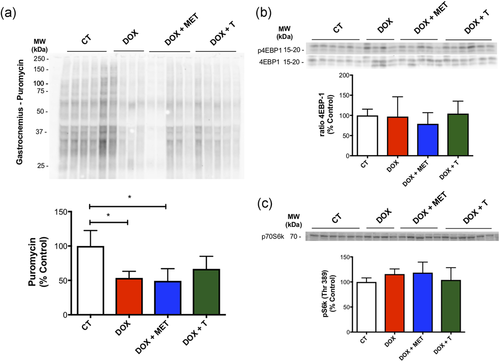
Effects of treatments on protein synthesis and its signaling in gastrocnemius muscle. (a) Representative image of western blot analysis using the surface sensing of translation method and protein synthesis in gastrocnemius muscle; (b,c). pS6K (Thr389), 4EBP1 (total and Thr37/46) protein expression and its quantification. *p < 0.05. CT, control; DOX, doxorubicin; MET, metformin; T, aerobic training [Color figure can be viewed at wileyonlinelibrary.com]
3.7 Effects on protein degradation pathways
Autophagy and proteasomal degradation could be other possible mechanisms that could be involved in muscle wasting previously observed. Thus, we investigated how the treatments could change the proteasomal activity. Treatment with DOX alone does not alter the proteasome activity. However, the DOX+MET group showed increase in the 20S β5 activity in comparison with all other groups (Figure 7a). There was no change in 26S β5 activity (Figure 7b). MAFbx/atrogin-1, an important protein expressed during muscular atrophy, was not changed either (Figure 7c).
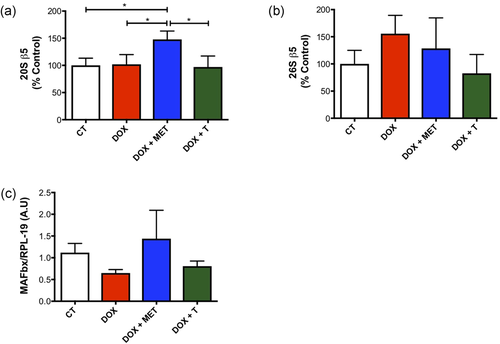
Proteasomal activity is increased with association between doxorubicin and metformin. (a) 20S β5 activity (ATP independent) in gastrocnemius muscle; (b) 26S β5 activity in gastrocnemius muscle (ATP dependent). (c) MAFbx/atrogin-1 gene expression in gastrocnemius muscle. *p < 0.05. CT, control; DOX, doxorubicin; MET, metformin; T, aerobic training [Color figure can be viewed at wileyonlinelibrary.com]
With regard to autophagy, we observed that aerobic exercise and metformin reduces the expression of proteins involved in this process. The expression of autophagic proteins Atg7 and Beclin was reduced by both exercise and metformin (Figure 8b,c), while the protein expression of LC3β was reduced just for the DOX+T group (Figure 8a). These results are caused by the treatments themselves since there was no statistical difference between the CT and DOX groups.
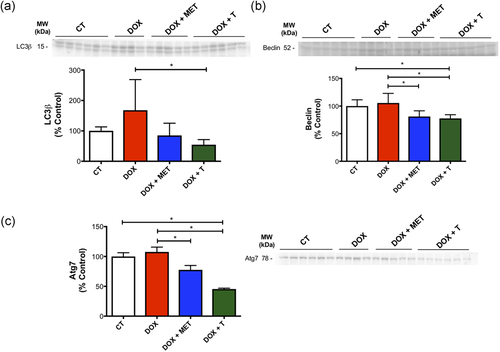
Aerobic exercise and metformin reduce the expression of proteins involved in autophagy caused by doxorubicin. (a) LC3β protein expression in gastrocnemius muscle; (b) Beclin protein expression in gastrocnemius muscle; (c) Atg7 protein expression in gastrocnemius muscle. *p < 0.05. n = 4–6. CT, control; DOX, doxorubicin; MET, metformin; T, aerobic training [Color figure can be viewed at wileyonlinelibrary.com]
4 DISCUSSION
Our findings showed that aerobic exercise associated with DOX treatment increased the maximal aerobic capacity without changing muscle mass or fiber CSA, rescuing the muscle fatigue observed with DOX treatment alone. In adjuvant treatment with metformin, this was not observed. The improvement caused by exercise was associated with AMPk activation, thus surpassing the negative effects of DOX on muscle performance and bioenergetics.
Our findings showed that the aerobic exercise increased AMPk phosphorylation in comparison with the DOX group in gastrocnemius. Moreover, we observed the improvement in aerobic muscle capacity. However, the CSA, skeletal muscle, and adipose tissue weight were not restored by aerobic exercise after DOX administration. Corticosterone levels increased after DOX treatment, and this effect was counter-regulated by aerobic exercise and metformin. Therefore, the aerobic exercise restored AMPk phosphorylation and improved the aerobic capacity in mice treated with DOX and reduced the autophagic flux, but did not prevent the decrease in CSA and protein synthesis. Although the coadministration with metformin had a few beneficial effects in the group treated with DOX (reducing corticosterone levels and the autophagic pathway induced by DOX), it increased the ubiquitin–proteasome activity and did not prevent muscle loss.
These results suggest that aerobic training could be considered an effective strategy to counteract the negative effects of DOX on AMPk. Considering that AMPk is a master regulator of cell metabolism, an increased expression of genes involved in increased energy metabolism is expected to occur (Hardie, 2014). In this regard, we measured the PGC-1α protein expression (a transcriptional coactivator that regulates the genes involved in aerobic metabolism), and interestingly, increased AMPk activation was not paralleled by increased PGC-1α protein expression in gastrocnemius muscle under exercise training. Our training protocol is the moderate (60% maximal velocity), and this model of training was not able to induce an increase in PGC-1α mRNA (Wen et al., 2014). Moreover, we measured the PGC-1α protein content in gastrocnemius muscle (a mixed, oxidative, and glycolytic muscle); the lack of molecular adaptation would be the reflex of a muscle specificity, given that oxidative muscles are more susceptible to endurance training effects (Hyatt et al., 2015; Pette, 1985). Although plausible, this is speculative since AMPk activation was robustly increased (even in gastrocnemius muscle).
Muscle metabolism is strictly linked to muscle mass control. Energy deficits frequently lead to muscle loss in several muscle diseases. DOX is a well-known chemotherapeutic drug that induces atrophy and reduces strength (de Lima et al., 2016; Gilliam & St Clair, 2011; van Norren et al., 2009). Skeletal muscle atrophy leads to decreases in muscle performance. In this regard, it was interesting to speculate if AMPk activators, such as metformin or aerobic exercise training, would reverse not only muscle performance but also the negative effects of DOX on muscle mass. We showed that although exercise training rescued muscle performance, the same was not true for muscle mass. This beneficial effect of exercise in muscle performance is similar to the finding of Bredahl et al. (2016), which showed that resistance and aerobic training before the DOX treatment minimized fatigue and preserved skeletal muscle function.
The maintenance of muscle mass is dependent on the ratio of protein synthesis to degradation. The effects of DOX on protein synthesis are controversial and are not well elucidated (Zähringer, 1981; Zima et al., 2001). Our results indicate that the treatment with DOX reduces protein synthesis, and metformin was not able to rescue this decrease. Aerobic exercise, however, attenuated this effect as the level of protein synthesis in the trained group was intermediate to control and other DOX-treated groups. When observing the effects of both treatments on muscle protein synthesis (MPS), DOX clearly decreased MPS, and this effect was partially reversed by exercise training (but not metformin), although both treatments were ineffective in restoring the muscle loss caused by DOX. It is unclear if increased exercise training (more weeks/sessions) would lead to an antiatrophic effect, but considering the MPS effect, this is a possibility to be considered. Nevertheless, we did not observe an effect on mammalian target of rapamycin (mTOR) downstream signaling (pS6k (Thr389); p4EBP1(Thr37/46):4EBP1 ratio). Recently, Dickinson et al. (2017) showed that chronic DOX administration reduces the mTOR activity via the elevation in REDD1 protein, and the high-intensity chronic exercise mitigates these effects. The conflicting results could be explained by differences in the animal model (Wistar rats vs. Sprague–Dawley rats), the gender (male vs. female ovariectomized), and/or the intensity and type of training between studies.
On the contrary, the DOX-induced protein degradation is well established (Gilliam & St Clair, 2011; Gilliam et al., 2012; Kavazis et al., 2014). Our results showed that the DOX treatment robustly decreased body weight and muscle mass.
In regard to the ubiquitin–proteasome pathway, the skeletal muscle DOX increases protein ubiquitination, leading to an increase in proteasomal activity (Sin et al., 2016). Treatment with DOX activates the FoxO pathway, causing increased expression of proteins involved in proteolysis, like MuRF-1 and atrogin-1/MaFbx (Kavazis et al., 2014). While we did not observe an effect of DOX treatment on this pathway, the association with metformin leads to increased 20S β5 proteasome subunit activity. Although the increase in AMPk activation in skeletal muscle can lead to increase in the expression of ubiquitin kinases (Krawiec, Nystrom, Frost, Jefferson, & Lang, 2007), we did not observe an increase in the MAFbx/atrogin-1 mRNA expression.
The autophagic flux was increased by DOX treatment in comparison with adjuvants treatments. Other authors have shown similar results, where DOX treatment increased the expression of autophagic proteins and that exercise can negatively regulate them (Campbell & Quadrilatero, 2016; Smuder, Kavazis, Min, & Powers, 2011). DOX-mediated autophagy is caused by increased mitochondrial uncoupling with the elevation of reactive oxygen species (ROS) production, and the exercise is able to increase antioxidant defense, leading to the reduction in ROS generation (Schwalm et al., 2015; Smuder et al., 2011)
DOX caused increase in corticosterone, as observed in previous studies (de Lima et al., 2016; Kwatra et al., 2016; Preziosi, Vacca, Ragazzoni, del Carmine, & Navarra, 1989). Increased corticosterone levels induce increase in both catabolic pathways (ubiquitin–proteasome and autophagy) in skeletal muscle. Interestingly, exercise training and metformin reversed the increase in corticosterone seen during DOX treatment, but the effects of this reversion on protein metabolism in muscle are not clear, because they were not correlated with glucose homeostasis improvements (insulin and/or blood glucose) or increase in muscle mass.
Overall, chemotherapeutic agents, such as DOX, are widely utilized in clinical practice, but the negative side effects of such agents impair its use, thus limiting its efficacy. As expected, we observed a robust effect of DOX in decreasing exercise performance and muscle mass, together via a decreased muscle protein synthetic effect. In contrast, aerobic exercise training was capable to restore the exercise capacity, oxidative metabolism via AMPk activation, and hormonal milieu (hypercortisolemia), without affecting muscle mass. Metformin was able to normalize the hypercortisolemia, without major effects on muscle mass or muscle performance. We conclude that moderate-intensity aerobic training, besides safe and effective, is capable of bringing important benefits to counteract DOX metabolic effects in the whole body and skeletal muscle. Those benefits seem to be linked to intrinsic alterations in muscle metabolism, thus bringing alterations to the muscle quality much more than in muscle mass. Finally, only exercise training seems to be capable to bring both metabolic and functional changes to DOX-treated muscle.
ACKNOWLEDGMENT
Research supported by Fundação de Amparo a Pesquisa do Estado de São Paulo. (FAPESP 2013/09367-4 and 2015/17068-2).
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interests.
AUTHOR CONTRIBUTIONS
J. C. R. N., E. A. L. Jr., and L. G. O. S. designed the study; E. A. L. Jr., L. G. O. S., and A .A. S. T. collected and processed the data; J. C. R. N., E. A. L. Jr., L. G. O. S., and N. E. Z. are responsible for data interpretation; and J. C. R. N., E. A. L. Jr., L. G. O. S., A. G. M., and N. E. Z wrote the manuscript.