P2Y12 shRNA treatment decreases SGC activation to relieve diabetic neuropathic pain in type 2 diabetes mellitus rats
Abstract
Diabetic neuropathic pain is a common complication of type 2 diabetes mellitus (DM). Activation of satellite glial cells (SGCs) in the dorsal root ganglia (DRG) plays a crucial role in neuropathic pain through the release of proinflammatory cytokines. The P2Y12 receptor is expressed in SGCs of the DRG. In this study, our aim was to investigate the role of the P2Y12 receptor on the pathological changes in diabetic neuropathic pain. The present study showed that diabetic neuropathic pain increased mechanical and thermal hyperalgesia in type 2 DM model rats. The results showed that the expression levels of P2Y12 messenger RNA (mRNA) and protein in DRG SGCs were increased in DM model rats compared with control rats. Glial fibrillary acidic protein (GFAP) and interleukin-1β (IL-1β) expression levels in the DRG were increased in DM rats. Upregulation of GFAP is a marker of SGC activation. Targeting the P2Y12 receptor by short hairpin RNA (shRNA) decreased the upregulated expression of P2Y12 mRNA and protein, coexpression of P2Y12 and GFAP, the expression of GFAP, IL-1β, and tumor necrosis factor-receptor 1 in the DRG of DM rats, and relieved mechanical and thermal hyperalgesia in DM rats. After treatment with the P2Y12 receptor shRNA, the enhancing integrated OPTICAL density (IOD) ratios of p-P38 MAPK to P38 mitogen activated protein kinase (MAPK) in the DM rats treated with P2Y12 shRNA were significantly lower than that in the untreated DM rats. Therefore, P2Y12 shRNA treatment decreased SGC activation to relieve mechanical and thermal hyperalgesia in DM rats.
1 INTRODUCTION
Type 2 diabetes mellitus (DM) is one of the fastest growing health problems worldwide (Ma & Chan, 2013; Tesfaye & Selvarajah, 2012; Whiting, Guariguata, Weil, & Shaw, 2011; Xu et al., 2013; Zychowska, Rojewska, Przewlocka, & Mika, 2013). Chronic complications of type 2 DM are related to causes of morbidity and mortality (Davies, Brophy, Williams, & Taylor, 2006; Xu et al., 2013; Zychowska et al., 2013). Diabetic neuropathic pain is a common complication of type 2 DM (Davies et al., 2006; Morales-Vidal, Morgan, McCoyd, & Hornik, 2012; Singh, Kishore, & Kaur, 2014). The symptoms of diabetic neuropathic pain are spontaneous pain, allodynia (pain to normally innocuous stimuli) and hyperalgesia (increased pain perception to noxious stimuli) (Callaghan, Cheng, Stables, Smith, & Feldman, 2012; Davies et al., 2006; Singh et al., 2014). Because the mechanisms of diabetic neuropathic pain are extremely complex, it is very difficult to treat (Schreiber, Nones, Reis, Chichorro, & Cunha, 2015; Tavakoli & Malik, 2008). Diabetic neuropathic pain is a chronic peripheral neuropathy and induces abnormalities of function in the dorsal root ganglia (DRG) (Li et al., 2017; Morales-Vidal et al., 2012; Obrosova, 2009; Rao et al., 2017; Schreiber et al., 2015; Wang et al., 2016). Numerous studies showed that chronic hyperglycemia is responsible for changes in the nerve tissue (Schreiber et al., 2015; Tesfaye & Selvarajah, 2012). The mechanisms of hyperglycemia-induced nerve damage are complicated, and the pathogenesis of diabetic neuropathic pain remains poorly understood.
Purinergic 2 receptors include metabotropic G-protein-coupled P2Y receptors and ionotropic P2X receptors (Burnstock, 2013; Burnstock, 2014; Idzko, Ferrari, Riegel, & Eltzschig, 2014; Magni & Ceruti, 2013). Extracellular purine (adenosine triphosphate [ATP] and adenosine diphosphate [ADP]) and/or pyrimidine (uridine triphosphate [UTP] and uridine diphosphate [UDP]) nucleotides can activate P2 receptors (Burnstock, 2013; Burnstock, 2014; Idzko et al., 2014; Magni & Ceruti, 2013). Both neurons and glial cells release ATP after nervous injury (Fields & Burnstock, 2006; Verderio & Matteoli, 2011; Sperlagh, Andras, & Vizi, 1997; Sperlagh, Kittel, Lajtha, & Vizi, 1995; Sperlagh, Magloczky, Vizi, & Freund, 1998). Satellite glial cells (SGCs) enwrap sensory neurons in the DRG (Costa, Moreira, Cavalcanti, Krinski, & Aoki, 2015; Hanani, 2005). After nerve damage or inflammation, SGC activation is characterized by the upregulation of glial fibrillary acidic protein (GFAP) expression and increased production of proinflammatory substances, such as cytokines (Hanani, 2005; Jasmin, Vit, Bhargava, & Ohara, 2010). The P2Y12 receptor is expressed in SGCs of the DRG (Katagiri et al., 2012; Kobayashi et al., 2008; Kobayashi, Yamanaka, & Noguchi, 2013). The P2Y12 receptor is involved in the nociceptive transmission of trigeminal ganglia (Burnstock, 2013; Horváth et al., 2014; Katagiri et al., 2012; Magni & Ceruti, 2013). In this experiment, our aim was to investigate the effects of the P2Y12 short hairpin RNA (shRNA) treatment on the mechanical and thermal hyperalgesia in type 2 DM rats.
2 MATERIALS AND METHODS
2.1 Animals and animal groups
Male Sprague–Dawley rats (180–230 g) were provided by the Center of Laboratory Animal Science of Nanchang University. The procedures were approved by the Animal Care and Use Committee of Nanchang University Medical School. The IASP's ethical guidelines for pain research in animals were followed. All animals were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research in China. The room was maintained at 22°C and 50% humidity with a 12:12 hr light/dark cycle and free feeding; cages and bedding were changed frequently. After regularly feeding for a week, rats were randomly divided into two groups: a control group (n = 8) and a type 2 DM group (n = 27).
The diabetic rat model was induced by an intraperitoneal (i.p.) injection of streptozocin (STZ) (high-calorie food and small doses of 30 mg/kg STZ injection for each rat). STZ was dissolved in citric acid and sodium citrate buffer (0.1 M). The control animals received the same volume buffer. After 7 days, blood samples were obtained from the tail vein, and blood glucose was determined using a glucometer. Postprandial blood glucose >11.1 mM or fasting plasma glucose >7.8 mM was considered type 2 DM. Spontaneous pain behaviors were measured on the seventh day after the STZ injection.
After the DM model was built successfully, rats were assigned in a random, blinded manner to four groups: control group, type 2 DM group, DM rats with the P2Y12 shRNA plasmid (Invitrogen) interference group (DM + P2Y12 shRNA group) and DM rats treated with no load plasmid as the negative control group (DM + NC [negative control]). Each group contained eight animals. The P2Y12 shRNA plasmid or no-load plasmid (10 μg dissolved in Entrater in vivo, Engreen Biosystem Co., Ltd, Beijing) was injected into the sublingual vein, and then spontaneous pain behaviors were measured 7–10 days after plasmid injection.
2.2 Thermal withdrawal latency
The latency to hind paw withdrawal from a thermal stimulus was determined by exposing the plantar surface of the hind paw to radiant heat using the Thermal Paw Stimulation System (BME-410C, Tianjin, China). Rats were placed in a transparent, square, bottomless acrylic box (22 cm × 12 cm × 22 cm) on a glass plate with a light source located underneath. After a 30-min habituation period, the plantar surface of the paw was exposed to a beam of radiant heat applied through the glass floor. The activation of the bulb simultaneously activated a timer, and both were immediately turned off by paw withdrawal or at the 30-s cutoff time. The hind paws were tested by a blinded observer eight times at 5-min intervals.
2.3 Mechanical withdrawal threshold
Determination of MWT was performed using a BME-404 electronic mechanical stimulator (Institute of Biomedical Engineering, Chinese Academy of Medical Sciences, Tianjin, China). This device had a test needle with a 0.6-mm end face diameter, a pressure measurement range of 0.1–50 g, and a pressure measurement resolution of 0.05 g. An organic glass box (22 × 22 × 12 cm) was placed on the sieve of the metal frame. The rat was placed in the box for 30 min of adaptation. The left hind paws were touched with the test needle until escape behavior was observed. An effective escape behavior response was defined as a rapid withdrawal and/or licking of the paw immediately on application of the stimulus. Whenever there was an effective response, the force of the test needle was adjusted to the next lowest setting. Whenever an invalid response occurred, the next highest force was applied. The pressure value was automatically recorded. Measurements were performed five times for each rat (interval ≥ 5 min), and the MWT was calculated as the mean of these measurements.
2.4 Quantitative real-time polymerase chain reaction
Rats in the four groups were anesthetized using 10% chloral hydrate (3 ml/kg, i.p.). The DRG was isolated immediately and flushed with ice-cold phosphate-buffered saline (PBS). Total RNA samples were prepared from the DRG of each group using TRIzol Total RNA Reagent (Beijing Tiangen Biotech Co.). Complementary DNA (cDNA) synthesis was performed with 2 μg of total RNA using a RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific Co., USA). Primers were designed with Primer Express 3.0 software (Applied Biosystems) using the following sequences: β-actin, forward 5′-TGTCACCAACTGGGACGATA-3′, reverse 5′-GGGGTGTTGAAGGTCTCAAA-3′ P2Y12, forward 5′-CTTCGTTCCCTTCCACTTTG-3′, and reverse 5′-AGGGTGCTCTCCTTCACGTA-3′. Quantitative polymerase chain reaction (PCR) was performed using SYBR® Green MasterMix in an ABI PRISM® 7500 Sequence Detection System (Applied Biosystems, Inc., Foster City, CA). Quantification of gene expression was performed using the ΔΔCT (CT means the number of cycles that occur when the fluorescence signal in each reaction tube reaches the set domain value) calculation with CT as the threshold cycle. The relative levels of target genes, normalized to the sample with the lowest CT, are given as 2−ΔΔCT. For the four groups, β-actin was used as an internal control. Relative expression levels of messenger RNA (mRNA) in the four groups were normalized to β-actin levels.
2.5 Western blot
The animals were anesthetized, and tissue collection was performed as described above, except that the tissue was snap frozen in tubes on dry ice during collection. Briefly, on the 10th day after plasmid injection, the animals were anesthetized with chloral hydrate, and the DRG was dissected. The DRG were isolated immediately and rinsed in ice-cold PBS. The ganglia were homogenized by mechanical disruption in the lysis buffer containing the following: 50 mM Tris-Cl (pH = 8.0), 150 mM NaCl, 0.1% sodium dodecyl sulfate (SDS), 1% Nonidet P-40, 0.02% sodium deoxycholate, 100 μg/ml phenylmethylsulfonyl fluoride, and 1 μg/ml aprotinin. The tissue fluid was incubated on ice for 50 min. The homogenates were then centrifuged at 12,000 rpm for 10 min, and the supernatants were collected. The quantity of total protein in the supernatants was determined using the Lowry method. After dilution with loading buffer (250 mM Tris-Cl, 200 mM dithiothreitol, 10% SDS, 0.5% bromophenol blue, and 50% glycerol) and heating to 100°C for 5 min, samples containing equal amounts of protein (20 μg) were separated by 10% SDS–polyacrylamide gel electrophoresis using a Bio-Rad system. The proteins were then transferred onto polyvinylidene fluoride (PVDF) membranes by electrophoretic transfer using the same system. The membrane was blocked with 5% nonfat dry milk in 25 mM Tris-buffered saline (pH = 7.2) and 0.05% Tween 20 (TBST) for 2 hr at room temperature; it was subsequently incubated with rabbit monoclonal anti-P2Y12 (diluted to a 1:1,000 ratio, Abcam), rabbit polyclonal anti-interleukin-1β (IL-1β) (1:1,000 concentration, Abcam), rabbit polyclonal antitumor necrosis factor-receptor 1 (TNFR1) (1:800 concentration, Abcam), mouse monoclonal anti-GFAP antibody (1:1,000 concentration, Millipore), rabbit polyclonal anti-phospho-P38 MAPK(1:500, Cell Signaling Technology), rabbit polyclonal anti-P38 (diluted to a 1:1,000 ratio, CST), and anti-β-actin antibody (1:1,000 concentration, Beijing Zhongshan Biotech Co, China) at 4°C overnight. The membranes were washed three times with TBST and incubated (1 hr, room temperature) with a horseradish-peroxidase-conjugated secondary antibody (goat anti-mouse IgG or goat anti-rabbit IgG, 1:2000, Beijing Zhongshan Biotech Co.) in blocking buffer. After another wash cycle, the labeled proteins were visualized by enhanced chemiluminescence (ECL; Thermo Fisher Scientific) on a high-performance film (Shanghai Pufei Biotech Co.). Chemiluminescent signals were collected on the autoradiography film, and band intensity was quantified using Image-Pro Plus software. The relative band intensity of the target proteins was normalized against the intensity of the respective β-actin internal control.
2.6 Double-labeled immunofluorescence
The DRGs were dissected and fixed in 4% paraformaldehyde (PFA) diluted in PBS (145 mM NaCl, 7.3 mM Na2HPO4, and 2.7 mM NaH2PO4 [pH = 7.2]) for 24 hr. The ganglia were dehydrated in 30% sucrose overnight at 4°C and then cut into 8-μm-thick sections. The sections were rinsed three times for 5 min in PBS and subsequently rinsed with 0.1% Triton X-100 in PBS for 30 min at room temperature. Nonspecific staining was blocked by incubation with 10% normal goat serum (Jackson ImmunoResearch Inc., West Grove, PA). The sections were then incubated with rabbit anti-P2Y12 (1:200, Abcam) and mouse anti-GFAP (1:150, Millipore) overnight at 4°C. Subsequently, sections were incubated for 1 hr at 37°C with the secondary antibodies, goat anti-rabbit IgG conjugated to tetraethyl rhodamine isothiocyanate (1:200, TRITC, EarthOx) and goat anti-mouse IgG conjugated to fluorescein isothiocyanate (1:200, FITC, EarthOx). The fluorescently stained sections were coverslipped and examined under a fluorescence microscope (Olympus, Tokyo, Japan). Neurons that were surrounded by GFAP-positive SGCs by more than 50% of their circumference were counted. The number of neurons surrounded with GFAP and P2Y12 positive SGCs in the DRG was analyzed.
2.7 Statistical analyses
The data were statistically analyzed on a computer (SPSS 11.5). All results are expressed as the mean ± standard error. Statistical significance was determined by one-way analysis of variance followed by Fisher's post hoc test for multiple comparisons, and p < 0.05 was considered significant.
3 RESULTS
3.1 Upregulated expression of the P2Y12 receptor in the DRG of DM rats
The expression of P2Y12 mRNA in the DRG was measured by real-time PCR. Using image analysis, the expression levels of P2Y12 receptor mRNA in the DM group were significantly higher than those in the control group (p < 0.01). The expression levels of P2Y12 mRNA in the DM + P2Y12 shRNA group were decreased compared with those in the DM group (p < 0.001) (Figure 1a). There was no significant difference between DM and DM + NC groups (p > 0.05) (n = 8 for each group).
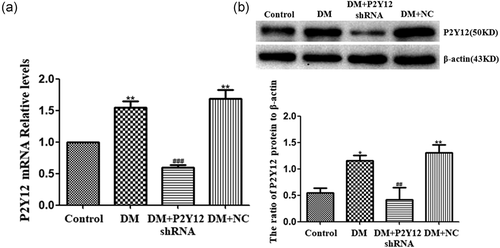
Upregulated expression of the P2Y12 receptor in the DRG of DM rats. (a) Expression of P2Y12 mRNA in the DRG was measured by real-time PCR. Expression in the DM group was higher than in the control group. In DM rats treated with P2Y12 shRNA, expression was significantly lower than in the DM rats that did not receive P2Y12 shRNA. The experiment was performed three times (n = 8 per group). Data are presented as the means ± SE. **p < 0.01 compared with the control group; ###p < 0.001 compared to the DM group. (b) Expression of the P2Y12 protein in the DRG was assessed by western blot. Protein expression in the DM group was increased compared with the control group. In DM rats treated with P2Y12 shRNA, expression was significantly lower than in DM rats that did not receive P2Y12 shRNA. Bar graphs show the ratio of the P2Y12 protein level to the β-actin level in each group. Data are displayed as the means ± SE. n = 8 for each group. *p < 0.05, **p < 0.01 compared with the control group; ##p < 0.01 compared to the DM group. DM, diabetes mellitus; DRG, dorsal root ganglia; mRNA, messenger RNA; PCR, polymerase chain reaction; SE, standard error; shRNA, short hairpin RNA
The expression levels of the P2Y12 receptor protein in the DRG were measured by western blot. The expression levels (the integrated optical density ratio, IOD ratio) of the P2Y12 receptor protein (normalized to each β-actin internal control) in the DM group were significantly higher than those in the control group (p < 0.05). The expression levels of the P2Y12 protein in the DM + P2Y12 shRNA group were decreased compared with those in the DM group (p < 0.01) (Figure 1b,c). No significant difference was found between DM+ noload plasmid (DM + NC) and DM groups (p > 0.05) (n = 8 for each group). Thus, the expression levels of the P2Y12 receptor in the DRG of DM rats were upregulated.
3.2 P2Y12 shRNA treatment reduced mechanical withdrawal threshold and thermal withdrawal latency in DM rats
The mechanical withdrawal threshold (MWT) was measured. The MWT in the DM group was significantly lower than that in the control group (p < 0.01). Ten days after treatment with P2Y12 shRNA, the MWT of rats in the DM + P2Y12 shRNA group was significantly higher than that in the DM group (p < 0.01) (Figure 2a). There was no difference between DM + NC and DM groups (p > 0.05) (n = 8 for each group). The above results showed that P2Y12 shRNA inhibited pain behaviors by increasing the MWT in DM rats.
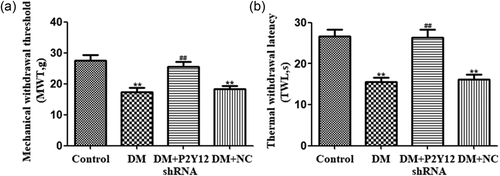
P2Y12 shRNA treatment reduced mechanical withdrawal threshold and thermal withdrawal latency in DM rats. (a) The mechanical withdrawal threshold (MWT) in the DM group was lower than in the control group. In DM rats treated with P2Y12 shRNA, the MWT was higher than in the DM group that did not receive P2Y12 shRNA. No difference was found between the DM + NC group (DM rats treated with no-load plasmid negative control group) and the DM group (p > 0.05). Each group consisted of eight rats. Data are displayed as the mean ± SE. n = 8 for each group. **p < 0.01 compared with the control group; ##p < 0.01 compared with the DM group. (b) The thermal withdrawal latency (TWL) in the DM group was gradually shorter than in the control group. In DM rats treated with P2Y12 shRNA, the TWL was higher than in the DM group that did not receive P2Y12 shRNA. No difference was found between the DM + NC group (DM rats treated with no-load plasmid negative control group) and the DM group (p > 0.05). Each group consisted of eight rats. The data are displayed as the means ± SE. n = 8 for each group. **p < 0.01 compared with the control group; ##p < 0.01 compared with the DM group. DM, diabetes mellitus; NC, negative control; SE, standard error; shRNA, short hairpin RNA
The thermal withdrawal latency (TWL) was also measured. The TWL in the DM group was significantly lower than that in the control group (p < 0.01). Ten days after treatment with P2Y12 shRNA, the TWL of rats in the DM + P2Y12 shRNA group was significantly higher than that in the DM group (p < 0.01) (Figure 2b). There was no difference between DM + NC and DM groups (p > 0.05) (n = 8 for each group). Our data suggest that P2Y12 shRNA treatment may relieve diabetic neuropathic pain.
3.3 P2Y12 shRNA treatment reduces the upregulated coexpression of P2Y12 and GFAP values in the DRG of DM rats
Coexpression of P2Y12 and GFAP in the DRG was measured by double immunofluorescence. GFAP is a marker of SGCs. The upregulation of GFAP in SGCs suggests the activation of DRG SGCs in DM rats. Coexpression of P2Y12 and GFAP in the DM group was higher than that in the control group (p < 0.01) . Silencing of P2Y12 was associated with lower coexpression of P2Y12 and GFAP in DM + P2Y12 shRNA rats relative to DM rats (p < 0.01) (Figure 3). There was no significant difference between the DM and DM + NC groups (p > 0.05) (Figure 3) (n = 8 for each group). The number of neurons surrounded with P2Y12 and GFAP-positive SGCs in DRG have be quantified. The results indicated that the upregulation of the P2Y12 receptor expressed in DRG SGCs of DM rats was involved in diabetic neuropathic pain.
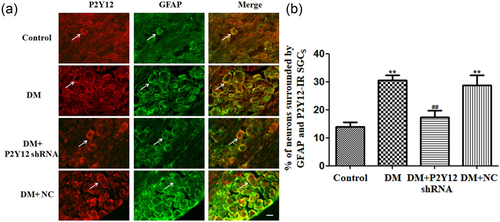
P2Y12 shRNA treatment reduces the upregulated coexpression of P2Y12 and GFAP values in the DRG of DM rats. Neurons that were surrounded by GFAP-positive SGCs by more than 50% of their circumference were counted. In the fields, a percentage of the total number of neurons was analyzed. Histogram in (b) showed the number of neurons surrounded with GFAP and P2Y12 positive SGCs in the DRG. Coexpression (the number of neurons surrounded with P2Y12 and GFAP-positive SGCs in DRG) of P2Y12 and GFAP in the DM group was higher than in the control group. P2Y12 shRNA treatment reduced coexpression of P2Y12 and GFAP in DM rats compared with DM rats that did not receive P2Y12 shRNA. There was no significant difference between the DM group and the DM + NC group (DM rats treated with no-load plasmid negative control group) (p > 0.05). Scale bar is 100 μm. The data are displayed as the mean ± SE. n = 8 for each group. **p < 0.01 compared to control group; ##p < 0.01 compared to the DM group. DM, diabetes mellitus; DRG, dorsal root ganglia; GFAP, glial fibrillary acidic protein; shRNA, short hairpin RNA; SE, standard error; SGC, satellite glial cell [Color figure can be viewed at wileyonlinelibrary.com]
3.4 P2Y12 shRNA treatment decreased the expression of GFAP and IL-1β protein in the DRG of DM rats
The expression levels of the GFAP protein in the DRG were measured by the western blot. The expression levels (IOD ratio) of the GFAP protein (normalized to each β-actin internal control) in the DM group were significantly higher than those in the control group (p < 0.01). The expression levels of the GFAP protein in the DM + P2Y12 shRNA group were decreased compared with those in the DM group (p < 0.001) (Figure 4a). No significant difference was found between DM + NC and DM groups (p > 0.05) (n = 8 for each group).

P2Y12 shRNA treatment reduces the expression of GFAP and IL-1β protein in the DRG of DM rats. (a) Expression of the GFAP protein (normalized to each β-actin internal control) in the DM group was higher than in the control group. In DM rats treated with P2Y12 shRNA, GFAP protein expression was lower than in the DM group that did not receive P2Y12 shRNA. No difference was found between the DM + NC group (DM rats treated with no-load plasmid as the negative control group) and the DM group (p > 0.05). Bar graphs show the ratio of GFAP protein level to β-actin level in each group. Data are displayed as the means ± SE (n = 8 per group). **p < 0.01 compared with the control group; ###p < 0.001 compared to the DM group. (b) Expression of the IL-1β protein (normalized to each β-actin internal control) in the DM group was higher than in the control group. In DM rats treated with P2Y12 shRNA, the expression levels of the IL-1β protein were lower than in the DM group that did not receive P2Y12 shRNA (p < 0.01). No difference was found between the DM + NC group (DM rats treated with no-load plasmid as the negative control group) and the DM group (p > 0.05). Bar graphs show the ratio of IL-1β protein to β-actin in each group. The data are displayed as the mean ± SE (n = 8 per group). **p < 0.01 compared with the control group; ##p < 0.01 compared to the DM group. DM, diabetes mellitus; DRG, dorsal root ganglia; GFAP, glial fibrillary acidic protein; IL, interleukin; NC, negative control; SE, standard error; shRNA, short hairpin RNA
The expression of the IL-1β protein in the DRG was measured by the western blot. The expression levels of the IL-1β protein in the DM group were significantly higher than those in the control group (p < 0.01). The expression levels of the IL-1β protein in the DM + P2Y12 shRNA group were decreased compared with those in the DM group (p < 0.01) (Figure 4b). There was no significant difference between the DM and DM + NC groups (p > 0.05) (n = 8 for each group). The present study indicated that P2Y12 shRNA treatment decreased the activation of DRG SGCs in DM rats and reduced the expression levels of GFAP and IL-1β protein in DM rats.
3.5 P2Y12 shRNA treatment decreased the expression of the TNFR1 protein in the DRG of DM rats
The expression levels of the TNFR1 protein in the DRG were measured by the western blot. The expression levels (IOD ratio) of the TNFR1 protein (normalized to each β-actin internal control) in the DM group were significantly higher than those in the control group (p < 0.05). The expression levels of the TNFR1 protein in the DM + P2Y12 shRNA group were decreased compared with those in the DM group (p < 0.05) (Figure 5). No significant difference was found between DM + NC and DM groups (p > 0.05) (n = 8 for each group). The data suggested that P2Y12 shRNA treatment decreased proinflammatory cytokines released from DRG SGCs and lowered the expression of the TNFR1 protein in the DRG of DM rats.
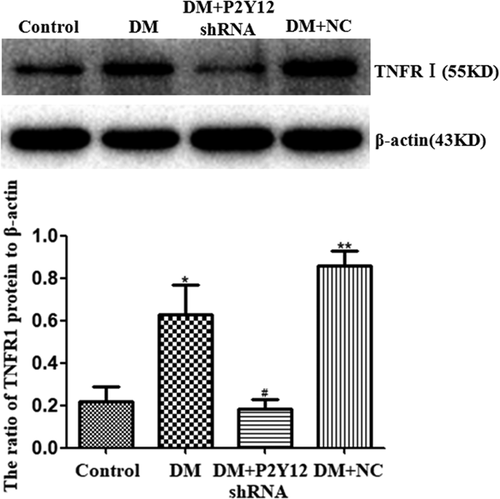
P2Y12 shRNA treatment reduces the expression of TNFR1 protein in the DRG of DM rats. Expression of the TNFR1 protein (normalized to each β-actin internal control) in the DM group was higher than in the control group. In DM rats treated with P2Y12 shRNA, TNFR1 protein expression was lower than in the DM group that did not receive P2Y12 shRNA. No difference was found between the DM + NC group (DM rats treated with no-load plasmid negative control group) and the DM group (p > 0.05). Bar graphs show the ratio of TNFR1 protein level to β-actin level in each group. Data are displayed as the mean ± SE (n = 8 per group). *p < 0.05 compared with the control group; #p < 0.05 compared with the DM group. DM, diabetes mellitus; DRG, dorsal root ganglia; SE, standard error; shRNA, short hairpin RNA
3.6 P2Y12 shRNA treatment inhibited the activation of the P38 MAPK pathway in the DRG of DM rats
Expression levels of phosphorylated P38 (p-P38) MAPK in the DRG were analyzed by western blot. We tested whether the administration of P2Y12 shRNA affected the phosphorylation of P38 MAPK in the DRG of the DM group. The IOD ratio of P38 MAPK protein to β-actin in the DM group was not different from that in the control group (p > 0.05). The IOD ratio of p-P38 MAPK protein to P38 MAPK in the DM group was significantly higher than that in the control group (p < 0.05) (Figure 6b,c). The IOD ratio of p-P38 MAPK protein to P38 MAPK in the DM rats treated with P2Y12 shRNA was significantly lower than that in the DM group that did not receive P2Y12 shRNA (p < 0.05) (Figure 6b,c). The results revealed that the phosphorylation and activation of P38 MAPK in the DRG is involved in mechanical and thermal hypersensitivity of DM rats. P2Y12 shRNA treatment inhibited the activation of the p38 MAPK pathway in the DRG of DM rats to reduce mechanical and thermal hypersensitivity in DM rats.
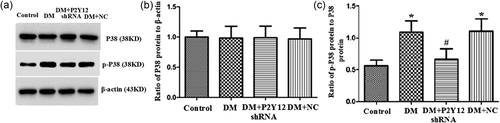
P2Y12 shRNA treatment inhibited the activation of the P38 MAPK pathway in the DRG of DM rats. The integrated optical density (IOD) ratio of P38 MAPK to β-actin was not significantly different between the DM group and the control group (p > 0.05). The IOD ratio of p-P38 MAPK to P38 MAPK was higher in the DM group than in the control group. The IOD ratio of p-P38 MAPK to P38 MAPK in the DM + P2Y12 shRNA-treated rats was significantly lower than in the DM group that did not receive P2Y12 shRNA. The data are displayed as the mean ± SE, n = 8 for each group. *p < 0.05 compared with the control group; #p < 0.05 compared with the DM group. DM, diabetes mellitus; DRG, dorsal root ganglia; MAPK, mitogen activated protein kinase; SE, standard error
4 DISCUSSION
Diabetic neuropathy affects peripheral nerves (Davies et al., 2006; Li et al., 2017; Morales-Vidal et al., 2012; Rao et al., 2017; Singh et al., 2014; Wang et al., 2016). Our results showed hyperglycemia-induced pathological changes in pain behaviors in DM rats. SGCs surrounding neurons in the DRG are activated by peripheral damage and inflamed (Hanani, 2005; Takeda, Takahashi, & Matsumoto, 2009). SGCs are endowed with the P2Y12 receptor (Katagiri et al., 2012; Kobayashi et al., 2013). Our experiments demonstrated that the P2Y12 receptor was increased in the DRG of DM rats, leading to enhanced mechanical and thermal hyperalgesia. It is possible that the P2Y12 receptor in the DRG may be involved in the nociceptive transmission of diabetic neuropathic pain in DM rats. Our results also revealed that the coexpression of P2Y12 and GFAP in the DM group was increased compared with that in the control group. Thus, the P2Y12 receptor expressed in DRG SGCs is involved in the transmission of diabetic neuropathic pain signals resulting from nerve damage and inflammation in DM rats.
A growing body of evidence indicates that the activation of SGCs plays a crucial role in neuropathic pain through the release of proinflammatory cytokines (Hanani, 2005; Takeda et al., 2007; Takeda et al., 2009). Increased expression levels of GFAP and IL-1β are markers of SGC activation (Hanani, 2005; Hanani, Blum, Liu, Peng, & Liang, 2014; Takeda et al., 2007). The present study showed that GFAP and IL-1β expression levels were increased in the DRG in DM rats. These changes in SGCs contribute to diabetic neuropathic pain (Hanani et al., 2014). The upregulation of GFAP and IL-1β expression in the DRG was associated with hypersensitivity of pain behaviors in DM rats. In DRG SGCs, P2Y12 shRNA treatment reduced the coexpression of P2Y12 and GFAP and the expression of GFAP and IL-1β protein in DM rats. Inhibition of SGC activation blocks hypersensitivity after nerve damage and inflammation (Hanani, 2005; Hanani et al., 2014; Takeda et al., 2007). Simultaneously, the mechanical withdrawal threshold (MWT) and TWL were increased in DM rats after P2Y12 shRNA treatment compared with DM rats that did not receive P2Y12 shRNA. Therefore, downregulation of the P2Y12 receptor decreased activation of DRG SGCs and improved MWT and TWL in DM rats.
TNF-α can activate TNFR1 in DRG neurons, which increases neuronal sensitization to nociceptive stimuli (Illes, Verkhratsky, Burnstock, & Franke, 2012; Kajander, Wakisaka, & Bennett, 1992). The current experiments also revealed that the expression levels of TNFR1 in the DRG of DM rats were increased compared with control rats. Upregulation of the TNFR1 protein in the DRG promoted hyperalgesia in DM rats. P2Y12 shRNA treatment lowered the expression levels of the TNFR1 protein in the DRG and decreased hyperalgesia in DM rats. Thus, the downregulation of the P2Y12 receptor decreased activation of DRG SGCs and then reduced the release of proinflammatory cytokines. Downregulated expression of the TNFR1 protein decreased abnormal signals of hypersensitivity between neurons and SGCs in the DRG as well as relieved neuropathic pain behaviors in DM rats.
The P38 MAPK pathway is involved in diabetes-induced hyperalgesia (Zychowska et al., 2013). In the present study, the integrated optical density (IOD) ratio of p-P38 MAPK to P38 MAPK in the DM group was higher than in the control group. After treatment with P2Y12 receptor shRNA, the IOD ratios of p-P38 MAPK to P38 MAPK in the DM rats treated with P2Y12 shRNA were significantly lower than that in the DM rats that did not receive P2Y12 shRNA. The G-protein coupled the P2Y12 receptor in activated microglia was one of the upstream molecules of phosphorylation of P38 MAPK after nerve injury (Kobayashi et al., 2008). Inhibition of P2Y12 signaling suppressed the activation of P38 MAPK in microglia (Kobayashi et al., 2008). In addition, P2Y12 receptors may activate P38 MAPK via Rho-associated coiled-coil-containing protein kinase in microglia after peripheral nerve injury (Tatsumi et al., 2015). Thus, the P2Y12 receptor shRNA treatment decreased the expression of the P2Y12 receptor as well as phosphorylation and activation of P38 MAPK in the DRG of DM rats. Blockade of P38 MAPK activation in the DRG can decrease mechanical and thermal hypersensitivity (Zychowska et al., 2013). The following downregulation of p-P38 MAPK in DM rats correlated well with the reduction in mechanical and thermal hyperalgesia after the P2Y12 receptor shRNA treatment. Therefore, the upregulation of the P2Y12 receptor was related to hyperalgesia involved in the P38 MAPK signaling pathway. Downregulation of P38 MAPK phosphorylation and activation in the DRG of DM rats decreased mechanical and thermal hyperalgesia.
In conclusion, the results demonstrated that upregulation of the P2Y12 receptor in DRG SGCs promoted the release of proinflammatory cytokines and enhanced mechanical and thermal hyperalgesia in a DM model. Upregulation of IL-1β, TNFR1, and GFAP may increase neuronal sensitization to nociceptive stimuli. Targeting the P2Y12 receptor in DRG SGCs by shRNA decreased upregulation of IL-1β, TNFR1, and GFAP, and reduced p-P38 MAPK in the DRG of DM rats. Therefore, downregulation of the P2Y12 receptor in DRG SGCs decreased mechanical and thermal hyperalgesia in a DM rat model.
ACKNOWLEDGMENTS
These studies were supported by grants from the National Natural Science Foundation of China (81570735, 31560276, 81560219, 81760152, 81701114, 81460200, and 81200853), the Technology Pedestal and Society Development Project of Jiangxi Province (20151BBG70250 and 20151BBG70253), the Natural Science Foundation of Jiangxi Province (20171BAB205025, 20142BAB205028, 20142BAB215027, and 20121512040234), Major Disciplines of Academic and Technical Leaders Project of Jiangxi Province (2014), and the Educational Department of Jiangxi Province (GJJ13155 and GJJ14319). Special fund project of graduate student innovation in Jiangxi Province (Project number YC2016-S058, YC2017-S078).
CONFLICTS OF INTEREST
The authors declare they have no conflict of interests.
AUTHOR CONTRIBUTIONS
S.L. designed the research. S.W., Z.W., L.L., Y.G, T.J., S.Z., H.Y., L.Z., L.S., S.L., B.W., Z.Y., H.L., Y.G., M.L., and C.Z. performed the research. S.W., J.M.D., and M.L. analyzed data. S.W. and S.L. wrote this paper. S.L. and J.M.D. revised this paper. All the authors read and approved the final manuscript.