A new copper ionophore DPMQ protects cells against ultraviolet B irradiation by inhibiting the TRPV1 channel
Abstract
Copper is more likely than iron to generate reactive oxygen species (ROS) in a redox reaction due to its higher electrochemical reactivity. This study examined the effect of a newly synthesized Cu2+ binding compound, (E)-2-(4-(dimethylamino)phenylimino)methyl)quinolin-8-ol (DPMQ), on ultraviolet B (UVB) irradiation–induced cytotoxicity in human dermal fibroblasts. DPMQ induced Cu2+ influx as effectively as disulfiram, a Cu2+ ionophore anticancer drug. However, disulfiram induced ROS generation, mitochondrial dysfunction, and apoptosis in fibroblasts in a Cu2+-dependent manner, whereas DPMQ was not only nontoxic, but protected cells against UVB irradiation–induced apoptosis in a Cu2+-independent manner. UVB irradiation induced a Ca2+-dependent increase in ROS generation, a decrease in Nrf2 levels, and activation of the mitochondrial apoptotic pathway, and these effects were prevented by DPMQ, which also increased Nrf2 nuclear translocation in a Cu2+-independent manner. UVB irradiation activated 12-lipoxygenase and 12-hydroxyeicosatetraenoic acid (12-HETE), a product of 12-lipoxygenase, activated the TRPV1 channel. DMPQ did not act as a Ca2+ chelator, but inhibited the cytosolic Ca2+ increase induced by 12-HETE or capsaicin, but not that induced by bradykinin or ATP. Blockade of Ca2+ influx by pharmacological inhibition or silencing of the TRPV1 channel or chelation of cytosolic Ca2+ inhibited the UVB irradiation–induced Nrf2 reduction, ROS generation, mitochondrial dysfunction, and apoptosis. Taken together, our results suggest that Ca2+ influx via the TRPV1 channel is responsible for UVB irradiation–induced cytotoxicity and that DPMQ protects cells against UVB irradiation by inhibiting the TRPV1 channel and stabilizing Nrf2, and could thus be a potentially useful compound for the treatment of free radical-induced diseases.
1 INTRODUCTION
Copper ion is present in many proteins responsible for redox reactions, such as superoxide dismutase and cytochrome c oxidase, and acts as a cofactor as it transfers electrons. Disruption of Cu2+ homeostasis is closely involved in many diseases. For example, dysregulation of Cu2+ is known to facilitate amyloid-β assemblies and this is one of the key features of Alzheimer’s disease (Budimir, 2011; Manto, 2014). In a search for a new drug to treat diseases involving Cu2+-containing proteins, the hybrid compound (E)-2-(4-(dimethylamino)phenylimino)methyl)quinolin-8-ol (DPMQ) was synthesized by conjugating N,N-dimethylanilide, an analog of amyloid-β imaging agents, to the hydroxyquinoline core of clioquinol, a Cu2+ ionophore (Fu, Hsu, Liao, & Hu, 2016). Clioquinol was previously used clinically as a topical formulation for the treatment of skin infections (Hervella-Garcés, García-Gavín, Silvestre-Salvador, & en representación del Grupo Español de Investigación en Dermatitis de Contacto y Alergia Cutánea GEIDAC, 2016; Hojyo, 1987) and indigestion and diarrhea (Cahoon, 2009), but was withdrawn from the market due to its neurological toxicity. However, recently, because of its Cu2+-binding ability, many researchers have considered its possible use in the treatment of Alzheimer’s disease (reviewed in Bareggi & Cornelli, 2012). DPMQ was found to exhibit Cu2+-binding ability and antioxidant capacity (Fu et al., 2016).
Potentially destructive hydrogen peroxide, the superoxide anion, and the hydroxyl radical are collectively referred to as reactive oxygen species (ROS). Hydrogen peroxide is generated within cells by xanthine oxidase and during oxidation of fatty acid in the peroxisome, whereas the superoxide anion is produced by NADPH oxidase. In addition, many enzymes, that is, cyclooxygenases, lipoxygenases, nitric oxide synthases, and mitochondrial respiratory chain enzymes, also generate hydrogen peroxide and superoxide anion as side products (Koskenkorva-Frank, Weiss, Koppenol, & Burckhardt, 2013). The harmful hydroxyl radical is generated from the superoxide anion and hydrogen peroxide in the presence of Fe3+/Fe2+ by a Fenton reaction and Haber–Weiss reaction recycling. Most of the aforementioned enzymes contain heme or quinone, both of which transfer electrons and are involved in cellular ROS generation. Acceleration of heme degradation by heme oxygenase 1 (HO-1) and of quinone reduction by NADPH quinone oxidoreductase 1 (NQO1) decreases cellular oxidative stress. The transcription factor Nrf2 regulates the expression of HO-1 and NQO1 (Zhang et al., 2013).
Copper ion is more likely than Fe2+ to aggravate cellular oxidative stress because of its higher electrochemical reactivity in the metal/ROS interaction in the Fenton reaction and Haber–Weiss reaction cycling (Öhrvik, Aaseth, & Horn, 2017). A side effect of Cu2+-containing enzymes is superoxide generation. In addition to the natural aging that occurs in all organs, the skin suffers extra damage due to exposure to sunlight, particularly ultraviolet B (UVB) irradiation (Yaar & Gilchrest, 2007). After exposure of dermal cells to UV irradiation, the structure of DNA is chemically altered (Dong, Damaghi, & Picart, 2008; Woollons et al., 1999). Cellular oxidative stress is also aggravated by UVB irradiation (Widel, Krzywon, Gajda, Skonieczna, & Rzeszowska-Wolny, 2014). Damaged DNA and accumulated ROS may cause apoptotic cell death or development of cancer. The hybrid compound DPMQ may therefore exert potential protective effects in free radical-induced skin diseases by its dual role as a ROS scavenger and a Cu2+ binder. In this study, we examined whether DPMQ protected dermal fibroblasts against UVB irradiation–induced oxidative stress. To understand the underlying mechanisms, we also compared its effects with those of the Cu2+ ionophore disulfiram. Our findings may have therapeutic implications for the treatment of free radical-induced diseases.
2 MATERIALS AND METHODS
2.1 Materials
Dulbecco’s modified Eagle’s medium (DMEM; high glucose), Ca2+-free DMEM, fetal bovine serum (FBS), penicillin, and streptomycin were obtained from Invitrogen (Carlsbad, CA). Fibroblast basal medium and fibroblast growth kit-low serum were purchased the from American Type Culture Collection (Manassas, VA). Dichlorodihydrofluorescein diacetate (H2DCFDA), tetramethylrhodamine ethyl ester (TMRE), fluo-3 acetoxymethyl ester, Fura-2 acetoxymethyl ester, calcein-acetoxymethyl ester, BAPTA-acetoxymethyl ester, and Alexa fluo 555 goat anti-rabbit immunoglobulin G (IgG) antibodies were obtained from Invitrogen Molecular Probes (Eugene, CA). Capsazepine, 12-HETE, capsaicin, arachidonic acid, ferrous sulfate, ammonium thiocyanate, bradykinin, ATP, N-acetylcysteine, disulfiram, tetrathiomolybdate (TTM), baicalein, and lysis buffer (CelLytic™ M cell lysis reagent for cultured mammalian cells) were purchased from Sigma (St. Louis, MO). R-phycoerythrin active caspase-3 apoptosis assay kits were obtained from BD Biosciences (San Jose, CA). Rabbit antibodies against TRPV1, Nrf2, NQO1, HO-1, or caspase-3 were purchased from GeneTex (Irvine, CA). TRPV1 channel and nontargeting control small interfering RNAs (siRNAs), transfection reagent, transfection medium, and horseradish peroxidase-conjugated anti-rabbit IgG antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit antibodies against Ki67 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were purchased from Cell Signaling Technology (Danvers, MA). DPMQ was synthesized as described previously (Fu et al., 2016).
2.2 Culture of cells
For expansion, primary human dermal fibroblasts (PCS-201-012; American Type Culture Collection) were plated at a density of 5 × 105 cells in 10 ml of fibroblast basal medium supplemented with 15% FBS, 2 mM of l-glutamine, 100 U/ml of penicillin, 100 μg/ml of streptomycin, and 2 μM of phenol red in a 100 mm culture dish and cultured at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Cells were passaged at approximately 80% confluence by incubation with 0.05% trypsin/EDTA for 5 min. For experiments, depending on needs, the cells were plated in 35, 60, or 100 mm dishes or on 24 mm coverslips (all at a density of 1 × 104 cells/cm2) and cultured for another 24 hr in the same medium, supplemented with 5% FBS.
Human HaCaT keratinocytes (a gift from Dr. Tsu-Chung Chang, Department of Biochemistry, National Defense Medical Center, Taipei, Taiwan) were cultured in high-glucose DMEM supplemented with 10% FBS, 2 mM of l-glutamine, 100 U/ml of penicillin, and 100 μg/ml of streptomycin at 37°C in a humidified atmosphere of 5% CO2 and 95% air.
2.3 Treatment of cells
Twenty-four hours after plating of primary human dermal fibroblasts, the medium was changed to high-glucose DMEM or Ca2+-free high-glucose DMEM supplemented with fibroblast growth kit-low serum, 2 mM of l-glutamine, 100 U/ml of penicillin, and 100 μg/ml of streptomycin in the presence or absence of the indicated drugs, and then the effects of UVB irradiation or disulfiram treatment were examined. For UVB irradiation, the cells were placed in the center of a UV box (UVILink CL508-G; UVItec Limited, Cambridge, UK) without a lid and UVB irradiation (1–20 mJ/cm2) was applied, with the UV box shutting off automatically when the accumulated irradiation reached the indicated dose. The cells were then incubated for another 30 min to 48 hr in the continued presence or absence of Ca2+ and the indicated drugs before various measurements were performed.
HaCaT keratinocytes were used to test the effect of 12-HETE, capsaicin, bradykinin, or ATP on intracellular Ca2+ levels.
Stock solutions of N-acetylcysteine, ATP, bradykinin, or TTM were prepared in Locke’s buffer (LB; in mM, 150 NaCl, 5 KCl, 1 MgCl2, 2.2 CaCl2, 5 glucose, and 10 HEPES, pH 7.4), whereas stock solutions of DPMQ, capsazepine, disulfiram, capsaicin, 12-HETE, baicalein, fluo-3-acetoxymethyl ester, and BAPTA-acetoxymethyl ester were prepared in dimethyl sulfoxide (DMSO); for use, all were diluted 100-fold with LB, and then a further 50–100-fold in growth medium or LB (when a stock solution in DMSO was used, the final DMSO concentration was 0.01–0.02%). Control cells were treated with diluted LB or DMSO without UVB irradiation or disulfiram treatment.
2.4 Determination of cell growth
After the indicated treatment, the morphology of cells in 35 mm dishes was analyzed by phase-contrast microscopy (Nikon TE200; Nikon, Tokyo, Japan). Cell number, used as an index of cell growth, was quantified in 9 randomly chosen fields in each dish, with 35–60 cells per field.
2.5 Determination of fluorescence quenching by Cu2+ influx
The fluorescence of calcein is strongly quenched by various transition metals and the relative quenching potency of transition metals is Cu > Ni > Co > Fe(II) (Breuer, Epsztejn, Millgram, & Cabantchik, 1995). An increase in the cytosolic Cu2+ concentration was measured by determining the quenching of the fluorescence of calcein. Cells were incubated with 2 μM of calcein-acetoxymethyl ester for 20 min at 37°C in LB, and were then detached by incubation with 0.05% trypsin/EDTA for 5 min and washed twice in LB; then, the pellet from one 100 mm dish (1–5 × 106 cells) was suspended in 1 ml of LB and the cells were counted. Subsequently, 3 × 105 cell aliquots were placed in a cuvette and the volume was made to 3 ml with LB. The quenching of fluorescence of the cell suspensions at an excitation wavelength of 485 nm and an emission wavelength of 515 nm was measured immediately after the addition of DPMQ or disulfiram in the presence or absence of TTM in a spectrofluorometer (LS55; PerkinElmer, Llantrisant, UK). Experiments were repeated five times using different batches of cells with similar results. The results for one representative experiment are shown.
2.6 Measurement of lipoxygenase activity
Lipoxygenase activity was measured by determining the generation of the red ferrithiocyanate complex as described previously (Lu et al., 2013). The principle of the colorimetric assay for lipoxygenase is that hydroperoxides, products of lipoxygenase, oxidize the ferrous ion (Fe2+) to the ferric ion (Fe3+), which then binds to thiocyanate to form a red ferrithiocyanate complex with an absorbance peak at 480 nm. Briefly, 12 hr after UVB irradiation, cells grown in 100 mm dishes were detached and thoroughly washed, and were then suspended in 200 μl of ice-cold lysis buffer (CelLytic™ M cell lysis reagent for cultured mammalian cells; Sigma), incubated for 30 min at 4°C, and centrifuged at 16,000 g for 30 min at 4°C; the supernatant was collected and the protein content was determined. Control cells without UVB irradiation were subjected to the same treatment. Lipoxygenase ferrithiocyanate assays were then performed in a 3 ml cuvette. Aliquots of cell lysate (about 0.5 mg of protein) were added to a cuvette containing 2 ml of 100 μM of arachidonic acid in 50 mM of Tris–HCl; then, after 1 min, the products were quantified by the addition of 1 ml of ferrithiocyanate reagent (equal volume mixture of reagent I [9 mM of ferrous sulfate in 0.2 M of HCl] and reagent II [6% ammonium thiocyanate in methanol]). The absorbance of the red ferrithiocyanate complex at 480 nm was then monitored for 3.5 min online with a spectrophotometer (Lambda 35; PerkinElmer). The experiments were repeated five times, with similar results. One representative trace is shown.
2.7 Measurement of the intracellular Ca2+ concentration
Changes in the intracellular Ca2+ concentration were measured using the fluorescent dyes, fluo-3 and fura-2. In all studies, except the silencing studies, cells grown on 100 mm dishes were harvested and incubated with 4 μM of fluo-3 acetoxymethyl ester in LB at 37°C for 30 min at a density of 1 × 106 cells/ml. After 3 washes with LB, the cells were suspended at a density of 1 × 106 cells/ml in LB. Immediately before each experiment, 100 μl aliquots were centrifuged and resuspended in 150 μl of normal or Ca2+-free LB with or without the indicated drug and placed in a well of a 96-well plate. The fluorescence was monitored for 5 min in response to the addition of various receptor agonists using a multifunctional ELISA reader (SynergyHT; BioTek, Winooski, VT) controlled by Gen5 software using an excitation wavelength of 490 nm and an emission wavelength of 526 nm. The change in the calcium concentration in the cells is expressed as the fluorescence at the indicated time (F) divided by the initial fluorescence (F0; F/F0).
For the TRPV1 silencing study, the Ca2+ change in single cells was measured using Fura-2 as previously described (Huang, Ma, Liu, Chen, & Chueh, 2016). Briefly, after loading with 2.5 μM of Fura-2 acetoxymethyl ester and UVB irradiation in the presence or absence of extracellular Ca2+, cells grown on coverslips were immediately mounted on the stage of a fluorescence microscope (model DMIRB; Leica, Heidelberg, Germany), and then the different agonists were added and the fluorescence intensity was measured for 5 min using a dual-excitation fluorometric Ca2+ imaging system equipped with a high-speed scanning polychromatic light source (model C7773; Hamamatsu Photonics, Hamamatsu, Japan) and a CCD camera (model C6790; Hamamatsu Photonics) controlled by Aquacosmos 2.5 software (Hamamatsu Photonics) using excitation wavelengths of 340 and 380 nm and an emission wavelength of 505 nm and a sampling rate of 1 Hz. Four cells per coverslip were selected and the 340–380 nm fluorescence ratio (F340/F380) was used to reflect intracellular Ca2+ changes. All experiments were performed four times, with similar results. Results from one representative experiment are illustrated graphically.
2.8 Immunoblotting
Immunoblotting was performed as described previously (Yeh, Ma, Liu, Kuo, & Chueh, 2015). After the indicated treatment, cells grown in 60 mm dishes were detached, thoroughly washed, suspended in 150 μl of ice-cold lysis buffer, incubated for 30 min at 4°C, and centrifuged at 16,000g for 30 min at 4°C, and then the supernatant was collected and the protein content was determined using the Bradford assay. Aliquots (10 μg of protein) were then mixed with an equal volume of double-strength reducing sodium dodecyl sulfate polyacrylamide gel electrophoresis sample buffer and boiled for 10 min. After electrophoresis on 4–12% gradient sodium dodecyl sulfate polyacrylamide gels, the proteins were electrophoretically transferred to a poly(vinylidene difluoride) membrane. After blocking overnight at 4°C with phosphate-buffered saline (PBS) containing 5% nonfat milk, the blots were incubated for 1 hr at room temperature with antibodies against Nrf2, HO-1, NQO1, TRPV1, caspase-3, or GAPDH (all diluted 1:1,000 in PBS), and bound antibodies were detected using horseradish peroxidase-conjugated anti-rabbit IgG antibodies diluted 1:1,000 in PBS and enhanced chemiluminescence substrate.
2.9 Immunofluorescence staining
Immunofluorescence staining was performed as described previously (Yeh et al., 2015). All incubations were performed at room temperature. Cells plated on 24 mm coverslips and treated as indicated were fixed in 3.7% formaldehyde in PBS for 10 min, rinsed twice in PBS, and permeabilized in PBS containing 0.1% Triton X-100 (PBST) for 20 min. After blocking by incubation for 60 min in PBST containing 3% bovine serum albumin, the cells were incubated overnight at 4°C with rabbit antibodies against Ki67 or Nrf2 diluted 1:100 in PBST. After washing with PBS, Alexa fluo 555 goat anti-rabbit IgG antibodies diluted 1:100 in PBST were added for 1 hr, and then the cells were washed several times with PBS. Hoechst 33258 (10 μM) was then added and the cells were incubated for 5 min; then, after a PBS wash, the cells were examined on a fluorescence microscope using a model Delta Vision Elite (GE Healthcare Life Science, Issaquah, WA). The percentage of Ki67-positive cells was calculated in nine randomly chosen fields, with about 13–28 cells in each field, by dividing the number of Ki67-positive cells by the total Hoechst 33258-stained cells in the chosen field. ImageJ (NIH, Bethesda, MD) was used to quantify the nuclear Nrf2 fluorescence intensity per cell in 35 randomly chosen fields in each dish. The experiments were repeated four times, with similar results. Results from one representative experiment were shown.
2.10 Measurement of apoptotic cells
Apoptotic cells were measured by quantifying condensed chromatin using the chromatin-specific dye Hoechst 33258 as described previously (Huang et al., 2016). Briefly, after the indicated treatment, cells grown in 60 mm dishes were harvested from the attached and floating fractions and fixed by 10 min of incubation with 3.7% formaldehyde in PBS, spread on slides, allowed to dry, and incubated for 20 min with 10 μM of Hoechst 33258 in LB, all at room temperature. Nuclear morphology was then examined on an Olympus IX-70, Tokyo, Japan fluorescence microscope and apoptotic and nonapoptotic nuclei were counted in nine randomly chosen fields per coverslip at a magnification of ×400. The percentage cell death was calculated by dividing the number of apoptotic cells by the total cell number.
2.11 Measurement of cellular ROS
The generation of intracellular ROS was measured using H2DCFDA. In brief, after the indicated treatment, cells grown in 60 mm dishes were harvested and incubated with 10 μM of H2DCFDA in LB for 30 min at 37°C, washed, and incubated for another 30 min at 37°C for de-esterification. The de-esterification product H2DCF is readily oxidized by intracellular ROS to fluorogenic DCF, which, in all studies, except the silencing study, was measured using a FACSCalibur cell sorter (Becton-Dickinson, San Jose, CA) with a 488 nm laser and a standard green 530/30 nm band pass filter. For each treatment, a minimum of 1 × 104 cells were analyzed. CellQuest Pro software (Becton-Dickinson) was used to obtain the mean fluorescence intensity. In the silencing study, DCF was measured using a spectrofluorometer (LS55; PerkinElmer), the emission fluorescence spectrum being scanned between 500 and 550 nm at an excitation wavelength of 485 nm and the increase in emission fluorescence at 525 nm associated with oxidation being used as a measure of the intracellular ROS levels.
2.12 Measurement of the mitochondrial membrane potential
The mitochondrial membrane potential was measured using TMRE. Accumulation of positively charged TMRE in functional mitochondria results in an increase in TMRE-associated red fluorescence. In brief, after the indicated treatment, cells grown in 60 mm dishes were harvested and incubated with 200 nM of TMRE in LB for 30 min at 37°C. After washing, cells were analyzed using a FACSCalibur cell sorter with a 488 nm laser and a standard red 670 nm long pass filter and CellQuest Pro software (Becton-Dickinson) to obtain the mean fluorescence intensity. For each sample, a minimum of 1 × 104 cells were analyzed.
2.13 Measurement of caspase-3 activity
In all studies, except the silencing study, caspase-3 activity was measured using R-phycoerythrin active caspase-3 apoptosis assay kits (BD Biosciences) according to the manufacturer’s instructions. In brief, after the indicated treatment, cells grown in 60 mm dishes were harvested and incubated sequentially with fixing–permeabilizing buffer for 20 min and with the R-phycoerythrin antiactive caspase-3 antibody for 30 min. After washing, the cells were analyzed using a FACSCalibur cell sorter with a 488 nm laser and a standard yellow 585/42 nm band pass filter and CellQuest Pro software (Becton-Dickinson, San Jose, CA) to obtain the mean fluorescence intensity. For each sample, a minimum of 1 × 104 cells were analyzed. In the silencing study, cleavage of caspase-3 was measured by immunoblotting.
2.14 Metal-binding assay
The relative metal (Cu2+ and Ca2+)-binding ability of DPMQ was examined using a UV–vis spectrophotometer (Lambda 35; PerkinElmer) as described previously (Fu et al., 2016; Sharma et al., 2012). First, the absorption spectrum of DPMQ (50 μM) alone was recorded at room temperature, and then 50 μM of CaCl2 (final concentration) in methanol was added and the spectrum was recorded. Finally, 50 μM of CuCl2 (final concentration) in methanol was added and a third spectrum was recorded. The resulting spectra were then examined as stated in the text to evaluate the metal-binding properties of DPMQ. The experiments were performed four times, with similar results. Results from one representative experiment are illustrated graphically.
2.15 siRNAs
Cells were cultured in 35 mm dishes in 1% FBS for 24 hr, and then transient transfection with siRNAs (50 nM final concentration) targeting the TRPV1 channel or a nontargeting control was carried out for 5 hr according to the manufacturer’s instructions (Santa Cruz Biotechnology). After transfection, the cells were incubated for 24 hr in 1% FBS, and then the effects of UVB irradiation on cell growth, intracellular Ca2+ concentration, ROS generation, caspase-3 activity, and apoptosis were assessed. The efficacy and specificity of TRPV1 knockdown were examined by immunoblot analysis.
2.16 Statistical analysis
The data are expressed as the mean ± standard deviation (SD). Statistically significant differences were determined using an unpaired Student’s t test.
3 RESULTS
3.1 The protective effect of DPMQ against UVB irradiation is independent of Cu2+, whereas the proapoptotic effect of disulfiram is Cu2+ dependent
Disulfiram is a Cu2+ ionophore, used as an anticancer drug (Brar et al., 2004; Liu et al., 2012; Roberts & Schilsky, 2008), and its therapeutic effect is inhibited by cotreatment of cells with TTM, a nonpermeable negatively charged Cu2+ chelator, suggesting that the proapoptotic effect of disulfiram requires Cu2+ (Calderon-Aparicio, Strasberg-Rieber, & Rieber, 2015). We therefore used TTM to examine the copper dependency of the effects of DPMQ. To select the optimal concentrations for use, we measured cell numbers after 24 hr exposure of fibroblasts to different concentrations of DPMQ, disulfiram, or TTM. As shown in Figure 1a, cell numbers were unaffected by exposure of cells to DPMQ or TTM at concentrations up to 3 µM, but were reduced to about 30% after treatment with 0.1 µM of disulfiram. We therefore chose to use 1 µM of DPMQ, 3 µM of TTM, and 0.3 µM of disulfiram in all subsequent studies unless otherwise specified. We next measured the effect of DPMQ on UVB irradiation–induced cytotoxicity. To select the optimal dose of UVB irradiation, we measured cell numbers 24 hr after exposure of cells to different doses of UVB irradiation and found that the dose of UVB irradiation resulting in 50% inhibition of cell growth (indicated by the number of cells) was 16.1 mJ/cm2 (data not shown). Therefore, the dose of 15 mJ/cm2 was used in this study. As shown in Figure 1b, in the control group, cell numbers increased continuously over 2 days, whereas, at 2 days after UVB irradiation, cell numbers were only about 50% of those in the control group. This decrease in cell numbers in UVB-treated cells was blocked by treatment of the cells with DPMQ during, and after, UVB irradiation and this inhibitory effect of DPMQ was Cu2+ insensitive as it was unaffected by the inclusion of TTM. Figure 1c shows the morphology of the cells after 24 hr incubation with buffer, DPMQ, or DPMQ + TTM with or without UVB irradiation. This decrease in cell numbers after UVB irradiation could reflect either increased apoptosis or decreased proliferation. We therefore measured the proliferation and apoptosis of dermal fibroblasts after UVB irradiation in the presence or absence of DPMQ. As shown in Figure 1d, the percentage of Ki67-positive cells, reflecting proliferation, was 73 ± 6% in control cells and was not significantly affected by UVB and/or DMPQ, showing that none of these treatments had any effect on proliferation. In contrast, apoptosis was observed in only 5% of the control cells (data not shown), whereas, as shown in Figure 1e, 68% of cells showed apoptosis 24 hr after UVB irradiation and this UVB irradiation–induced apoptosis was dose dependently inhibited by cotreatment of cells with DPMQ (50% of maximal effective protection at 0.34 µM), and this protective effect was not affected by the presence of TTM. These results indicate that the protective effect of DPMQ against UVB irradiation–induced apoptosis does not depend on Cu2+. We next examined whether the toxic effect of disulfiram was Cu2+ dependent. As shown in Figure 1f, after 24 hr treatment of fibroblasts with 0.3 μM of disulfiram, cell numbers were reduced (panel b vs. a) and this effect was blocked by cotreatment of the cells with 3 μM of TTM (panel d vs. b). As shown in Figure 1g, when cells were incubated for 24 hr with concentrations of 0–1 μM disulfiram, apoptosis occurred, with 50% of the maximal effect being observed at 0.06 µM of disulfiram, and this effect was markedly inhibited by cotreatment of the cells with 3 μM of TTM. Thus, disulfiram-induced apoptosis of fibroblasts requires Cu2+ and it is possible that Cu2+ influx may be activated by disulfiram. We therefore examined whether disulfiram caused Cu2+ influx using fluorescence quenching of calcein as an indicator as calcein fluorescence is rapidly and stoichiometrically quenched by Cu2+, Co2+, or Ni2+ at neutral pH and is minimally affected by a change in Ca2+ or Mg2+ concentration (Breuer et al., 1995). As shown in Figure 1h, in control fibroblasts, the fluorescence remained the same for 10 min after the addition of LB containing 0.02% DMSO (vehicle), whereas fluorescence quenching was observed 4–5 min after addition of disulfiram in DMSO, indicating an influx of Cu2+, and this effect was blocked by TTM, which, alone, had no effect. The addition of 1 μM of DPMQ also caused a large fluorescence quenching and this effect was also blocked by TTM. Thus, both DPMQ and disulfiram caused Cu2+ influx, that is, acted as Cu2+ ionophores. Our data show that both DPMQ and disulfiram induce similar levels of Cu2+ influx and that the protective effect of DPMQ against UVB irradiation–induced apoptosis does not depend on Cu2+, whereas the proapoptotic effect of disulfiram on fibroblasts does.
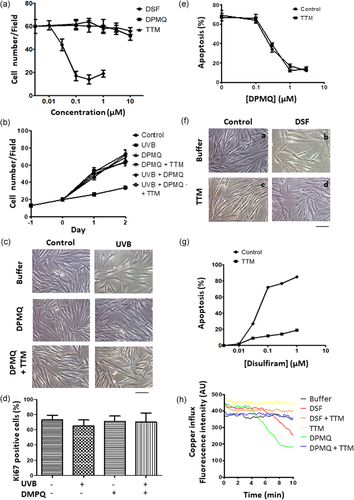
Effect of (E)-2-(4-(dimethylamino)phenylimino)methyl)quinolin-8-ol (DPMQ) and disulfiram on cell growth, copper influx, or apoptosis in dermal fibroblasts. (a) Cells were treated with different concentrations of DPMQ, disulfiram (DSF), or tetrathiomolybdate (TTM) as indicated. Cell numbers in the dish were calculated after 24 hr incubation. (b) Twenty-four hours after plating, cells were left untreated (control) or were treated with 15 mJ/cm2 ultraviolet B (UVB) irradiation in the presence and absence of 1 μM of DPMQ with or without 3 μM of TTM and incubated for another 48 h. The quantitative results for the number of cells attached to the dish are shown. The morphology of cells in each group after 24 hr incubation is shown in (c). (d) Cells were left untreated or were treated with 15 mJ/cm2 UVB irradiation in the presence or absence of 1 μM of DPMQ. Percent of Ki67-positive cells was measured after 24 hr incubation. (e) Cells were treated with 15 mJ/cm2 UVB irradiation in the presence or absence of the indicated concentration of DPMQ with or without 3 μM of TTM. Apoptotic cell death was measured after 24 hr incubation. (f) Cell morphology at 24 hr treated with or without 0.3 μM of DSF in the presence or absence of 3 μM of TTM. (g) Cells were treated with the indicated concentration of DSF with or without 3 μM of TTM. Apoptotic cell death was measured after 24 hr incubation. (h) Aliquots of calcein-loaded cells were suspended in Locke’s buffer (LB) in a cuvette, and then, LB (buffer), 0.3 μM of DSF, 0.3 μM of DSF plus 3 μM of TTM (DSF + TTM), 3 μM of TTM, 1 μM of DPMQ, or 1 μM of DPMQ plus 3 μM of TTM (DPMQ + TTM) was added, and fluorescence quenching indicating the Cu2+ influx was measured immediately. Scale bar = 100 μm in (c) and (f). The data are the mean ± SD for four to six independent experiments in (a), (b), (d), (e), and (g) [Color figure can be viewed at wileyonlinelibrary.com]
3.2 UVB irradiation or disulfiram treatment stimulates the mitochondrial death pathway
Increased ROS generation, reduction of the mitochondrial membrane potential, and caspase-3 activation are components of the mitochondrial death pathway. We therefore examined whether these processes were induced by UVB irradiation or by disulfiram treatment and the role of Cu2+ and Ca2+ in these processes. As shown in Figure 2, at 30 min after UVB irradiation (Figure 2a), or after 24 hr of disulfiram treatment (Figure 2b), increased intracellular ROS levels were observed, and both increases were inhibited by cotreatment of cells with N-acetylcysteine, a cellular ROS scavenger. UVB irradiation–induced ROS generation was inhibited by the lack of extracellular Ca2+ and was unaffected by TTM cotreatment. UVB irradiation–induced ROS generation was also inhibited by cotreatment of cells with DPMQ and this inhibition was insensitive to TTM cotreatment (Figure 2a). In contrast, as shown in Figure 2b, disulfiram-induced ROS generation was unaffected when cells were suspended in a Ca2+-free solution, but was inhibited by TTM cotreatment. The quantitative data for ROS levels induced by UVB irradiation and by disulfiram treatment expressed relative to those in control cells are shown in Figure 2c,d, respectively.
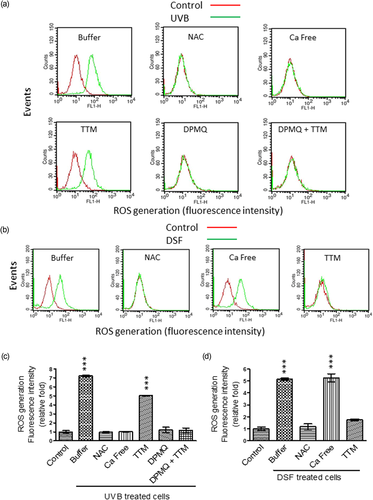
Effect of N-acetylcysteine (NAC), Ca2+ deprivation, tetrathiomolybdate (TTM), or (E)-2-(4-(dimethylamino)phenylimino)methyl)quinolin-8-ol (DPMQ) on reactive oxygen species (ROS) generation induced by ultraviolet B (UVB) irradiation or by disulfiram treatment in dermal fibroblasts. (a) Cells were left untreated (control) or were treated with 15 mJ/cm2 UVB irradiation in the absence (buffer) or presence of 10 μM of NAC, 3 μM of TTM, 1 μM of DPMQ, or 1 μM of DPMQ plus 3 μM of TTM (DPMQ + TTM), or in the absence of extracellular Ca2+ (Ca free). (b) Cells were left untreated (control) or were treated with 0.3 μM of disulfiram (DSF) in the absence (buffer) or presence of 10 μM of NAC or 3 μM of TTM, or in the absence of extracellular Ca2+ (Ca free). The extent of cellular ROS generated was measured by a FACSCalibur cell sorter 30 min after UVB irradiation or 24 hr after DSF treatment. The mean fluorescence intensity obtained using CellQuest Pro software in each group of UVB-irradiated cells or DSF-treated cells relative to that in control cells is shown in (c) and (d), respectively. The data are the mean ± SD for five independent experiments. ***p < 0.001 compared with control cells [Color figure can be viewed at wileyonlinelibrary.com]
Similar results were observed when the mitochondrial membrane potential (Figure 3) and caspase-3 activity (Figure 4) were measured. Both UVB irradiation (Figures 3a and 4a) and disulfiram treatment (Figures 3b and 4b) reduced the mitochondrial membrane potential and activated caspase-3, and these effects were reversed by N-acetylcysteine cotreatment. In addition, these two effects of UVB irradiation were inhibited by the lack of Ca2+, but were unaffected by TTM cotreatment. The effects of UVB irradiation were also inhibited by cotreatment of cells with DPMQ with or without TTM (Figures 3a and 4a). In contrast, these effects of disulfiram were unaffected by the absence of Ca2+, but were inhibited by TTM cotreatment (Figures 3b and 4b). These results show that UVB irradiation–induced ROS generation requires Ca2+, but not Cu2+, whereas disulfiram-induced ROS generation requires Cu2+, but not Ca2+. Increased ROS generation subsequently leads to mitochondrial dysfunction, caspase-3 activation, and apoptosis, and DPMQ inhibits UVB irradiation–induced ROS generation and apoptosis.
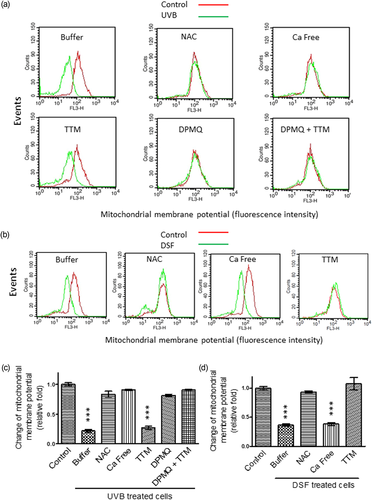
Effect of N-acetylcysteine (NAC), Ca2+ deprivation, tetrathiomolybdate (TTM), or (E)-2-(4-(dimethylamino)phenylimino)methyl)quinolin-8-ol (DPMQ) on mitochondrial membrane potential reduction induced by ultraviolet B (UVB) irradiation or by disulfiram treatment in dermal fibroblasts. (a) Cells were left untreated (control) or were treated with 15 mJ/cm2 UVB irradiation in the absence (buffer) or presence of 10 μM of NAC, 3 μM of TTM, 1 μM of DPMQ, or 1 μM of DPMQ plus 3 μM of TTM (DPMQ + TTM), or in the absence of extracellular Ca2+ (Ca free). (b) Cells were left untreated (control) or were treated with 0.3 μM of disulfiram (DSF) in the absence (buffer) or presence of 10 μM of NAC or 3 μM of TTM, or in the absence of extracellular Ca2+ (Ca free). The amplitude of the mitochondrial membrane potential was measured by a FACSCalibur cell sorter 12 hr after UVB irradiation or 24 hr after disulfiram treatment. The mean fluorescence intensity obtained using CellQuest Pro software in each group of UVB-irradiated cells or DSF-treated cells relative to that in control cells is shown in (c) and (d), respectively. The data are the mean ± SD for five independent experiments. ***p < 0.001 compared with control cells [Color figure can be viewed at wileyonlinelibrary.com]

Effect of N-acetylcysteine (NAC), Ca2+ deprivation, tetrathiomolybdate (TTM), and (E)-2-(4-(dimethylamino)phenylimino)methyl)quinolin-8-ol (DPMQ) on caspase-3 activation induced by ultraviolet B (UVB) irradiation or by disulfiram treatment in dermal fibroblasts. (a) Cells were left untreated (control) or were treated with 15 mJ/cm2 UVB irradiation in the absence (buffer) or presence of 10 μM of NAC, 3 μM of TTM, 1 μM of DPMQ, or 1 μM of DPMQ plus 3 μM of TTM (DPMQ + TTM), or in the absence of extracellular Ca2+ (Ca free). (b) Cells were left untreated (control) or were treated with 0.3 μM of disulfiram (DSF) in the absence (buffer) or presence of 10 μM of NAC or 3 μM of TTM, or in the absence of extracellular Ca2+ (Ca free). The activity of caspase-3 was measured by a FACSCalibur cell sorter 24 hr after both treatments. The mean fluorescence intensity obtained using CellQuest Pro software in each group of UVB-irradiated cells or DSF-treated cells relative to that in control cells is shown in (c) and (d), respectively. The data are the mean ± SD for four independent experiments. ***p < 0.001 compared with control cells [Color figure can be viewed at wileyonlinelibrary.com]
3.3 UVB irradiation activates 12-lipoxygenase, whereas DPMQ inhibits the TRPV1 channel
It has been shown that UVB irradiation increases the expression and activity of epidermal lipoxygenases (Burrall & Ziboh, 1986; Rhodes et al., 2009) and that both 12-hydroperoxyeicosatetraenoic acid, generated from arachidonic acid by 12-lipoxygenase, and its metabolite 12-HETE are agonists of the TRPV1 channel (Gregus et al., 2012; Xie & Wang, 2011). We therefore examined whether UVB irradiation activated 12-lipoxygenase in dermal fibroblasts using a new colorimetric method (Lu et al., 2013). Aarachidonic acid was added to a cuvette, followed by cell lysate from control or UVB-irradiated cells; then, after incubation for 1 min, a mixture of ferrous sulfate and ammonium thiocyanate was added and the formation of red ferrithiocyanate was monitored for 3.5 min at 480 nm, reflecting the activity of 12-lipoxygenase. Figure 5a shows that lysates from UVB-irradiated cells resulted in a significantly greater increase in absorbance compared with those from control cells. This result shows that 12-lipoxygenase in dermal fibroblasts was activated by UVB irradiation. We then examined whether 12-HETE caused an increase in the intracellular Ca2+ concentration, measured using Fluo-3, and the effect of DPMQ. As shown in the top row of traces in Figure 5b, the intracellular Ca2+ concentration increased after the addition of 12-HETE and this effect was blocked by the lack of extracellular Ca2+ or in the presence of the TRPV1 channel blocker capsazepine, showing that the 12-HETE-induced Ca2+ concentration increase was due to Ca2+ influx via activation of the TRPV1 channel. The increase was also inhibited in the presence of DPMQ or DPMQ plus TTM, but was insensitive to disulfiram. Similar results were observed using capsaicin to activate the TRPV1 channel (Figure 5b, middle traces). To determine whether the effect of DPMQ was due to chelation of intracellular Ca2+ or inhibition of the TRPV1 channel, we examined its effect on the bradykinin-induced Ca2+ increase. Bradykinin, a G protein–coupled receptor agonist, induces an increase in Ca2+ by activating Ca2+ release from intracellular Ca2+ stores via inositol 1,4,5-trisphosphate receptor activation (Chin, Hwang, & Chueh, 2002). As shown in the bottom traces in Figure 5b, bradykinin induced an increase in the intracellular Ca2+ concentration and the amplitude of the increase was unaffected by the lack of extracellular Ca2+ or the presence of capsazepine, DPMQ, DPMQ plus TTM, or disulfiram, thus ruling out the possibility that DPMQ acts as a Ca2+ chelator and suggesting that it inhibits the TRPV1 channel. It has been shown that UVB irradiation activates the TRPV1 channel in HaCaT keratinocytes (Huang, Ma, Liu, Chen, & Chueh, 2017; Lee et al., 2009). We therefore examined whether DPMQ had an inhibitory effect on TRPV1 channel activation in HaCaT cells. As shown in Figure 5c, in HaCaT cells, 12-HETE (top left traces) and capsaicin (bottom left traces) induced an intracellular Ca2+ increase via the TRPV1 channel, whereas bradykinin (top right traces) and ATP (bottom right traces) induced increases via, respectively, the inositol 1,4,5-trisphosphate receptor or ATP-gated channels. DPMQ or DPMQ plus TTM treatment of cells inhibited the 12-HETE- or capsaicin-induced intracellular Ca2+ increase, but had no effect on that induced by bradykinin or ATP, further suggesting that DPMQ specifically inhibits the TRPV1 channel. To directly confirm that DPMQ did not act as Ca2+chelator, we next measured its ability to bind Ca2+ or Cu2+ using UV–vis spectroscopy (Fu et al., 2016; Sharma et al., 2012). As shown in Figure 5d, in the absence of metal, the spectrum of DPMQ showed peaks at 268 and 417 nm. When 50 μM of CaCl2 was then added to the cuvette containing DPMQ (DPMQ + 50 μM of Ca2+), the spectrum was not altered, whereas, when 50 μM of CuCl2 was added to the mixture of DPMQ and Ca2+ (DPMQ + 50 μM of Ca2+ + 50 μM of Cu2+), the absorption peaks shifted from 268 and 417 nm to 305 and 504 nm, showing negligible formation of the DPMQ–Ca2+ complex, but formation of the DPMQ–Cu2+ complex. In the converse experiment, the shift of the two peaks was observed when Cu2+ was first added to DPMQ and was unaffected by the subsequent addition of Ca2+ (data not shown). In addition, no binding of Ca2+ to DPMQ was observed at a CaCl2 concentration as high as 0.5 mM (data not shown), ruling out weak binding. Taken together, our data suggest that 12-lipoxygenase is activated by UVB irradiation and that DPMQ inhibits the TRPV1 channel.
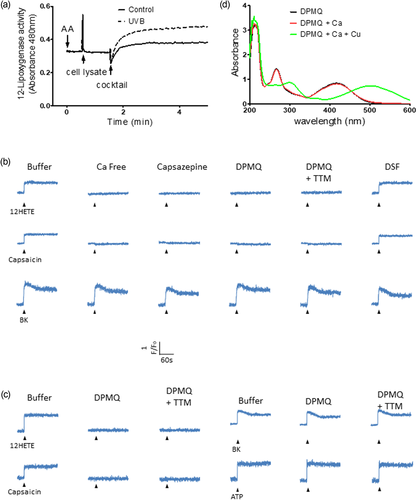
Ultraviolet B (UVB) irradiation stimulates lipoxygenase in fibroblasts and (E)-2-(4-(dimethylamino)phenylimino)methyl)quinolin-8-ol (DPMQ) inhibits TRPV1 channels in fibroblasts and keratinocytes. (a) Aliquots of cell lysate (about 0.5 mg of protein) isolated from control or UVB (15 mJ/cm2)-irradiated fibroblasts were added to a cuvette containing the substrate of lipoxygenase (100 μM of arachidonic acid). After 1 min of an enzymatic reaction, the products were quantified by adding a reactant cocktail of colorimetric reaction. Aliquots of fluo-3-loaded dermal fibroblasts (b) or HaCaT keratinocytes (c) were suspended in Ca2+-free Locke’s buffer (LB; Ca free) or in normal LB in the absence (buffer) or presence of 30 μM of capsazepine, 1 μM of DPMQ, 1 μM of DPMQ plus 3 μM of tetrathiomolybdate (TTM; DPMQ + TTM), or 0.3 μM of disulfiram as indicated. Fluorescence intensity was measured as the index of change in the cytosolic Ca2+ level (F/F0). 12-HETE (10 μM), capsaicin (30 μM), bradykinin (BK; 1 μM), or ATP (30 μM) was added as indicated by arrowheads. For simplicity, the name of the same agonist added was omitted, except the first leftmost one. (d) UV spectra of 50 μM of DPMQ alone, treated with 50 μM of CaCl2 (DPMQ + Ca), followed by 50 μM of CuCl2 (DPMQ + Ca + Cu) [Color figure can be viewed at wileyonlinelibrary.com]
3.4 UVB irradiation decreases Nrf2 levels via TRPV1 channel activation
It is possible that the TRPV1 channel may play a critical role leading to apoptosis after UVB irradiation and that DPMQ inhibits the TRPV1 channel to protect fibroblasts from UVB irradiation–induced cytotoxicity. If this were the case, inhibition of the TRPV1 channel should block UVB irradiation–induced cytotoxicity. As shown in Figure 6a, in the presence of the TRPV1 channel blocker capsazepine, UVB irradiation failed to increase ROS generation 30 min later or decrease the mitochondrial membrane potential or activate caspase-3 after 24 hr incubation. Since cellular oxidative status is regulated by Nrf2, we next examined the cellular Nrf2 distribution before and after UVB irradiation with or without TRPV1 channel inhibition by immunofluorescence staining and immunoblotting. As shown in Figure 6b, in control cells, Nrf2 was present in the cytoplasm and the nucleus and cellular Nrf2 levels decreased after UVB irradiation as Nrf2 staining in both the cytosol and the nucleus was markedly decreased after 24 hr of incubation. Cotreatment of cells with DPMQ during, and after, UVB irradiation prevented the decrease in Nrf2 levels and stimulated translocation of Nrf2 into the nucleus, and these effects were unaffected by the addition of TTM. Identical results were obtained in the absence of extracellular Ca2+ or in the presence of capsazepine during and after UVB irradiation. The quantitative data for the fluorescence intensity of nuclear Nrf2 in control and UVB-irradiated cells under different incubations are summarized in Figure 6c. These results show that Nrf2 levels and Nrf2 translocation into the nucleus were only reduced by UVB irradiation when the TRPV1 channel was functionally active. Similar results were observed when Nrf2 levels in cell lysates were measured by immunoblotting; Figure 6d shows that Nrf2 levels were unaffected by UVB irradiation if 12-lipoxygenase was inhibited by baicalein, the TRPV1 channel was inhibited by either DPMQ plus TTM or capsazepine, or when cytosolic Ca2+ was chelated by the cell-permeant chelator BAPTA during UVB irradiation. Consistent with this, as shown in Figure 6e, the expression of the Nrf2-targeting genes HO-1 and NQO1 was decreased after UVB irradiation and this effect was blocked by DPMQ with or without TTM during UVB irradiation.
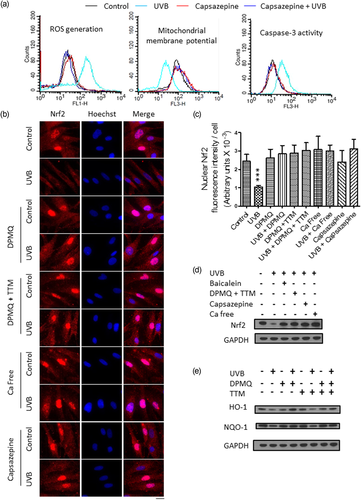
Ultraviolet B (UVB) irradiation induces reactive oxygen species (ROS) generation and Nrf2 degradation via TRPV1 channel activation. (a) Cells were left untreated (control) or were treated with 15 mJ/cm2 UVB irradiation in the absence or presence of 30 μM of capsazepine as indicated. Cellular ROS generation was measured after 30 min incubation, whereas the mitochondrial membrane potential and caspase-3 activity were measured after 24 hr incubation by a FACSCalibur cell sorter. (b) Cells grown in coverslips were left untreated (control) or treated with 15 mJ/cm2 UVB irradiation in the absence or presence of 1 μM of (E)-2-(4-(dimethylamino)phenylimino)methyl)quinolin-8-ol (DPMQ), 1 μM of DPMQ plus 3 μM of tetrathiomolybdate (TTM; DPMQ + TTM), or 30 μM of capsazepine, or in the absence of extracellular Ca2+ (Ca free). After 24 hr incubation, localization of Nrf2 was visualized by immunofluorescence staining with nuclei counterstained by Hoechest 33258; the overlay of the two images is shown on the right. Scale bar = 30 μm. (c) The nuclear Nrf2 intensity per cell shown in (b) was quantified using ImageJ (NIH, Bethesda, MD) in 35 randomly chosen fields in each dish. The data are the mean ± SD for 269–318 cells from one representative experiment. ***p < 0.001 compared with control cells. (d,e) Cells were left untreated or treated with 15 mJ/cm2 UVB irradiation in the absence or presence of 8 μM of baicalein, 1 μM of DPMQ plus 3 μM of TTM, or 30 μM of capsazepine, or in the absence of extracellular Ca2+ plus 2 μM of BAPTA-AM (Ca free) as indicated. Cells lysates were prepared after 24 hr incubation and immunoblotted with antibodies against Nrf2 or GAPDH (d), HO-1, NQO1, or GAPDH (e). GAPDH was used as the loading control [Color figure can be viewed at wileyonlinelibrary.com]
3.5 UVB irradiation–induced cytotoxicity is blocked by silencing of the TRPV1 channel
We next examined whether silencing of the TRPV1 channel inhibited UVB irradiation–induced cytotoxicity. As shown in Figure 7a, transfection with siRNA targeting TRPV1 almost completely silenced TRPV1 expression as the intensity of the band of the TRPV1 channel on immunoblots was markedly reduced by the TRPV1-targeting siRNA (si TRPV1), but not by control siRNA (si control). As shown in Figure 7b, in control siRNA cells, the intracellular Ca2+ concentration increased after the addition of capsaicin to control cells, whereas, in UVB-irradiated cells, a higher basal Ca2+ concentration with spontaneous Ca2+ waves was observed; however, none of these effects were observed in the TRPV1 channel-silenced cells. In the absence of extracellular Ca2+, no increase in Ca2+ was induced by capsaicin in control siRNA cells and TRPV1 channel-silenced cells. In fibroblasts silenced for the TRPV1 channel, the UVB irradiation–induced decrease in cell numbers (Figure 7c,d) and increase in ROS generation (Figure 7e), caspase-3 activation (Figure 7f), and apoptosis (Figure 7g) observed in si control cells were all essentially abolished. Thus, UVB irradiation–induced Ca2+ influx via the TRPV1 channel is responsible for UVB irradiation–induced cytotoxicity.
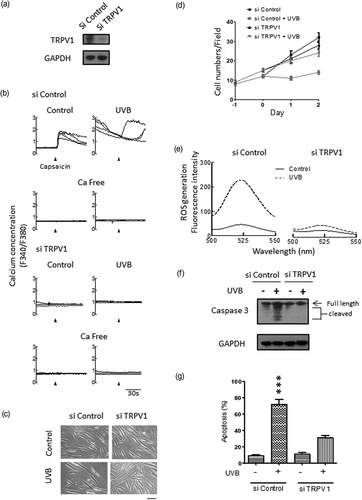
Effect of TRPV1 channel knockdown on ultraviolet B (UVB) irradiation–induced cytotoxicity in dermal fibroblasts. Cells were treated with control (si control) or TRPV1 channel siRNA (si TRPV1) as described in Section 2. (a) The si control and si TRPV1 cells were immunoblotted with the TRPV1 antibody. (b) The si control and si TRPV1 cells were left untreated (control) or treated with 15 mJ/cm2 UVB irradiation in the presence or absence of extracellular Ca2+ (Ca free) as indicated. Cytosolic Ca2+ change in a single cell was measured immediately after UVB irradiation with fura-2. Capsaicin (30 μM) was added to cells as indicated by the arrowheads. Four cells per coverslip were selected for experiments and shown. (c–g) Twenty-four hours after plating, the si control and si TRPV1 cells were left untreated (control) or were treated with 15 mJ/cm2 UVB irradiation as Day 0 in (d). The morphology of cells after 48 hr incubation is shown in (c) and the statistical results for the number of cells attached to the dish are shown in (d). Reactive oxygen species (ROS) generation (e) was measured after 30 min, whereas caspase-3 activation (f) and apoptosis (g) were measured after 24 hr incubation. UVB irradiation–induced increases in cytosolic Ca2+, ROS generation, caspase-3 activation, and apoptosis were all inhibited by TRPV1 silencing. The data are the mean ± SD for three independent experiments in (d) and (g). ***p < 0.001 compared with control cells. Scale bar = 100 μm in (c)
4 DISCUSSION
Although Cu2+ is highly electrochemically active and free Cu2+ ions would aggravate cellular oxidative stress, this is avoided because Cu2+ is always bound to proteins within the cell (Denoyer, Masaldan, La Fontaine, & Cater, 2015). Thus, free intracellular Cu2+ ion levels are maintained very low (Rae, Schmidt, Pufahl, Culotta, & O’Halloran, 1999). Although DPMQ acted as a Cu2+ ionophore, it protected cells against the effects of UVB irradiation, including apoptosis, and this effect was unaffected by the Cu2+ chelator TTM, showing that Cu2+ was not required or was bound to DPMQ for the protective effect. UVB irradiation activated 12-lipoxygenase (Figure 5a) and 12-HETE, the product of 12-lipoxygenase, activated the TRPV1 channel (Figure 5b), and then the increased cytosolic Ca2+ concentration aggravated oxidative stress and activated the mitochondrial apoptotic death pathway (Figures 2-4). DPMQ did not bind and scavenge Ca2+ (Figure 5d), but inhibited the TRPV1 channel (Figure 5b) to protect cells from UVB irradiation–induced damage. In addition, DPMQ may decrease ROS generation by its known antioxidant property (Fu et al., 2016). In contrast, disulfiram-induced cytotoxicity depends on Cu2+. The fact that the cytosolic free Cu2+ ion concentration is negligible (Rae et al., 1999) suggests that the disulfiram–Cu2+ coordinated complex, rather than disulfiram or Cu2+ itself, interacts with target proteins to cause cell death. A simplified depiction of the proposed mechanism for the protective effect of DPMQ against UVB irradiation–induced cytotoxicity and the cytotoxic effect of disulfiram is shown in Figure 8.
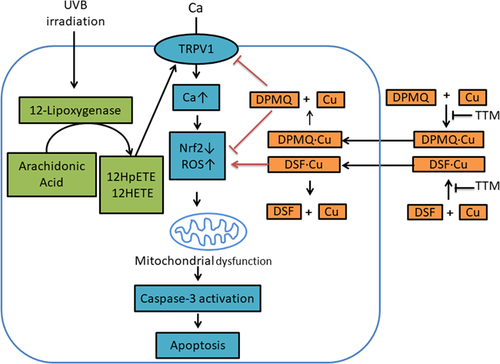
Simplified depiction of the proposed mechanism involved in the protective effect of (E)-2-(4-(dimethylamino)phenylimino)methyl)quinolin-8-ol (DPMQ) against ultraviolet B (UVB) irradiation and the proapoptotic effect of disulfiram. Treatment with UVB irradiation results in increased reactive oxygen species (ROS) generation and decreased Nrf2 levels by a mechanism involving lipoxygenase stimulation and TRPV1 channel activation. Increased ROS results in mitochondrial dysfunction and apoptosis. DPMQ inhibits TRPV1 channel and scavenges ROS, and hence protects cells against UVB irradiation–induced cytotoxicity, whereas disulfiram induces copper influx and increases ROS generation, and therefore causes apoptosis [Color figure can be viewed at wileyonlinelibrary.com]
The TRPV1 channel is a member of a nonselective cation channel family and is the primary cellular sensor for the thermal and UV radiation after exposure to the sun (Lee, Kang, & Chung, 2012). Activation of the TRPV1 channel induces apoptosis in different types of cells, and distinct mechanisms of apoptosis have been proposed to be coupled to TRPV1 occupancy, that is, an increased cytosolic Ca2+ concentration (Agopyan, Head, Yu, & Simon, 2003; Xie et al., 2016), activation of p38 MAPK (Amantini et al., 2007), endoplasmic reticulum stress (Thomas et al., 2012), increased generation of ROS (Farfariello, Amantini, & Santoni, 2012), and autophagy (Chien et al., 2013). Our results (Figures 2a, 3a, and 4a) showed that, in control cells, ROS generation, mitochondrial membrane potential, and caspase-3 activity were not altered after the removal of extracellular Ca2+, whereas, in UVB-irradiated cells, the UVB-induced increase in ROS generation, decrease in mitochondrial membrane potential, and increase in caspase-3 activity were abolished. These results suggested that a Ca2+ influx mechanism, inactive in the basal state, is activated by UVB irradiation. We therefore examined the possible Ca2+ influx mechanisms that could be activated by UVB irradiation, leading to apoptosis. Our results support a scheme involving lipoxygenase stimulation and TRPV1 channel activation. UVB irradiation–induced apoptosis was also blocked by capsazepine (Figure 6a) or silencing of the TRPV1 channel (Figure 7), further supporting the idea that the TRPV1 channel is activated by UVB and is the primary trigger in UVB irradiation–induced apoptosis. Thus, DPMQ blocks the TRPV1 channel and inhibits UVB-induced apoptosis.
The transcription factor Nrf2 is the key regulator of cellular oxidative stress. In the basal state, Nrf2 is degraded via the proteasomal pathway after ubiquitination, whereas, under oxidative stress, it dissociates from Keap1 and is stabilized, translocates into the nucleus, and initiates the transcription of antioxidant enzymes, including HO-1, NQO1, and glutathione synthetase, which mitigate the cellular oxidative stress (reviewed in Boutten, Goven, Artaud-Macari, Boczkowski, & Bonay, 2011; Zhang et al., 2013). Previous studies (Cao et al., 2017; Santofimia-Castano et al., 2015) have shown that an increase in intracellular Ca2+ levels is indispensable for increased activation of the Nrf2-ARE signaling pathway and that increased intracellular Ca2+ levels stabilize Nrf2. However, we found that the UVB irradiation–induced decrease in Nrf2 levels required Ca2+ (Figure 6). A newly synthesized calcium stabilizer, ITH14001, a hybrid of the L-type Ca2+ channel blocker nimodipine and the mitochondrial Na+/Ca2+ exchanger inhibitor CGP37157, prevents cytosolic Ca2+ overload by inhibiting both parental targets and also activates the Nrf2-ARE transcription pathway and increases HO-1 expression (Buendia et al., 2017). This suggests that there may be a Ca2+-dependent Nrf2 degradation mechanism. Apart from Keap1-dependent degradation, Nrf2 can also be degraded via the SCF/β-TrCP-dependent proteasomal pathway. Phosphorylation of Ser342 and Ser347 in the Neh6 domain of Nrf2 catalyzed by GSK3β leads to ubiquitination by the β-TrCP–cullin-1 E3 ligase complex (Rada et al., 2011). In pheochromocytoma PC12 cells, Ca2+-dependent dephosphorylation is involved in a rotenone-induced cytosolic Ca2+ increase and activation of GSK3β, which then stimulates phosphorylation and aggregation of α-synuclein (Yuan et al., 2015). Thus, the Ca2+-GSK3β-Nrf2-β-TrCP–cullin-1 pathway may explain Ca2+-dependent Nrf2 degradation.
We observed that the increased ROS generation observed after UVB irradiation was also closely regulated by the increased intracellular Ca2+ concentration (Figure 2). Ca2+ signaling regulates cellular ROS generation by NADPH oxidases and mitochondria (Hempel & Trebak, 2017). It is known that NADPH oxidase isoforms Nox5, Duox1, and Duox2 are activated by Ca2+ and produce superoxide anion (Bánfi et al., 2004; Rigutto et al., 2009). In addition, the mitochondrial tricarboxylic acid cycle and respiration chain are accelerated when intracellular Ca2+ levels are increased and may, in turn, drive ROS production in the mitochondria (Rizzuto, De Stefani, Raffaello, & Mammucari, 2012). In NG108-15 neuroblastoma × glioma hybrid cells, treatment of cells with hydrogen peroxide increases ROS generation via 12-lipoxygenase (Yeh et al., 2015). In the current study, UVB irradiation activated 12-lipoxygenase, and 12-HETE, the product of 12-lipoxygenase, activated the TRPV1 channel and increased cytosolic Ca2+ levels (Figures 5 and 7). 12-Lipoxygenase contains nonheme iron. It is feasible that more ROS are generated via the Fenton reaction when 12-lipoxygenase is activated. Thus, in the current study, 12-lipoxygenase and the TRPV1 channel might be responsible for the UVB irradiation–induced ROS generation.
DPMQ inhibited the Ca2+ increase induced by capsaicin or 12-HETE, but not that induced by bradykinin or ATP (Figure 5b), showing that DPMQ specifically inhibits the TRPV1 channel. The mechanism by which DPMQ blocks the TRPV1 channel is unclear. In human vascular smooth muscle cells and cardiomyocytes, bioavailable Cu2+ is increased after acute treatment with the Cu2+ ionophore CuII ATSM. After binding Cu2+, Cu2+ chaperone DJ-1 then displaces Keap1 from Nrf2, thus stabilizing Nrf2, and also binds to superoxide dismutase 1 and activates it by transfer of Cu2+ (Srivastava et al., 2016). In HeLa cells, the histamine-induced increase in Ca2+ levels caused by the triggering of Ca2+ release from the endoplasmic reticulum via inositol 1,4,5-trisphosphate receptor is mitigated by DJ-1 overexpression, which leads to increased mitochondrial Ca2+ uptake by increasing contact sites between the endoplasmic reticulum and the mitochondria (Ottolini, Cali, Negro, & Brini, 2013). Furthermore, because DJ-1 enhances the expression of two uncoupling proteins (UCP4 and UCP5) in the mitochondria, the mitochondrial oxidative stress induced by the activation of the L-type Ca2+ channel is attenuated by DJ-1 (Guzman et al., 2010). Thus, DJ-1 shapes cytosolic Ca2+ increases (Shtifman, Zhong, Lopez, Shen, & Xu, 2011). In our study, it is possible that DPMQ may not directly inhibit the TRPV1 channel, but may enhance DJ-1 activity by increasing bioavailable Cu2+ to lower cytosolic Ca2+ levels.
Taken together, our results suggest a Ca2+-dependent Nrf2 degradation mechanism. Apart from its inhibitory effect on Nrf2 degradation by blockade of the TRPV1 channel, DPMQ may stabilize Nrf2 by carrying the Cu2+ ion into the cell. Activation of the TRPV1 channel plays a pivotal role in skin photoaging and nociception transduction after sun exposure (Lee et al., 2012). DPMQ may therefore have therapeutic implications for the treatment of free radical-induced diseases, counteracting photoaging, and alleviating pain.
ACKNOWLEDGMENTS
We thank Dr. Thomas Barkas for helpful discussion. Contract grant sponsor: Chi Mei Medical Center, Taiwan, Republic of China; Contract grant number: CMNDMC10605. Contract grant sponsor: Ministry of National Defense, Medical Affairs Bureau, Taiwan, Republic of China; Contract grant number: MAB-106-073.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.