Retracted: Effect of microRNA-186 on oxidative stress injury of neuron by targeting interleukin 2 through the janus kinase-signal transducer and activator of transcription pathway in a rat model of Alzheimer’s disease
Abstract
Recent studies have proposed that microRNAs (miR) function as novel diagnostic and prognostic biomarkers and therapeutic targets in Alzheimer’s disease (AD), a common disease among the elderly. In the current study, we aim to explore the effect of miR-186 on oxidative stress injury of neuron in rat models of AD with the involvement of the interleukin-2 (IL2) and the Janus kinase/signal transducers and activators of transcription (JAK–STAT) pathways. AD rat models were established, and dual-luciferase reporter assay and online software were used to confirm the targeting relationship between miR-186 and IL2. Immunohistochemistry was used evaluating the positive rate of IL2. Afterward, to define the role of miR-186 in AD, miR-186, IL2, and JAK–STAT related protein (JAK2, STAT3) expressions were quantified. Cell proliferation was measured by 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyl tetrazolium bromide, and cell apoptosis was detected by flow cytometry. We observed downregulated miR-186 and IL2 and upregulated JAK–STAT signaling pathway related genes in AD. The overexpression of miR-186 was shown to significantly promote cell proliferation while suppressing cell apoptosis along with the expression of the IL2 and JAK–STAT signaling pathway related protein. Collectively, the key findings obtained from the current study define the potential role of miR-186 as an inhibitor of AD development by downregulation of IL2 through suppression of the JAK–STAT signaling pathway.
1 INTRODUCTION
Alzheimer’s disease (AD) is regarded as a chronic neurodegenerative disorder. It is considered to be the most common cause of dementia among aging people (Giri, Shah, Upreti, & Rai, 2017). AD is usually characterized by aggregations and depositions of many misfolded proteins and progressive cognitive dysfunction, which eventually lead to dementia and mortality (Bertram, Lill, & Tanzi, 2010). Statistics have shown that there were 24.3 million people with dementia around the world in 2001, and it is predicted that the number will rise to 42.3 million in 2020 and to 81.1 million by 2040 (Ballard et al., 2011). A previous study indicated that the major cause of AD pathogenesis was abnormal aggregation and clearance of Aβ by apolipoprotein E, and age, race, and region could significantly influence the morbidity of AD (Kim, Basak, & Holtzman, 2009). Recently, there has been a controversy over neurodegenerative disorders: whether oxidative stress (OS) is the reason or the consequence of the changes that contributes to neurodegeneration (Manzanero, Santro, & Arumugam, 2013). In this context, a research demonstrated that microRNAs (miRs) play an important role in AD incidence and progression (Lukiw, Andreeva, Grigorenko, & Rogaev, 2012).
miRs are endogenous noncoding RNAs that are approximately 22 nucleotides in length, and several miRNAs have been reported to be associated with various diseases like cancer, hematopoietic diseases, and skeletal disorders (Calin et al., 2002, Du et al., 2014; Goldberger & Hunter, 2009; Waki et al., 2015). A previous research pointed out that miR-186 is a novel tumor suppressor gene (Wang et al., 2017). miR-186 is also found to suppress cell proliferation in human esophageal squamous cell carcinoma (He et al., 2016) and to suppress aerobic glycolysis in gastric cancer through hypoxia inducible factor-1 alpha (HIF-1α) regulation (Nie, Liu, & Zhang, 2016). A previous study also revealed that miR-186 was decreased in the aged brain and suppressed β-secretase (BACE1) expression, which suggested that miR-186 might be one of the putative molecular links between aging and AD development (Kim et al., 2016). The association between miR-155 and interleukin-2 (IL2) has been found with sound evidence (Lashine, Salah, Aboelenein, & Abdelaziz, 2015). IL2 is a pleiotropic cytokine produced after antigen activation and plays a pivotal role in immune response (Liao, Lin, & Leonard, 2013). Several interleukin members have been proposed to be involved in AD progression (Dursun et al., 2015; L. Liu & Chan, 2014; Y. Liu et al., 2014). For instance, a prior study indicated that IL2 deficiency causes hippocampal modifications and dysfunction of learning and memory ability in mice with AD (Alves et al., 2017). IL2 has a correlation with Janus kinase/signal transducers and activators of transcription (JAK/STAT) that, as a former study suggested, activation of the JAK–STAT pathway results in the transcription of target genes, which influences IL2-dependent biologic actions (Kovanen et al., 2005). The JAK/STAT pathway is regarded to be necessary for various developmental and homeostatic processes like hematopoiesis, immune cell development, and organogenesis (Harrison, 2012). Moreover, the JAK/STAT3 pathway is a common inducer of astrocyte reactivity in AD (Ben Haim et al., 2015). From all of the above, we speculate that there may be an effect of miR-186 on OS injury of neuron by targeting IL2 through the JAK–STAT pathway in AD rats.
2 MATERIAL AND METHODS
2.1 Ethical statements
All animal experiments in this study were in line with the Requirements and Regulations for Animal Experiments of the National Health Ministry (Article No. 55, 2001) and the principles of the Ethics Committee of the Affiliated Municipal Hospital of Xuzhou. All efforts were made to minimize the pain of the animals.
2.2 Study subjects
A total of 72 Sprague–Dawley (SD) male rats (12 months, 290–360 g) and some healthy SD rats born within 24 hr (5–8 g; both purchased from the Experimental Animal Center of the Southern Medical University, Guangzhou, China) were selected 1 week before the experiment to adapt to the environment. The SD male rats were divided into three groups (each containing 24 rats): the normal group, the sham group, and the model group. With free access to water and feed, the rats were housed at 22–24°C with 50%–60% humidity and a 12 hr light/12 hr dark cycle.
2.3 Dual-luciferase reporter assay
TargetScan (http://www.targetscan.org/vert_71/) was chosen for the analysis of the target gene of miR-186 (Accession of miR-186: MI0000931; ID: rno-mir-186; Linsen, de Wit, de Bruijn, & Cuppen, 2010; X. He, Zhang, Liu, & Pan, 2007). Human embryonic kidney 293T (HEK-293T) cells (AT-1592; ATCC, Manassas, VA) were incubated in a 24-well plate and cultured for 24 hr. Total RNA was extracted and reversely transcribed into complementary DNA (cDNA). Then, the full-length sequence in the 3′-untranslated region (3′-UTR) of the IL2 was obtained with polymerase chain reaction (PCR) amplification using cDNA as a template. The primers were designed and synthesized according to the IL2 sequence and amplified using the genome extracted from HEK-293T cells as a template. After amplification, the product was cloned and detached to the downstream of the luciferase reporter gene in the pmiRRB vector for the IL2 reporter gene vector (pmiRRB-IL2-3′-UTR). Then, pmiRRB-IL2-3′-UTR was cotransfected with a miR-186 mimic, a miR-186 inhibitor or negative control (NC) into the HEK-293T cells. After 48 hr of transfection, the medium was removed, and the cells were washed two times with phosphate buffer saline (PBS). Then the cells were collected and lysed. The activity of luciferase was detected by a dual-luciferase reporter assay system (Dual-Luciferase® Reporter Assay System; E1910; Promega, Madison, WI). In every 10 μl cell sample, 50 μl of a firefly luciferase working fluid was added for detecting the activity of firefly luciferase and 50 μl of a renilla luciferase working fluid was added for detecting the activity of renilla luciferase. The ratio of the firefly luciferase activity to the renilla luciferase activity served as the relative luciferase activity. The experiment was carried out in triplicate.
2.4 Establishment of the AD rat model
Pentobarbital sodium (300 μl/100 g) purchased from Jiangsu Hengrui Medicine Co., Ltd. (Jiangsu, China) was injected into the abdominal cavity of the SD rats in the model group. The rats were fixed on a stereotaxic apparatus with the hair near the head shaved, and disinfected with the skin cut. Next, according to the map of the stereotaxic apparatus, the craniums were drilled at 2.5 mm behind the anterior fontanel and at 2.5 mm near both sides of the midline. Then, the injector needle was pushed vertically from the surface of the brain for approximately 2.9 mm (injection rate: ~0.2 μl/min), and 1 μl Aβ1–42 amyloid protein (amyloid beta peptide fragment 1–42, Aβ1–42, 5 μg/μl; Sigma-Aldrich Chemical Company, St Louis, MO) was injected into the rats. The needle was slowly withdrawn 5 min later. Then the skin was sutured, and 20,000 IU penicillin (19985; Shanghai Runwell technology Co., Ltd., Shanghai, China) was smeared locally to prevent infection. The sham group and the model group were subjected to the same surgical procedure. Both sides of the hippocampus were injected with an equal volume of 0.9% sterile saline. The normal group was fed regularly without any treatment and used in subsequent experiments after 4 weeks. The Morris water maze (MWM) was used to determine whether the model was successfully established, and the successfully modeled rats were assigned in the model group and normal rats were used as control for following experiments (Coleman, Liang, Patel, Ali, & Mukherjee, 2017; Yin et al., 2013).
2.5 Morris water maze
The place navigation experiment was conducted on all enrolled rats. This experiment consisted of space exploration, and hidden platform. The detection system consisted of a removable transparent platform, an automatic recording system, and a circular pool (120 cm in diameter, 50 cm in height, and 30 cm in depth; 2 cm above the platform). Water was maintained at room temperature. The platform was put in the first quadrant, and two entry points with the same distance from the platform were selected in the two opposite sides of the platform. The rats were put into the maze 1 day before the experiment to adapt to the environment. During the test, the position of the platform was fixed. The rats faced the pool wall and were slowly placed in water from three starting points (except the entry point in the fourth quadrant) during the training. The recording device was started, the escape latency was recorded, and the rats were allowed to stay on the platform for 10 s. If the rats could not find the platform within 60 s, the latency was recorded as 60 s, and then rats were trained in the next starting point. The first training was when rats entered the pool from the three points respectively, and the mean latency was regarded as the escape latency of the navigation test. The spatial learning and memory abilities of rats were determined by the latency, and the shorter the latency: the better the spatial learning and memory abilities were. All rats were experimented before modeling, and 3 days after the training, the latency of rats was recorded two times/day for 5 days. The daily average latency was taken as the escape latency value of the day. Finally, based on the changes in the rats’ memory ability in each group measured by the latency time of rats in each group before and after modeling, it was determined whether the model was successfully established.
2.6 Hematoxylin–eosin staining
After that, six rats from each group were randomly selected and injected with pentobarbital sodium (300 μl/100 g) in the abdominal cavity. After the anesthesia, the thoracic cavity was cut open to expose the heart. Then, the right atrial appendages were cut after a pin was placed into the aorta from the left ventricle. Next, 100 ml of PBS was added to the rats and fixed with 100 ml of 4% paraformaldehyde, and the brains were collected immediately after the rats were euthanized. After being fixed in 4% paraformaldehyde for 8 hr, the tissues located from the chiasma opticum to the papillary were put into the embedding box, dehydrated with gradient ethanol (70%, 80%, 90%, 95%, and 100%, respectively) one time for 1 min, cleared with xylene two times (5 min each), dipped in wax, and embedded with paraffin. Hematoxylin–eosin (HE) staining was performed later. First, prepared sections (~4 μM) were dewaxed with xylene two times (5 min each), dehydrated with ethanol (100%, 95%, 80%, and 75%) for 1 min, respectively, and washed with running water for 2 min. Next, the sections were stained with hematoxylin for 2 min and washed with running water for 10 s, and the color was separated with 1% hydrochloric acid-ethanol for 10 s. Then, the sections were washed with distilled water for 1 min, stained with eosin for 1 min, and washed with 95% and 100% ethanol for two times (1 min each), respectively, after washing with distilled water for 10 s. Finally, the samples were sealed with neutral balsam after clearing with xylene. Sections were observed and photographed under a microscope (wi102502; Beijing Ruoshuihe Technology Co., Ltd., Beijing, China). The experiment was carried out in triplicate.
2.7 Immunohistochemistry
A total of six rats from each group were randomly selected and euthanized for hippocampus section preparation. The sections were baked in an incubator at 60°C for 1 hr. After that, sections were dewaxed with xylene in three cylinders for 30 min (each for 10 min), dehydrated with gradient ethanol (95%, 80%, and 75%) in three cylinders for 1 min each, washed with running water for 1 min and then incubated in 3% H2O2 (84885; Sigma-Aldrich, San Francisco, CA) at 37°C for 30 min. After being washed with PBS, the sections were boiled in 0.01 M citric acid buffer solution at 95°C for 20 min and cooled to room temperature, washed with PBS, and sealed in normal goat serum at 37°C for 10 min. Subsequently, primary antibodies rabbit antimouse IL2 (1:500; ab92381; Abcam Inc., Cambridge, MA) and Cleaved-Caspase-3 (1:500; ab2302; Abcam Inc.) were added for incubation overnight at 4°C. After washing with PBS, the horseradish peroxidase (HRP)-labeled secondary antibody goat anti-rabbit (DF7852; Shanghai Yao Yun Biological Technology Co., Ltd, Shanghai, China) was added and incubated at room temperature for 30 min. Then, the sections were visualized with diaminobenzidine (DAB; ab64238; Abcam Inc.), restained with hematoxylin and mounted. PBS replaced the primary antibody as an NC. Positive cells/all cells >10% were recorded as positive (+); others were recorded as negative (−).
2.8 Activity of glutathione peroxidase, superoxide dismutase, lactic dehydrogenase and the levels of reactive oxygen species and malondialdehyde in hippocampus
A total of six rats from each group were randomly selected and euthanized for collecting hippocampus. The activity of superoxide dismutase (SOD) in hippocampus was determined by xanthine oxidase. The activity of glutathione peroxidase (GSH-PX) and the level of reactive oxygen species (ROS) were determined by the Fenton reaction. The level of malondialdehyde (MDA) was determined by thiobarbituric acid (TBA) assay. The activity of lactic dehydrogenase (LDH) was determined by enzyme-linked immunosorbent assay (ELISA). SOD Kit (Item No. A001), GSH-PX Kit (Item No. A005), LDH Kit (Item No. A020-2), ROS Kit (Item No. A003-2), and MDA Kit (Item No. A003-1) were purchased from Nanjing Jiancheng Bioengineering Company (Nanjing, Jiangsu, China).
2.9 Enzyme-linked immunosorbent assay
A total of six rats from each group were randomly chosen and euthanized for blood collection. The samples were detected by the ELISA kit (ml038325; Shanghai Meilian biotechnology company, Shanghai, China). The epidermal growth factor (EGF; ab84253; Abcam), platelet-derived growth factor (PDGF; ab51090; Abcam), interferon-γ (IFN-γ; ab113349; Abcam), and growth hormone (GH; ab52551; Abcam) antibodies were diluted to 1–10 μg/ml with buffer solution, 0.1 ml of which was then added to each well and reacted overnight at 4°C. The samples were washed with PBS-Tween20 (PBST) three times the next day, and then 0.1 ml diluted supernatant was added to the covered wells and incubated at 37°C for 1 hr. After being washed with PBST three times, the samples were added with 0.1 ml diluted enzyme labeled secondary antibody (Abcam Inc), incubated at 37°C for 35–60 min and washed. The samples were washed with ddH2O (PER 018-1; Beijing Dingguochangsheng Biotechnology Co., Ltd, Beijing, China) three times at least. Next, 0.1 ml temporary formulation tetramethylbenzidine (TMB; EL0001; InnoReagents, Zhejiang, China) was added to each well and cultured at 37°C for 10–30 min, and 50 μl of stop solution was added to terminate coloration. The optical density (OD) value of each well at the wavelength of 450 nm was determined within 20 min.
2.10 Isolation, purification, and culture of hippocampal neuronal cells
The neonatal SD rats were killed by immersion in iodine. The hippocampus was isolated from the brain under aseptic conditions and repeatedly washed in an iced glass dish containing with a dissection fluid, which could wash out the blood cells and other impurity cells. The brain tissues were fully cut up and added with 0.25% trypsin detached for 20–30 min, until the tissues became flocculent. The samples were fully percussed with a straw and filtered using a 200-mesh filter. After the filtration, the cells were centrifuged at 1,000 rpm for 10 min with the supernatant removed. The inoculum was added, and the cells were blown off. Then the cell density was calculated, and the cells were inoculated into a six-well culture plate at the density of 1 × 107/ml and incubated in a 5% CO2 incubator at 37°C. The previous culture solution was replaced by the hippocampal neuronal culture solution 24 hr later. After 3–4 days, 1 μg/μl cytarabine was added and the cytarabine was removed 48 hr later by replacing the solution. Then, the solution was changed every 3–4 days, and an obvious nerve cell morphology could be observed about 6 or 7 days later for follow-up experiments.
2.11 Establishment of hippocampal neuronal cell injury induced by Aβ1–42
The hippocampal neuronal cells were grouped into normal and model groups, and incubated at 37°C for 2 hr. Aged Aβ1–42 was added into the model group to make a final concentration of 5 μmol/L, and the same volume of culture medium was added into the normal group. An Aβ1–42-induced hippocampal neuronal cell injury model was established after 24 hr incubation (Yang et al., 2015).
2.12 Identification of hippocampus neurons
The hippocampal neuronal cells cultured for 8 days were washed two times with 0.1 mol/L of PBS with the medium removed, fixed with 4% paraformaldehyde for 30 min, washed two times with PBS, and air dried for staining. The fixed cells were washed with 0.01 mol/L PBST three times (5 min each), oxidized with 80% methanol solution containing 0.3% H2O2 for 10 min, washed with running water for 5 min and washed with PBST three times (5 min each). Afterward, the cells were added with the primary antibody (neuron-specific enolase [NSE], mouse anti-human antibody; ab16808; Abcam Inc) for incubation overnight at 4°C. PBS was used as NC instead of the primary antibody. Subsequently, the cells were washed by PBST three times (5 min each) and added with a secondary antibody (donkey antirat; ab150109; Abcam) for incubation for 90 min at room temperature. The cells were washed with PBST three times again (5 min each), and HRP-labeled streptavidin (Beijing Zhongshangjingqiao Biotechnology Co. Ltd,. Beijing, China) was added for incubation at room temperature for 90 min. The cells were visualized with a 0.05% DAB-0.03% H2O2 coloring solution for 5–10 min after washing with PBST, and mounted with glycerogelatin after washing with running water. Finally, a light microscope was used to observe the samples.
2.13 Cell transfection and grouping
According to the GeneBank gene sequence, the forward and reverse primers were designed for IL2 according to Invitrogen’s online design software (http://rnaidesigner.invitrogen.com/rnaiexpress/). The full-length sequence was amplified by PCR, and the PCR products were verified by nucleic acid electrophoresis and purified by gel extraction. The lentiviral vector pcDNATM6.2-GM/EmGFP-siRNA (Invitrogen, Co., Ltd, Carlsbad, CA) and the target gene were detached with endonuclease Nhe I and Bam I. The products were recycled by nucleic acid electrophoresis, added into the competence bacteria E.coli TG1, transformed, sequenced, and filtrated to construct the lentiviral vectors of pcDNATM6.2-GM/EmGFP-IL2-siRNA. The blank vector was pcDNATM6.2-GM/EmGFP-siRNA. Both the miR-186 mimic and the miR-186 inhibitor were purchased from Guangzhou Ruibo Biotechnology Co. Ltd. (Guangzhou, China). Hippocampal neuronal cells were assigned into a blank group (without any transfection), an NC group (transfected with NC), a miR-186 mimic group (transfected with miR-186 mimic), a miR-186 inhibitor group (transfected with miR-186 inhibitor), small interfering RNA (siRNA)-IL2 group (transfected with IL2 interference plasmid) and a miR-186 inhibitor + siRNA-IL2 group (cotransfected with miR-186 and IL2 interference plasmids). After being detached with 0.25% trypsin and resuspended with M199 culture medium containing 10% fetal bovine serum into 1 × 105/ml, the cells were inoculated into a six-well culture plate and the original medium was replaced by a serum-free M199 medium after the cell confluence reached 70%. The culture condition had continuous access to nitrogen and loss of oxygen. The transfection was performed after 24 hr of incubation. Then 6 μl lipofectamin 2000 (11668-019; Invitrogen, Co., Ltd.) was diluted with 200 μl serum-free Opti-minimal essential medium (MEM) medium (31985070; Gibco Inc.; Grand Island, NY), and 4 μg plasmids were diluted with 200 μl of serum-free Opti-MEM medium, which were mixed respectively and allowed to stand at room temperature for 10 min. Next, the two were mixed and incubated at room temperature for 20 min. The original culture medium in the six-well plate was discarded, and 60 μl of Opti-MEM medium was added to each well. The transfected complex was added to the corresponding cell culture wells. Finally, the cells were incubated in a 5% CO2 incubator at 37°C for 6–8 h, and the former medium was replaced by fresh complete culture medium. Forty-eight hours after transfection, the cells were collected.
2.14 Reverse transcription quantitative PCR
Total RNA was extracted from the hippocampus and the transfected cells in each group using an RNA extraction kit (10296010, Invitrogen, Co., Ltd.). miRNA-186 and U6 were reverse-transcribed using stem ring reverse transcription primers. These primers were synthesized by the Shanghai Sangon Company (Shanghai, China). According to the instructions of The RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA), the RNA was reverse-transcribed to cDNA. After that, the reagents were gently shaken, quickly centrifuged, incubated at 42℃ for 60 min, and heated in a water bath for 5 min at 70℃. Then, reverse transcription was terminated, and the collected cDNA was stored at −80℃ for further experiments. The detection of IL2, JAK2, STAT3, Bcl-2, Bax, and β-actin messenger RNA (mRNA) was conducted with the routine extraction of RNA, and the RNA was reverse-transcribed to cDNA according to instructions with random six polymers as reverse transcription primers. The primers of miR-186, IL2, JAK2, STAT3, B-cell lymphoma-2 (Bcl-2), Bcl-2 associated X protein (Bax) and β-actin were designed and synthesized by Takara Holdings Inc. (Kyoto, Japan; Table 1), using the SYBR® Premix Ex TaqTM II Kit (RR820A, Takara, Kyoto, Japan). The reaction system was 50 μl, including 25 μl of SYBR® Premix Ex TaqTM II (2×), 2 μl of the PCR forward primer, 2 μl of reverse primer, l μl of ROX Reference Dye (50×), 4 μl of DNA template and 16 μl of ddH2O. Fluorescence quantitative PCR was performed by an ABI7500 PCR instrument (7500; ABI Company; Oyster Bay, NY). The reaction conditions were as follows: predenaturation at 95°C for 30 s, and 40 cycles of denaturation at 95°C for 5 s, and annealing and extension at 60°C for 30 s. U6 small nuclear RNA (U6) was the internal reference for miR-186 relative expression and β-actin was the internal reference for IL2, JAK2, STAT3, Bcl-2, and Bax relative expression. The relative expression of each target gene was calculated by 2−ΔΔCt (Ayuk, Abrahamse, & Houreld, 2016), and each experiment was repeated in triplicate.
| Genes | Primer sequences |
|---|---|
| miR-186 | F: GCGGCGCAAAGAATTCTCCT |
| R: GTGCAGGGTCCGAGGT | |
| IL2 | F: CAGCGTGTGTTGGATTTGAC |
| R: TGATGCTTTGACAGATGGCTA | |
| JAK2 | F: TTTGAAGACAGGGACCCTACACAG |
| R: TCATAGCGGCACATCTCCACA | |
| STAT3 | F: CACCCATAGTGAGCCCTTGGA |
| R: TGAGTGCAGTGACCAGGACAGA | |
| Bcl-2 | F: CCTGCCCCAAACAAATATGAAAAG |
| R: TTGACCATTTGCCTGAATGTGTG | |
| Bax | F: AACAACATGGAGCTGCAGAGG |
| R: GAAGTTGCCGTCTGCAAACATC | |
| β-Actin | F: GAGCGTGGCTACAGCTTCACCAC |
| R: TACTCCTGCTTGCTGATCCACAT | |
| miR-186 | F: GCGGCGCAAAGAATTCTCCT |
| R: GTGCAGGGTCCGAGGT | |
| U6 | F: CTCGCTTCGGCAGCACA |
| R: ACGCTTCACGAATTTGCGT | |
| IL2 | F: CAGCGTGTGTTGGATTTGAC |
| R: TGATGCTTTGACAGATGGCTA | |
| JAK2 | F: TTTGAAGACAGGGACCCTACACAG |
| R: TCATAGCGGCACATCTCCACA | |
| STAT3 | F: CACCCATAGTGAGCCCTTGGA |
| R: TGAGTGCAGTGACCAGGACAGA | |
| Bcl-2 | F: CCTGCCCCAAACAAATATGAAAAG |
| R: TTGACCATTTGCCTGAATGTGTG | |
| Bax | F: AACAACATGGAGCTGCAGAGG |
| R: GAAGTTGCCGTCTGCAAACATC | |
| β-Actin | F: GAGCGTGGCTACAGCTTCACCAC |
| R: TACTCCTGCTTGCTGATCCACAT |
- Note. Bax, Bcl-2 associated X protein; Bcl-2, B-cell lymphoma-2; F, forward; IL2, interleukin-2; JAK2, Janus kinase 2; miR-186, microRNA-186; R, reverse; RT-qPCR, reverse transcription quantitative polymerase chain reaction; STAT3, signal transducer and activator of transcription 3.
2.15 Western blot assay
Using a lysate, hippocampus was homogenized and lysed for 30 min, centrifuged at 3,000g for 15 min at 4℃ and then the supernatant was collected and stored at −20℃ for further experiments. The transfected cells were collected and added with protein lysate (R0010; Beijing Solabio Life Sciences Co., Ltd, Beijing, China) and centrifuged at 3000 rpm until the cells were completely lysed. The cells were then placed in an ice bath at 4°C for 30 min and centrifuged at 5000 rpm for 15 min with the supernatant collected. The protein concentration was accurately measured by bicinchoninic acid and spectrophotometry and adjusted to 1 μg/μl. The protein was then added to the loading buffer wells (20 μg/well) and subjected to electrophoresis with 10% sodium dodecyl sulfate polyacrylamide gel (P1200; Beijing Solabio Life Sciences Co., Ltd., Beijing, China). First, electrophoresis was performed at 80 v/cm, and then the separation gel was subjected to electrophoresis at 120 v/cm, and terminated when the bottom of the separation gel was closed to the sample. After that, the protein samples were transferred on polyvinylidene fluoride (PVDF) membrane by the semidry electrophoretic transfer method (HVLP04700; Millipore, Bedford, MA), stained with Ponceau (P0012; Beijing Solabio Life Sciences Co., Ltd., Beijing, China), and the transfer process was observed. The membranes were washed with Tris-Buffered Saline Tween (TBST) two times, sealed with 5% skim milk powder at room temperature for 2 hr and washed with TBST three times. Then, diluted primary antibody rabbit antirat IL2 (1:200; ab202911; Abcam), JAK2 (1:5,000; ab108596), STAT3 (1:2,000; ab68153; Abcam), p-STAT3 (1:5,000; ab32143; Abcam), p-JAK2 (1:5,000; ab32101; Abcam), Cleaved-Caspase-3 (1:500; ab2302; Abcam), Bcl-2 (1:500; ab59348; Abcam), and Bax (1:2,000; ab32503; Abcam) were added in membranes for incubation overnight at 4°C. Then, the membranes were washed with PBS five times (5 min each), immersed in enhanced chemiluminescence (ECL) solution (WBKLS0500; Pierce, Rockford, IL), and the results were observed and photographed in a dark room.
2.16 3-(4,5-Dimethylthiazol-2-yl)2,5-diphenyl tetrazolium bromide assay
Cells were collected and calculated after transfection and culture for 48 hr, incubated into a 96-well plate at 3 × 103–6 × 103 cells/well, and the volume of each well was 0.1 ml with six duplicated wells in an incubator. Three time-points were set: 24, 48, and 72 hr for the following experiments. First, 20 μl of the prepared 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) solution (5 mg/ml) was added into each well, incubated at 37°C for 2 hr and then the culture was terminated with the supernatant in the wells removed. Next, 150 μl of dimethylsulfoxide was added to each well. The OD value at 570 nm was determined by an ELISA reader (NYW-96M; Beijing Nuoya Wei Instrument Co., Ltd., Beijing, China). Each experiment was repeated three times. The cell viability curve was drawn with the time point as the X-axis, and OD value as the Y-axis. The experiment was conducted three times to obtain the mean value.
2.17 Flow cytometry
After transfection for 48 hr, the cells were detached with trypsin without ethylenediamine tetraacetic acid and collected in a flow tube and centrifuged with the supernatant discarded. Then the cells were washed with cooling PBS three times, centrifuged, and the supernatant was discarded. According to the instructions of the Annexin-V-fluorescein isothiocyanate (Annexin-V-FITC) apoptosis detection Kit (Sigma-Aldrich, St. Louis, MO), the Annexin-V-FITC, propidium iodide (PI) and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer solution were mixed as an Annexin-V-FITC/PI dye solution in a ratio of 1:2:50. Every 100 μl of the dye was used for resuspending 1 × 106 cells and shaken well. Then 1 ml of HEPES buffer solution was added and shaken after the cells were incubated at room temperature for 15 min. The fluorescence of FITC and PI was detected through 525 nm and 620 nm of bandpass filters excited at 488 nm for detection of cell apoptosis. The experiment was conducted three times to obtain the mean value.
2.18 Measurement of activity of GSH-PX, SOD, LDH and the levels of ROS and MDA in hippocampal neuronal cell
The supernatant in each group was collected. The activity of GSH-PX, SOD, LDH, and the levels of ROS and MDA were assessed as suggested by the GSH-PX Kit (Item No. A005; Nanjing Jiancheng Bioengineering Company), SOD Kit (Item No. A001; Nanjing Jiancheng Bioengineering Company), LDH Kit (Item No. A020-2; Nanjing Jiancheng Bioengineering Company), ROS Kit (Item No. A003-2; Nanjing Jiancheng Bioengineering Company), and MDA Kit (Item No. A003-1; Nanjing Jiancheng Bioengineering Company), respectively.
2.19 Statistical analysis
All data were processed by SPSS21.0 (IBM Corp. Armonk, NY). Measurement data were expressed as mean ± standard deviation. Comparisons between two groups were made by t-test and comparisons among multiple groups by one-way analysis of variance. p < 0.05 was considered a significant difference.
3 RESULTS
3.1 IL2 is a direct target gene of miR-186
First, the target gene of miR-186 was analyzed by TargetScan (http://www.targetscan.org/vert_71/). The prediction results suggested that there were binding sites between miR-186 and IL2 3′-UTR (Figure 1); thus IL2 was a target gene of miR-186. Compared with the NC group, the relative luciferase activity intensity in the miR-186 mimic group was significantly decreased, and the relative luciferase activity intensity in the miR-186 inhibitor group was significantly increased (p < 0.05). These findings suggested that miR-186 inhibited the activity of IL2.
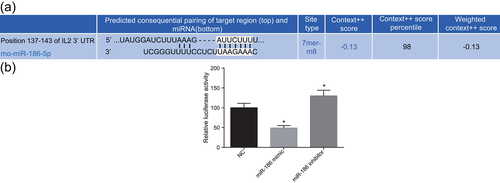
IL2 is a target gene of miR-186. (a) Online prediction of target gene; (b) comparison of luciferase experiments. Measurement data was presented as mean ± standard deviation; one-way analysis of variance was used to compare multigroup data; the experiment was carried out in triplicate. *p < 0.05, versus the NC group. IL2, interleukin-2; miR-186, microRNA-186; NC, negative control [Color figure can be viewed at wileyonlinelibrary.com]
3.2 Serious memory injury in the rat model of AD
With the aim to observe whether rats in the model group induced by Aβ had an serious memory injury, MWM was conducted. The results showed that the escape latency in the normal group, the sham group and the model group was gradually decreased from the first day to the fifth day, which indicated that the rats had some learning ability within the 5 days of training. Compared with the normal group, the escape latency of rats in the model group was prolonged obviously (p < 0.05; Table 2). Taken together, these results suggested that the rats in the model group had serious memory injury and the success rate of the model was 100%.
| Groups | Number | First day | Second day | Third day | Fourth day | Fifth day |
|---|---|---|---|---|---|---|
| Normal | 24 | 46.2 ± 7.5 | 36.0 ± 4.7 | 33.5 ± 3.3 | 32.1 ± 3.1 | 27.5 ± 4.1 |
| Sham | 24 | 42.3 ± 4.7 | 39.2 ± 5.0 | 37.1 ± 3.5 | 35.0 ± 4.2 | 30.1 ± 3.8 |
| Model | 24 | 52.5 ± 5.3* | 49.1 ± 5.1* | 47.5 ± 5.4* | 46.1 ± 5.3* | 45.0 ± 4.9* |
- Note. *versus the normal group, p < 0.05, n = 6; measurement data were expressed as mean ± standard deviation; comparison among multiple groups was compared by one-way analysis of variance; the experiment was conducted in triplicate.
3.3 Significant pathological changes were found in the hippocampus of AD rats after HE staining
Next, the changes in the hippocampus morphology in the AD rat model induced by Aβ were observed with HE staining. The results of HE staining (Figure 2) showed that in the normal group, the hippocampal neuronal cells were abundant, arranged in neat rows, with morphological integrity and zonal distribution. And there were plenty of cells with round or oval nuclei, and stained homogeneously. But no significant can be found between the normal group and the sham group. Compared with the normal group, the hippocampal neuronal cells in the model group were largely missing, irregularly arranged, the cell volume and the cytoplasm were shrunk, and the cell layers were decreased.
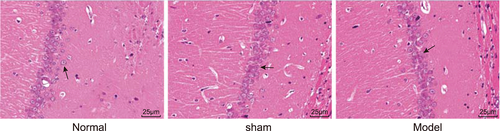
HE staining is used for observing pathological changes of hippocampus, and the model group has largely missing, irregularly arranged hippocampal neuronal cells (×400), scale = 25 um. The arrow directed to neurons; HE, hematoxylin–eosin [Color figure can be viewed at wileyonlinelibrary.com]
3.4 IL2 and Cleaved-Caspase-3 are expressed at a higher level in AD rat
Afterward, the expression of IL2 and Cleaved-Caspase-3 in the rat model of AD induced by Aβ was measured by immunohistochemistry. Immunohistochemistry results showed that positive expression of IL2 was mainly located in the cell cytoplasm and cell interstitial with brown positive particles (Figure 3a). The positive expression rate of IL2 protein in the sham group was 19.0% and it showed no significant difference from that in the normal group (16.67%; p > 0.05), but was significantly lower than that in the model group (79.63%; p < 0.05; Figure 3c). The Cleaved-Caspase-3 positive cells had brown particles in the cell nucleus (Figure 3b); no difference was found between the positive rate of Cleaved-Caspase-3 between the sham group (5.0%) and the normal group (3.24%; p < 0.05). The positive expression rate of Cleaved-Caspase-3 protein in the model group was 15.42%, which was significantly higher than that in the normal group (3.24%; p < 0.05; Figure 3d). Taken together, AD led to a higher expression level of IL2 and Cleaved-Caspase-3.
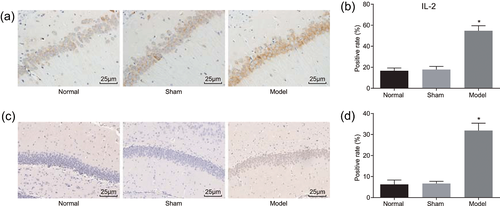
Higher IL2 and Cleaved-Caspase-3 expression in the rat model of AD is detected by immunohistochemistry staining (×400), n = 6; scale = 25 µm. (a, c) IL2 expression was increased in the rat model of AD; (b, d) immunohistochemistry verified that Cleaved-Caspase-3 expression level was decreased in the rat model of AD. Measurement data were presented as mean ± standard deviation; one-way analysis of variance was used to compare multigroup data; the experiment was carried out in triplicate. *p < 0.05, versus the normal group. AD, Alzheimer’s disease; IL2, interleukin-2 [Color figure can be viewed at wileyonlinelibrary.com]
3.5 Increased activity of LDH, levels of ROS and MDA, and decreased activity of GSH-PX and SOD in hippocampus of the rat model of AD
Subsequently, the activity of SOD in hippocampus was determined by xanthine oxidase. The activity of GSH-PX and the level of ROS were determined by Fenton reaction. The level of MDA was determined by TBA assay. The activity of LDH was determined by ELISA. Compared with the normal group, the activity of GSH-PX and SOD in hippocampus of the model group was significantly decreased (p < 0.05), where the activity of LDH, and the levels of ROS and MDA in hippocampus of the model group were significantly increased (p < 0.05; Table 3). In the sham group, no significant changes in SOD activity, GSH-PX activity, ROS level, MDA level, and LDH activity were observed when compared with the normal group.
| Groups | Normal (n = 6) | Sham (n = 6) | Model (n = 6) |
|---|---|---|---|
| GSH-PX | 26.61 ± 3.11 | 26.42 ± 3.09 | 18.15 ± 3.32* |
| SOD | 69.97 ± 6.30 | 69.56 ± 6.11 | 50.89 ± 5.41* |
| LDH | 73.65 ± 5.25 | 73.02 ± 5.12 | 164.35 ± 12.15* |
| MDA | 56.34 ± 8.11 | 56.01 ± 8.02 | 113.17 ± 10.25* |
| ROS | 2.01 ± 0.21 | 2.00 ± 0.19 | 4.23 ± 0.47* |
- Note. GSH-PX, glutathione peroxidase; LDH, lactic dehydrogenase; MDA, malondialdehyde, ROS, reactive oxygen species, SOD, superoxide dismutase. *versus the normal group, p < 0.05, n = 6, measurement data were expressed as mean ± standard deviation, comparison among multiple groups was compared by one-way analysis of variance, the experiment was conducted in triplicate.
3.6 Lower EGF expression and higher PDGF, IFN-γ, and GH expression in hippocampus of the rat model of AD
In addition, the expressions of EGF, PDGF, IFN-γ, and GH was measured by ELISA in the rat model of AD. The results of ELISA (Table 4) demonstrated that compared with the normal group, the expression of EGF was significantly decreased (p < 0.05), and the expression of PDGF, IFN-γ, and GH was significantly increased in the model group (p < 0.05). In the sham group, no remarkable changes of EGF, PDGF, IFN-γ, and GH expression occurred in comparison with the normal group.
| Groups | Normal (n = 6) | Sham (n = 6) | Model (n = 6) |
|---|---|---|---|
| EGF | 179.26 ± 6.55 | 178.63 ± 6.42 | 128.58 ± 5.43* |
| PDGF | 104.61 ± 10.45 | 104.12 ± 10.12 | 152.21 ± 16.59* |
| IFN-γ | 34.02 ± 12.35 | 33.96 ± 12.04 | 162.50 ± 43.68* |
| GH | 1.05 ± 0.33 | 1.04 ± 0.31 | 2.78 ± 0.53* |
- Note. EGF, epidermal growth factor; GH, growth hormone; IFN, interferon; PDGF, platelet-derived growth factor. *versus the normal group, p < 0.05, n = 6; measurement data were expressed as mean ± standard deviation; comparison among multiple groups was compared by one-way analysis of variance; the experiment was conducted in triplicate.
3.7 Lower miR-186, Bcl-2 expression and higher IL2, JAK2, STAT3, Bax, and Cleaved-Caspase-3, expression in hippocampus of the rat model of AD
Furthermore, reverse transcription quantitative PCR (RT-qPCR) and western blot assay were applied for the measurement of miR-186, Bcl-2, IL2, JAK2, STAT3, Bax Cleaved-Caspase-3, p-STAT3, and p-JAK2 expression. The results revealed that compared with the normal group, the expression of miR-186 (Figure 4a) and the protein and mRNA expression of Bcl-2 (Figure 4b,d) in the model group were markedly decreased (p < 0.05), the protein and mRNA expression of IL2 (Figure 4a,c), JAK2 (Figure 4a,c), STAT3 (Figure 4a,c), Bax (Figure 4b,d), as well as Cleaved-Caspase-3 protein expression (Figure 4b,d) and the extent of p-STAT3 and p-JAK2 (Figure 4d) in the model group were markedly increased (p < 0.05). However, the expression of miR-186, Bcl-2, IL2, JAK2, STAT3, Bax, Cleaved-Caspase-3, p-STAT3, and p-JAK2 did not differ notably in the sham group (p < 0.05) when compared with the normal group. Taking all the results above into consideration, AD was a negative factor for miR-186 and Bcl-2 expression, yet a positive factor for IL2, JAK2, STAT3, Bax, and Cleaved-Caspase-3 expression.
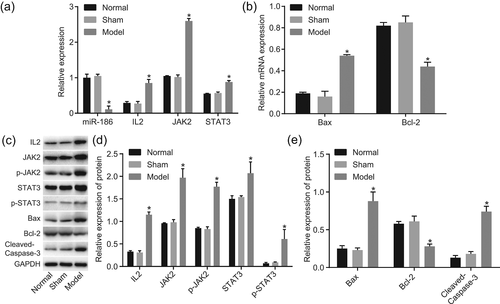
miR-186 and Bcl-2 expression is lower and expression of IL2, JAK2, STAT3, Bax, Cleaved-Caspase-3, p-STAT3 and p-JAK2 is higher in hippocampus in the rat model of AD (n = 6). (a, b) RT-qPCR proved that miR-186 expression and Bcl-2 mRNA expression were declined while IL2, JAK2, STAT3, and Bax mRNA expression was facilitated in the rat model of AD; (c, d) western blot assay validated that Bcl-2 protein expression was higher while the protein expression of Bax, Cleaved-Caspase-3 was lower in the rat model of AD; (e) gray value analysis of IL2, JAK2, STAT3, Bax, Bcl-2, Cleaved-Caspase-3, p-STAT3 and p-JAK2. Measurement data was presented as mean ± standard deviation; one-way analysis of variance was used to compare multigroup data; the experiment was carried out in triplicate. *p < 0.05, versus the normal group. Bax, Bcl-2 associated X protein; Bcl-2, B-cell lymphoma-2; IL2, interleukin-2; JAK2, Janus kinase 2; miR-186, microRNA-186; p-JAK2, phosphorylation of JAK2; p-STAT3, phosphorylation of STAT3; RT-qPCR, reverse transcription quantitative polymerase chain reaction; STAT3; signal transducer and activator of transcription 3
3.8 Pathological changes of hippocampus neurons
Additionally, NSE immunofluorescence was utilized for hippocampus neurons assessment. The results of NSE (Figure 5) demonstrated that the neurons NSE in the normal group were mainly located in the endochylema with a clear color: the color of the nucleus was light, the cells were arranged in a certain density, and the nerve fiber had a lighter color than the cell body. No significance can be found between the normal group and the sham group. Compared with the normal group, the neurons in the model group were lighter with a vague morphology and disordered arrangement, and the color of the nerve fibers was not changed significantly.

NSE staining is utilized for the observation of pathological changes of hippocampus neurons, and the model group has lighter neurons with vague morphology (×400). NSE, nonspecific esterase [Color figure can be viewed at wileyonlinelibrary.com]
3.9 miR-186 negatively regulates the JAK–STAT signaling pathway by downregulating IL2
In the following experiments, we applied RT-qPCR and western blot assay for measuring miR-186, Bcl-2, IL2, JAK2, STAT3, Bax, Cleaved-Caspase-3, p-STAT3 and p-JAK2 expression in vitro. The results (Figure 6) revealed that compared with the blank and NC groups, the miR-186 expression in the miR-186 mimic group was markedly increased (p < 0.05), yet no significant change could be found in the siRNA-IL2 group (p > 0.05). The protein and mRNA expression of Bcl-2 were markedly increased in the miR-186 mimic and the siRNA-IL2 groups (p < 0.05), and the protein and mRNA expression of IL2, JAK2, STAT3 and Bax, along with protein expression of Cleaved-Caspase-3, p-STAT3 and p-JAK2, were markedly decreased (p < 0.05). The miR-186 expression and Bcl-2 protein and mRNA expression in the miR-186 inhibitor group were markedly decreased (p < 0.05), but the protein and mRNA expression of IL2, JAK2, STAT3 and Bax, along with Cleaved-Caspase-3, p-STAT3 and p-JAK2 protein expression were markedly increased (all p < 0.05) when compared with the blank and NC groups. Compared with the blank and NC groups, in the miR-186 inhibitor + siRNA-IL2 group, miR-186 expression was markedly decreased (p < 0.05), whereas the protein and mRNA expressions of IL2, JAK2, STAT3, and Bax expression as well as Cleaved-Caspase-3, p-STAT3, and p-JAK2 protein expression had no difference (p > 0.05). These findings indicated that by downregulation of IL2, miR-186 played a negative role in the modulation of the JAK–STAT signaling pathway.
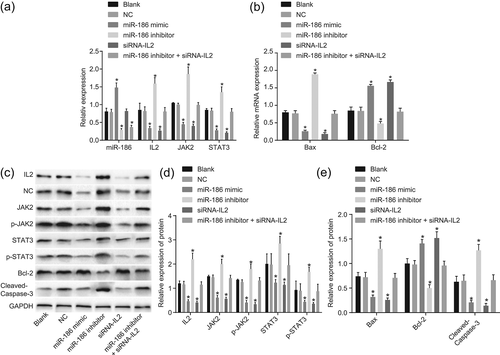
miR-186 acts as a negative regulator in the JAK–STAT signaling pathway by downregulating IL2. (a,b) RT-qPCR proved that miR-186 expression and Bcl-2 mRNA expression were declined, while IL2, JAK2, STAT3, and Bax mRNA expression was facilitated in vitro; (c,d) western blot assay validated that miR-186 expression and Bcl-2 protein expression were higher while the protein expression of IL2, JAK2, STAT3, Bax, Cleaved-Caspase-3, p-STAT3, and p-JAK2 were lower in vitro; measurement data were presented as mean ± standard deviation; one-way analysis of variance was used to compare multigroup data; the experiment was carried out in triplicate. *p < 0.05, versus the blank group and the NC group. Bax, Bcl-2 associated X protein; Bcl-2, B-cell lymphoma-2; IL2, interleukin-2; JAK2, Janus kinase 2; miR-186, microRNA-186; mRNA, messenger RNA; NC, negative control; p-JAK2, phosphorylation of JAK2; p-STAT3, phosphorylation of STAT3; RT-qPCR, reverse transcription quantitative polymerase chain reaction; siRNA, small interfering RNA; STAT3, signal transducer and activator of transcription 3
3.10 Overexpression of miR-186 promotes hippocampus viability
To determine the effect of miR-186 and IL2 on hippocampus viability, MTT assay was applied. The results of the MTT assay (Figure 7) showed that compared with the blank and NC groups, cell proliferation had no difference in the miR-186 inhibitor + siRNA-IL2 group (p > 0.05); cell proliferation was promoted in the miR-186 mimic and siRNA-IL2 groups, and the OD values at 48 hr and 72 hr were increased (all p < 0.05); but cell proliferation was inhibited in the miR-186 inhibitor group and the OD values at 48 hr and 72 hr were decreased (all p < 0.05). It was concluded that miR-186 improved hippocampus viability via IL2 downregulation.
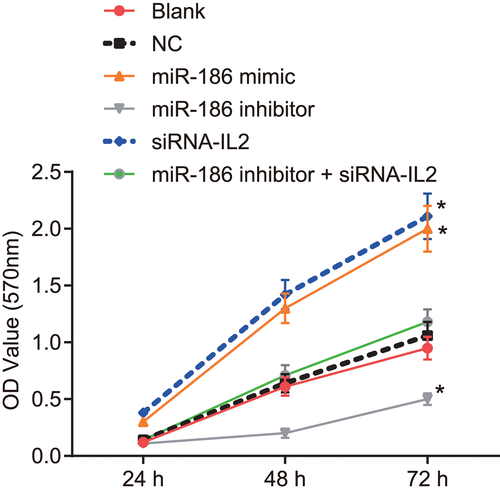
Hippocampus viability is improved by high expression of miR-186. Measurement data were expressed as mean ± standard deviation; comparison among multiple groups was compared by one-way analysis of variance; the experiment was conducted in triplicate. *p < 0.05, versus the normal and NC groups at 24 hr and 48 hr. IL2, interleukin 2; miR-186, microRNA-186; NC, negative control; siRNA, small interfering RNA [Color figure can be viewed at wileyonlinelibrary.com]
3.11 Overexpression of miR-186 inhibits hippocampus apoptosis
Later, the effect of miR-186 and IL2 on hippocampus apoptosis was validated by flow cytometry. The results (Figure 8) demonstrated that compared with the blank and NC groups, cell apoptosis rate was markedly increased in the miR-186 inhibitor group (all p < 0.05), and decreased in the miR-186 mimic and siRNA-IL2 groups (all p < 0.05). The miR-186 mimic and siRNA-IL2 groups had no difference (all p > 0.05). In conclusion, miR-186 conferred resistance to hippocampus apoptosis by negatively regulating the IL2 expression.
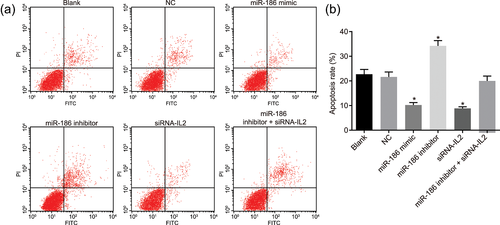
Hippocampus apoptosis is inhibited by high expression of miR-186. (a, b) Flow cytometry results confirmed that miR-186 was a negative factor in cell cycle. Measurement data were expressed as mean ± standard deviation; comparison among multiple groups was compared by one-way analysis of variance; the experiment was conducted in triplicate. *p < 0.05, versus, the normal and NC groups at 24 hr and 48 hr. FITC, fluorescein isothiocyanate; IL2, interleukin 2; miR-186, microRNA-186; NC, negative control; siRNA, small interfering RNA [Color figure can be viewed at wileyonlinelibrary.com]
3.12 Overexpression of miR-186 increases the activity of GSH-PX and SOD and decreases the activity of LDH and the levels of ROS and MDA
Finally, the activity of SOD, GSH-PX, and LDH as well as the levels of ROS and MDA after transfection were detected. The results (Table 5) revealed that compared with the blank and NC groups, the activity of GSH-PX and SOD was decreased, yet the activity of LDH and the levels of ROS and MDA were elevated in the miR-186 inhibitor group (all p < 0.05); the activity of GSH-PX and SOD was increased, yet the activity of LDH and the levels of ROS and MDA were decreased in the miR-186 mimic group and the siRNA-IL2 group (all p < 0.05). The miR-186 mimic and siRNA-IL2 groups had no difference (all p > 0.05). In summary, miR-186 overexpression positively regulated the activity of GSH-PX and SOD, while negatively regulating the activity of LDH and the levels of ROS and MDA.
| Group | GSH-PX | SOD | LDH | MDA | ROS |
|---|---|---|---|---|---|
| Blank | 32.26 ± 5.12 | 39.22 ± 5.41 | 121.57 ± 11.23 | 93.17 ± 10.25 | 5.50 ± 0.72 |
| NC | 31.86 ± 4.98 | 39.02 ± 5.37 | 120.68 ± 10.72. | 92.84 ± 10.12 | 7.46 ± 0.68 |
| miR-186 mimic | 109.46 ± 10.90* | 105.09 ± 10.80 | 75.61 ± 3.12* | 8.12 ± 2.24* | 1.23 ± 0.45* |
| miR-186 inhibitor | 9.68 ± 5.01* | 9.21 ± 6.12* | 162.83 ± 9.12* | 177.45 ± 17.82* | 18.15 ± 1.93* |
| siRNA-IL2 | 107.34 ± 9.21* | 101.61 ± 8.73* | 72.35 ± 2.26* | 7.92 ± 1.72* | 1.04 ± 0.40* |
| miR-186 inhibitor + siRNA-IL2 | 31.79 ± 5.01 | 38.88 ± 5.34 | 120.16 ± 9.98 | 92.37 ± 10.61 | 5.45 ± 0.70 |
- Note. GSH-PX, glutathione peroxidase; IL2, interleukin-2; LDH, lactic dehydrogenase; MDA; malondialdehyde; miR-186, microRNA-186; NC, negative control; ROS, reactive oxygen species; siRNA, small interfering RNA; SOD, superoxide dismutase. *versus the blank and NC groups, p < 0.05.
4 DISCUSSION
AD, the most common disease in the industrialized world, is a multifactorial and fatal neurodegenerative disorder for which the mechanisms leading to profound neuronal loss are not completely recognized (Bertram et al., 2010; Wong et al., 2013). Increased production and clearance of Aβ in AD pathological reactions aggravated blood–brain barrier dysfunction, neuronal apoptosis, and neuroinflammation, resulting in apoptosis of uninjured neurons (Yin et al., 2013). Recently, another study showed that miRs played potential roles as diagnostic biomarkers and therapeutic applications in AD (Chen, Zhao, Zhao, & Li, 2017). The aim of this study was to investigate the mechanism of miR-186 and its target gene IL2 in the development of AD by regulating the JAK–STAT signaling pathway.
In our study, cells transfected with miR-186 mimic and siRNA-IL2 had decreased expression of IL2, JAK2, STAT3, Bax, Cleaved-Caspase-3, p-STAT3, and p-JAK2 and increased expression of Bcl-2. MiR-186 was previously shown to be altered in AD specimens, and miR-186 could be an important player in AD pathology (Delay et al., 2014). Caspase-3 had been significantly increased in AD (Louneva et al., 2008). A study has revealed that IL2–Caspase-3 chimeric protein might also provide a novel approach to the therapy of the human inflammatory bowel disease (Shteingart et al., 2009). Cui et al. (2017) showed that the expression of Bcl-2 had decreased and the expression of Bax had increased in AD rats compared with the normal rats. Xie, Tian, Wei, & Liu (2015) also demonstrated that Aβ treatment significantly induced Bax and downregulated Bcl-2 in the rat hippocampus with AD. Chiba, Yamada, & Aiso (2009) found the inactivation of STAT3 in AD and demonstrated that p-STAT3 levels were markedly decreased in hippocampal neuronal cells of clinically and pathologically diagnosed AD. Y. Zhang et al. (2013) also revealed that phosphorylation levels of JAK2 and STAT3 and the protein level of suppressor of cytokine signaling 1 (SOCS-1) were markedly reduced in AD rats. A prior report proposed that the JAK–STAT pathway can associate with the cell membrane (receptor) and the nucleus (gene expression) directly, and by correlation with cytokines, such as IL2, IL3, IL5, IL7, IL9, IL1, IL17E, IL4R, and IL6, cell growth and differentiation were identified to be regulated by several members of the mammalian STAT family proteins, including Stat4, Stat6, Stat5a, and Stat5b (Dittrich et al., 2012; Kurgonaite et al., 2015; Luo et al., 2016; Takeda & Akira, 2000). Meanwhile, previous studies on human and animals found that STAT3 activity significantly changed in the hippocampus of AD (Chiba et al., 2009, Park et al., 2013). Besides, STAT3 played an important mediating role in nicotine inhibition of AD inflammatory response (Hosur & Loring, 2011; Kawamata & Shimohama, 2011). In addition, a research has verified that selective targeting of JAK/STAT signaling was potentiated by Bcl-xL blockade in IL2-dependent adult T-cell leukemia (M. Zhang et al., 2015; Q. Zhang et al., 2015). The current study revealed that the overexpression of miR-186 and IL2 silencing decreased the expression of IL2, JAK2, STAT3, Bax, Cleaved-Caspase-3, p-STAT3, and p-JAK2, whereas increased the expression of Bcl-2 in AD rats.
Besides, cells transfected with miR-186 mimic and siRNA-IL2 had decreased the activity of LDH and the levels of ROS and MDA, and increased the activity of GSH-PX and SOD. Manton, Volovik, & Kulminski (2004) demonstrated that ROS had effects on neurodegeneration in AD and related disorders. Evidence has shown that the cooperative action of miR-186 with miR-337-3p and miR-216b might induce replicative senescence through CK2α downregulation-dependent ROS generation (Lee, Kim, & Bae, 2014). A work of Li et al. revealed that administration of Aβ-40 significantly decreased the levels of SOD and GSH-PX, while it increased the MDA level in the hippocampus in AD rats (M. Zhang et al., 2015; Q. Zhang et al., 2015). Growing evidence suggests that cerebral the Aβ accumulation contributes to the AD pathology (Holtzman, Morris, & Goate, 2011; Perrin, Fagan, & Holtzman, 2009). In line with our study, overexpression of miR-186 markedly decreased Aβ level (Kim et al., 2016). Therefore, our study revealed that the overexpression of miR-186 and IL2 silencing decreased the activity of LDH and the levels of ROS and MDA, and increased the activity of GSH-PX and SOD.
Furthermore, our study showed that the miR-186 mimic and siRNA-IL2 groups increased proliferation and decreased apoptosis of hippocampal neuronal cells. Soluble Aβ oligomers can induce OS, neuronal apoptosis, memory deficits, and synaptic dysfunction which are present in AD (Braidy, Poljak, Zarka, Bridge, Sachdev, 2013). According to a previous investigation, the Aβ level could be markedly decreased by overexpressed miR-186 (Kim et al., 2016). Furthermore, accumulated evidence demonstrated that miR-186 inhibited cell proliferation in prostate cancer by targeting Golgi phosphoprotein 3 (Hua et al., 2016). A previous study had shown that miR-186 acted as a tumor suppressor by downregulating the proapoptotic genes, and the change of miR-186 expression in AD influenced brain regions had been reported (Ben Halima, Siegel, & Rajendran, 2016). Former studies have investigated the implication of IL2 in proliferation and proposed that proliferation may be accelerated by IL2 overexpression (Eguizabal et al., 2007; Guma, Lee, Ling, Gordon, & Kleinerman, 2014). In hippocampus, IL2 plays a critical role in neurodegenerative processes and neuronal injury responses and increases the survival (de Araujo, da Silva, & Dos Santos, 2009). Qiu, Yuan, & Lu (2016) revealed that miR-186 promoted cell proliferation in human melanoma. Our current study supposed that the overexpression of miR-186 promoted proliferation and inhibited apoptosis of hippocampal neuronal cells by downregulating IL2.
In conclusion, the current study demonstrated that overexpressed miR-186 and siRNA-IL2 alleviates OS injury of neuron through inhibition of the JAK–STAT pathway. Although the mechanism underlying AD progression still needs to be discussed, our findings provide new insights for novel AD therapies.
ACKNOWLEDGMENTS
This study was supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the 2016 “333 Project’’ Award of Jiangsu Province, the 2013 “Qinglan Project” of the Young and Middle-aged Academic Leader of Jiangsu College and University, the National Natural Science Foundation of China (81571055, 81400902, 81271225, 31201039, 81171012, and 30950031), the Major Fundamental Research Program of the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (13KJA180001), and grants from the Cultivate National Science Fund for Distinguished Young Scholars of Jiangsu Normal University. We would like to show sincere appreciation to the reviewers for critical comments on this study.
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interests.