NLRP3 inflammasome mediates chronic intermittent hypoxia-induced renal injury implication of the microRNA-155/FOXO3a signaling pathway
Abstract
Chronic intermittent hypoxia (CIH), as the foremost pathophysiological change of obstructive sleep apnea (OSA), contributes to continued deterioration in renal function. Nucleotide-binding domain like receptor protein 3 (NLRP3) inflammasome is a multiprotein complex that triggers innate immune responses to infection and cell stress through activation of caspase-1 and maturation of inflammatory pro-interleukin-1β cytokine. Emerging evidence indicates that inhibition of the NLRP3 inflammasome ameliorates renal injury. Nevertheless, it is uncertain whether NLRP3 inflammasome participates in CIH-induced renal injury. The molecular mechanisms modulating NLRP3 inflammasome activation remain to be elucidated. Compared with wild-type mice, NLRP3 knockout mice dramatically protected them from kidney injury, as indicated by the restoration of creatinine levels, lessened histopathological alterations, and the suppression of macrophages infiltration stained with F4/80. NLRP3 deficiency notably reversed CIH-induced oxidative stress (malondialdehyde and superoxide dismutase), concomitantly with the abrogated apoptosis-related proteins and proinflammatory signaling pathway. Consistently, NLRP3-deficient tubular cells remarkably inhibited reactive oxygen species generation and NLRP3 inflammasome activation. Furthermore, our study revealed that microRNA-155 (miR-155) was augmented in the renal tissue and HK-2 cells exposed to CIH. In addition, we investigated the role of miR-155 in the regulation of NLRP3 inflammasome. Inhibition of miR-155 suppressed the CIH-induced NLRP3 inflammasome activation in renal tubular cells, whereas overexpression of miR-155 promoted oxidation and enhanced NLRP3 pathway. Collectively, we demonstrated that miR-155 might be a positive-regulator of NLRP3 pathway by inhibiting the targeted FOXO3a gene. These results established a link between the miR-155/FOXO3a pathway and the NLRP3 inflammasome, suggesting pharmacological blockage of NLRP3 as a potential therapeutic strategy for OSA-associated chronic kidney disease.
1 INTRODUCTION
Characterized by repetitive upper airway collapse and recurrent nocturnal hypoxemia, obstructive sleep apnea (OSA) is a chronic inflammatory disease, in which chronic intermittent hypoxia (CIH) has been recognized as the major characteristic of pathogenesis (Chiang, 2006), proving to be a risk factor for a variety of diseases. The patients with untreated OSA have shown an increased risk of advanced chronic kidney disease (CKD) over the past two decades (Sim et al., 2009). Accumulating evidence indicates that OSA contributes to CKD through a direct effect of intrarenal hypoxia on the kidney and activation of the systemic and the renal renin–angiotensin system (Hanly & Ahmed, 2014). Determining a new therapeutic approach in the development of OSA-associated CKD may have significant implication for clinical practice in an effort to reduce the burden of population health (Adams et al., 2017).
Recent studies provide new insights into the nucleotide-binding domain like receptor protein 3 (NLRP3) inflammasome, which activates upon signs of cellular “danger” to trigger innate immune defense and has been implicated in sterile inflammatory response (Place & Kanneganti, 2017). As the best-characterized inflammasome, NLRP3 is a cytoplasmic multiprotein complex composed of the NLRP3 scaffold, adapter protein ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain), and pro-caspase-1 (Schroder & Tschopp, 2010). Since recruited caspase-1 is known as an interleukin (IL)-1β-converting enzyme, the activation of the inflammasome leads to the maturation and secretion of some proinflammatory cytokines, such as IL-1β and IL-18, thereby causing tissue damage (Martinon, Petrilli, Mayor, Tardivel, & Tschopp, 2006). Growing evidence has shown that inhibition of the NLRP3 inflammasome ameliorates renal injury in a variety of animal models (Chow, Ozols, Nikolic-Paterson, Atkins, & Tesch, 2004; Lorenz, Darisipudi, & Anders, 2014). Studies on humans demonstrated increased NLRP3 in a variety of diabetic kidney diseases and CKD (Lee et al., 2013; Vilaysane et al., 2010). Evidenced by renal dysfunction and histopathological assessment, our group previously reported CIH induced prominent inflammatory cell infiltration and severe tubular cell apoptosis (X. Wu et al., 2016). The coexistence of cellular injury and inflammation suggests a possible contribution of NLRP3 inflammasome to the progression of CIH-induced renal injuries. Whether NLRP3 regulates CIH-induced renal injuries and the underlying molecular mechanisms needs to be explored.
The belief that the activation of NLRP3 inflammasome is stimulated by increased reactive oxygen species (ROS) generation is widely accepted. MicroRNA-155 (miR-155) can target multiple components, playing a critical role in the inflammatory and immune regulatory functions (Ceppi et al., 2009; Wan et al., 2016). As a hypoxia-inducible miRNA, miR-155 can control HIF-1α activity to form a negative feedback loop and is thus responsible for oxygen homeostasis (Robertson, Wasylyk, Ye, Jung, & Wasylyk, 2014). Furthermore, miR-155 has been identified to promote ROS generation and oxidative damage through inhibition of FOXO3a expression (Wang et al., 2015). A previous research by Wu et al. (2016) found that exogenous miR-155 regulated pyroptosis-related proteins, including caspase-1, IL-1β in response to renal injury. We previously discovered hypoxic upregulation of miR-155 in cultured HK-2 cells and renal tissues from mice exposed to CIH. Presumably, oxidative stress and redox modulation seem to bridge the induction of miR-155 and NLRP3 inflammasome initiation in CIH-induced renal injuries. Moreover, the integrated molecular signals required to activate the NLRP3 inflammasome during CIH remain undefined. Therefore, we developed the hypothesis that miR-155 served as an endogenous regulator of the crucial NLRP3 inflammasome under hypoxic conditions.
In this study, we used NLRP3 knockout mice to explore the involvement of NLRP3 inflammasome in CIH-induced renal injury and the potential contribution of miR-155 to NLRP3 inflammasome activation. These data would provide valuable insights into the pathophysiologic processes driving renal inflammation and CKD progression and identify NLRP3 as a novel target for therapeutic intervention.
2 MATERIALS AND METHODS
2.1 Animal and experimental model of CIH
NLRP3−/− and wild-type (WT) male mice on C57BL/6 background (Jackson Laboratory, Sacramento, CA) were housed under standard conditions with a 12-hr light/12-hr dark cycle at 22–24°C and allowed free access to water and food. The project was approved by the Medical Experimental Animal Administrative Committee of the Shanghai Medical College of the Fudan University, in accordance with the guidelines implemented by the National Institutes of Health Guide regarding the care and use of animals for experimental procedures. All effects were made to minimize animal suffering. The standard CIH protocol was modeled as described in our previous study (Wu et al., 2016). The mice exposed to intermittent hypoxia (IH) were placed inside custom-made (28.5 × 30.0 × 51.5 cm) chambers where flows of oxygen and nitrogen were controlled to obtain the desired profile of changes in oxygen level. CIH was administered for 10 hr/day, from 7:00am to 5:00pm, with the oxygen level oscillating between 24% and 7% with a period of 60 s. The oxygen concentration was measured automatically using an oxygen analyzer (Corporation, Shanghai, China). All mice were randomized into four experimental groups of six animals each: the normal air (NA) control group and the CIH group of WT mice; and the NA group and CIH group of NLRP3−/− mice.
2.2 Histopathology analysis
The renal tissue harvested from the mice was washed with 0.9% saline, fixed in 10% neutral buffered formalin, and then embedded in 10% paraffin. Sections (5 μm thick) were stained with hematoxylin-eosin (H&E) for further analysis. Kidney injuries were examined from 10 fields per kidney under a microscope according to the modified 0–5 Jablonsky grading scale, as described previously.
Blood samples were collected to measure the concentration of creatinine (Cr) in the serum using detection kits (Nanjing Jiancheng, Nanjing, China) according to the manufacturer’s instruction. The renal tissues were homogenized and dissolved in an extraction buffer to determine the malondialdehyde (MDA) and superoxide dismutase (SOD) content using a commercial kit (Beyotime Institute of Biotechnology, Changsha, China), according to the manufacturer’s instructions. The MDA content was expressed as nanomoles per milligram of proteins.
2.3 Immunohistochemistry
Paraffin-embedded sections were used to deparaffinize and rehydrate, and to be prepared as previously described in immunohistochemistry. The sections were then incubated with a primary antibody against NLRP3 (Santa Cruz Biotechnology, Santa Cruz, CA; 1:100 dilution) and an anti-F4/80 rat monoclonal antibody (1:100; Santa Cruz Biotechnology) at 4°C overnight, washed, and then incubated with a secondary antibody at room temperature for 30 min. 3,3′-diaminobenzidine tetrahydrochloride was used for signal development after washing with phosphate buffer saline (PBS) three times, and then the sections were counterstained with 20% hematoxylin. The stained sections were digitalized and analyzed using a microscope (FSX-100; Olympus, Tokyo, Japan). The F4/80-positive area was measured as a percentage of positively stained cells multiplied by the staining intensity using ImageJ 1.47v (U.S. National Institute of Health, Bethesda, MD).
2.4 Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling apoptosis assay
Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) assay was performed to detect cell apoptosis using an in situ cell death detection kit (Roche, Netley, NJ) according to the manufacturer’s protocol. Immunofluorescent staining of NLRP3 was also performed in renal tissues. Cell nuclei were counter-stained with 4′,6-diamidino-2-phenylindole (DAPI). The number of apoptotic cells was calculated as TUNEL+/DAPI+ cells in random 10 (×200) fields per section.
2.5 In vitro CIH model and siNLRP3 transfection
The human renal proximal tubular cell line human kidney-2 (HK-2) was obtained from American Type Culture Collection (Manassas, VA) and cultivated in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum at 37°C in humidified 5% CO2. The medium was replaced every other day. HK-2 cells were cultivated to 60%–80% confluence in culture medium containing no penicillin or streptomycin. Application of CIH or NA in vitro was done using a custom-designed computer-controlled incubator chamber attached to an external O2–CO2 computer-driven servo-controller (Biospherix, Lacona, NY) as previously described (Almendros et al., 2014). The selected IH profile consisted of maintaining cells at 37°C in a custom-made chamber in which O2 concentration was alternated, by injecting nitrogen or oxygen, between 0% and 22% every 30 min, in 5% CO2. The dissolved O2 inside the culture medium was monitored by a laser O2 probe (Biospherix) and the IH reached to 5% O2 and 21% O2 as hypoxic and normoxic values sensed by the cells. The NA conditions corresponded to 21% O2 and 5% CO2. For gene knockdown studies, NLRP3 small interfering RNA (siRNA; sense: 5′-GGUGUUGGAAUUAGACAAC-3′; antisense: 5′-GUUGUCUAAUUCCAACACC-3′) and control siRNA (sense: 5′-UUCUCCGAACGUGUCACGUTT-3′; antisense: 5′-ACGUGACACGUUCGGAGGAGAATT-3′) were synthesized by GenePharma (Shanghai, China). Transfection was done by Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. In particular, the HK-2 cells were transfected with NLRP3 siRNA or control siRNA 48 hr before hypoxia treatment. miR-155 mimics (miR-mimics), anti-miR of miR-155 (anti-miR), and miRNA control (control) were purchased from GenePharma. HK-2 cells were transfected with miR-mimics (20 nM), miRNA control or anti-miR (50 nM) using Lipofectamine 2000. Successful depletion of NLRP3 protein was confirmed by immunofluorescent staining.
2.6 Immunofluorescent staining for NLRP3
After reaching more than 80% confluence, HK-2 cells were transferred to a serum-free culture medium for 24 hr and then exposed to hypoxia. After stimulation, the cells were fixed by 1 ml 4% paraformaldehyde at room temperature for 15 min, permeabilized with 0.1% Triton X-100 for 5 min and washed with PBS three times, and then stained with primary antibodies overnight at 4°C. The cells were washed a further three times, and then the secondary Alexa Fluor® 488-conjugated antibody (Santa Cruz) was added and incubated for 1 hr at room temperature. Eventually, the cells were treated with DAPI. Images were acquired with a fluorescence microscope.
2.7 Measurement of ROS production
Briefly, the cells were stained with 50 μM of 2′,7′-dichlorofluorescin diacetate (DCFH-DA; Sigma) at 37°C in a dark place for 30 min. After washing with PBS, the cells were detected by the quantitation of fluorescence intensity under a fluorescence microscope.
2.8 Cell apoptosis detected by flow cytometry
HK-2 cells were collected and washed with PBS three times, and then incubated with Annexin V–fluorescein isothiocyanate (FITC) and propidium iodide (PI) according to the manufacturer’s instruction (FITC apoptosis detection kit; BD Biosciences, San Diego, CA). Quantification was then conducted by FACSCalibur (BD Biosciences). The lower and upper right quadrants show the proportions of the early (Annexin V+/PI−) and the late (Annexin V+/PI+) apoptotic cells, respectively.
2.9 RNA extraction and reverse transcription-quantitative polymerase chain reaction
For quantitation of miR-155, total miRNA extraction was conducted using the RNAeasy Small RNA Isolation Kit (Beyotime Biotechnology). The expression of miR-155 was performed in triplicate using the ployA assays and the respective nmiRNA primer purchased from Guangzhou RiboBio Co., Ltd. (Guangzhou, China). Then the samples were normalized by evaluating the U6 expression. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was performed on an ABI 7500 with an SYBR green mastermix (Takara Bio Inc., Shiga, Japan). Data were normalized using the 2−∆∆Ct method for relative quantification.
2.10 Luciferase assay
FOXO3a 3′-untranslated regions (UTRs) containing conserved miR-155 binding sites as well as 3′-UTRs with mutated sites were synthesized by GenePharma and amplified by qPCR. The forward primer was 5′-AGAAGTGTATGAGTGAGAGGCA-3′, and the reverse primer was 5′-CTGGCTCACTGACAAGCAGA-3′. The 3′-UTR luciferase vector (150 ng) was cotransfected into HK-2 cells with either a miR-155 mimic or inhibitor using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA) and 20 ng of Renilla luciferase reporters were used as an internal control. After 48 hr, the cells were collected and lysed. A luciferase activity assay was performed using the Dual Luciferase Reporter Assay System (Promega Biotech Co., Ltd.) according to the manufacturer’s instructions.
2.11 Western blot
Western blot analysis was carried out on both the cultured HK-2 cells and the mice tissue lysates. All denatured proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE gel) and transferred to polyvinylidene difluoride membranes (Roche). The membranes were blocked with 5% bovine serum albumin (BSA) in Tris-buffered saline and then incubated with primary antibodies as follows: FoxO3a (cs-12829, Cell Signaling Technology, Beverly, MA, USA), anti-Bcl-2 (cs-3498), anti-Bax (ab32503, Abcam, Cambridge, MA), NLRP3 (ab214185), pro-caspase1 and cleaved p20 (sc-1218; Santa Cruz), ASC (sc-376916; Santa Cruz), IL-1β (sc-52012; Santa Cruz) GAPDH (cs-5174) and anti-β-actin (Sigma-Aldrich; 1:2,000 dilution). The samples were then incubated with a horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibody after thoroughly washing three times with phosphate buffered saline with Tween-20 (PBST). The bands were visualized using the chemiluminescence (ECL) detection system (Thermo Fisher Scientific) and quantified by Image J gel analysis software.
2.12 Statistical analysis
Data were expressed as mean ± standard error of the mean. A two-tailed Student t test was used to compare two groups. Analysis of variance with Bonferroni correction was used to assess the statistical significance for comparisons between multiple groups. The differences were considered statistically significant when the P value < 0.05.
3 RESULTS
3.1 NLRP3 deficiency modulates CIH-induced renal injury in vivo
With H&E staining, we found that CIH induced remarkable renal structure damage in WT mice, such as inflammatory cellular infiltration, extensive tubular vacuolization, tubular epithelial cell exfoliation, and thickening of the glomerular basement membrane. And these alterations were notably alleviated in NLRP3−/− mice, which indicated that renal injury was significantly hampered in NLRP3−/− mice compared with the controls (Figure 1a). Moreover, we found that exposure of mice to CIH increased the serum levels of Cr, a marker of renal function, whereas the serum Cr levels were relatively but not significantly lower in NLRP3 deficient mice. The oxidative stress biomarkers, SOD and MDA, were also examined in renal tissues. The SOD activity of the WT mice in the CIH model group was decreased compared with that of the mice in the normal group, whereas the MDA content was significantly increased (P < 0.05). Compared with the WT mice exposed to CIH, the NLRP3 knockout mice blunted the changes in SOD activity and MDA content in renal tissues. NLRP3 deficiency not only reduced CIH-induced MDA formation but also showed a tendency to lessen the SOD depletion (Figure 1b).
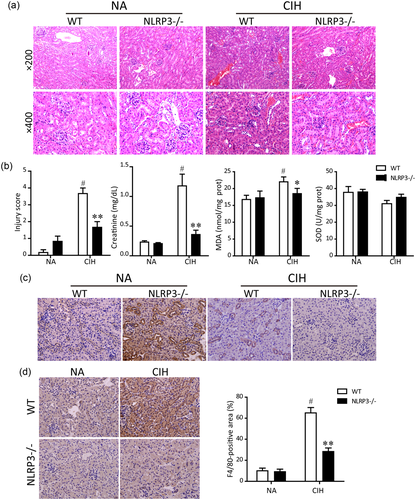
Protective effects of NLRP3 deficiency on CIH-induced renal injury in vivo. (a) Representative histological kidney sections from normal air (NA) group, CIH + WT group, CIH + NLRP3 knockout group, and negative control (NA + NLRP3 knockout) group stained by hematoxylin and eosin (H&E; magnification ×200 and ×400). (b) Quantitative assessment of renal injury score based on the 0–5 Jablonski grading scale. Renal function was determined by plasma creatinine levels. MDA and SOD activities were measured in kidney homogenates. Representative photographs of immunostaining for NLRP3 (c) and F4/80 (d) in the cortex were shown (magnification, ×400). F4/80 staining of kidney tissue digitally analyzed as percentage of positive staining per high-power field. Data are presented as means ± SEM (n = 6 in each experimental group). #P < 0.01 versus NA group; *P < 0.05 and **P < 0.01 versus CIH + WT group. CIH, chronic intermittent hypoxia; NA, normal air; MDA, malondialdehyde; NLRP3, nucleotide-binding domain like receptor protein 3; SEM, standard error of the mean; SOD, superoxide dismutase; WT, wild type [Color figure can be viewed at wileyonlinelibrary.com]
As illustrated in Figure 1c, NLRP3 was highly expressed in renal tubular epithelium of WT mice. As expected, the expression of NLRP3 detected by immunohistochemical staining was deleted in NLRP3−/− mice. In addition, CIH-induced renal damage was accompanied by activation of the NLRP3 inflammasome. Because NLRP3 inflammasome was mainly activated in inflammatory cells, we further examined the macrophage infiltration by immunohistochemical analysis using the macrophage marker F4/80. The CIH group showed a significant increase in inflammatory cell infiltration in the cortex of WT after 5 weeks of IH, whereas genetic disruption of NLRP3 notably reversed CIH-induced F4/80 expression (Figure 1d).
To investigate the effect of NLRP3 on CIH-induced renal tubular apoptosis, serial sections stained of TUNEL colocalized with NLRP3 were conducted in kidney tissues. NLRP3 was clearly stained in the cortical tubules of WT mice. In contrast, NLRP3−/− mice only expressed faint NLRP3+ cells. In WT mice kidneys, TUNEL+ cells were widely noted at the corticomedullary section upon CIH treatment, whereas TUNEL+ cells were prominently ameliorated by the deletion of NLRP3 gene in mice (Figure 2a). Quantification of TUNEL+/DAPI+ immunofluorescent staining depicted the proportion of cell apoptosis. Following CIH exposure, the absence of NLRP3 in mice underwent a 43% reduction of the apoptotic cells compared with the WT mice (Figure 2b).
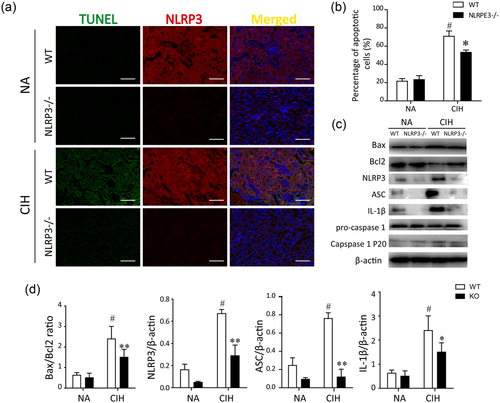
Effects of NLRP3 deficiency on renal tubular injury in vivo. (a) Apoptosis was assayed with TUNEL by costaining with NLRP3 immunofluorescence staining. Representative micrographs for TUNEL (green), NLRP3 (red), and the merged pictures from kidney tissues of each group (magnification, ×200). (b) The CIH-stimulated apoptosis was obviously abrogated in NLRP3 knockout mice. Data are presented as a percentage of apoptotic cells (TUNEL+/DAPI+ cells) in 10 random fields. (c) Protein expressions of Bax, Bcl2, NLRP3, ASC, procaspase-1, IL-1β, and cleaved caspase-1 in NLRP3−/− mice and WT mice were measured by western blot analysis. Quantification of relative protein expression was performed by densitometric analysis and β-actin acted as an internal control. Similar results were obtained from three independent experiments. All data are presented as means ± SEM. #P < 0.01 versus NA group; *P < 0.05 and **P < 0.01 versus CIH + WT group. ASC, apoptosis-associated speck-like protein containing a caspase recruitment domain; CIH, chronic intermittent hypoxia; DAPI, 4′,6-diamidino-2-phenylindole; NA, normal air; NLRP3, nucleotide-binding domain like receptor protein 3; SEM, standard error of the mean; TUNEL, terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling; WT, wild type [Color figure can be viewed at wileyonlinelibrary.com]
In response to CIH stimulation, NLRP3 inflammasome recruits and activates caspase-1 either directly or indirectly through the ASC adapter, leading to caspase-1 cleavage and subsequent activation of IL-1β. Furthermore, a correlation analysis was performed to detect whether the silence of NLRP3 would modulate their downstream component ASC, caspase-1, and IL-1β. Western bot analysis showed that the NLRP3 inflammasome member ASC as well as its downstream effectors were significantly augmented following the CIH treatment. Consistent with the above results, the ratio of Bax to Bcl2, a key factor in the regulation of apoptosis, was significantly increased in the kidneys of WT mice than that in the NLRP3−/− mice (Figure 2c). Moreover, silence of NLRP3 in mice reversed its downstream signaling and apoptotic proteins, similarly to the levels of normal control. Taken together, CIH facilitated phenotypic alteration and apoptosis of the renal cells. In addition, these findings indicated the involvement of the NLRP3 inflammasome in the pathogenesis of CIH-induced renal injuries.
3.2 Silence of NLRP3 alleviates CIH-induced apoptosis in HK-2 cells
NLRP3 inflammasome under CIH stimulation was further evaluated in vitro. First, we chose IH exposure of 48 hr as an in vitro model, and real-time RT-PCR analysis revealed that the expression of NLRP3 mRNA was markedly increased in the HK-2 cells after exposure to hypoxia for 48 hr (data not shown). Then, we silenced NLRP3 via siRNA strategy, and then performed immunofluorescent analysis for NLRP3. Obvious robust NLRP3 staining was induced by CIH in HK-2 cells, indicative of the efficiency of hypoxia in the in vitro model (Figure 3a). Meanwhile, we confirmed that NLRP3 was deleted successfully in HK-2 cells. Western blot showed that the tubular cell apoptosis induced by CIH was associated with Bax upregulation. On the contrary, suppressed expression of antiapoptosis molecule Bcl-2 was ameliorated by NLRP3 deficiency under CIH condition. In line with the in vivo experiments, CIH also promoted the member ASC, caspase-1 and IL-1β expressions in HK-2 cells following the endogenous NLRP3 protein activation. However, accumulation of proapoptotic protein Bax stimulated by hypoxia was substantially abolished by deletion of NLRP3. Moreover, the levels of ASC, activated caspase-1 and IL-1β subsequently decreased as well (Figure 3b). Afterward, we monitored the levels of ROS (Figure 3d) and determined cell apoptosis by Annexin V/flow cytometry (Figure 3e). As illustrated in Figure 3d, IH enhanced the oxidative stress as compared to the NA group, whereas si-NLRP3 effectively reduced CIH-triggered ROS generation. Also, the percentage of early apoptotic cells (Annexin V+/PI−) was the highest following CIH exposure. Conversely, the number of apoptotic cells was significantly lower in HK-2 cells with NLRP3 deficiency exposed to hypoxia. Consistent with the results in NLRP3−/− mice, our in vitro data suggested that transfection of HK-2 cells with specific NLRP3 siRNA dramatically ameliorated CIH-induced cell apoptosis compared with si-NC (P < 0.001). Accordingly, these results suggest that NLRP3 inflammasome-mediated oxidative stress and apoptosis in the in vitro model of CIH.
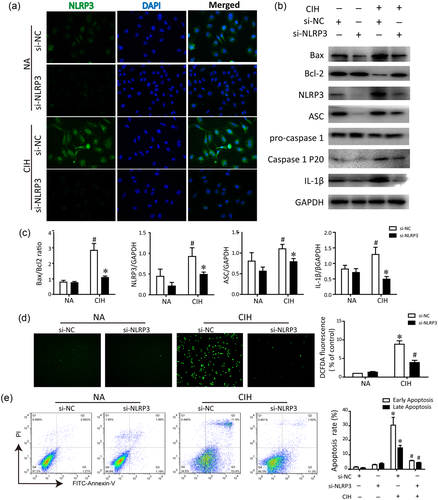
Effects of NLRP3 deficiency on CIH-induced renal tubular cell apoptosis in vitro. (a) Gene-modified HK-2 cells were stained with NLRP3 (green fluorescence) and colocated with DAPI (blue). (b) Western blot analysis of NLRP3, ASC, procaspase-1, IL-1β, caspase-1, Bax, and Bcl-2 protein expression in comparison with GAPDH used as a loading control in a vitro model of CIH. The results of statistical analysis were shown three independent replicates. #P < 0.01 versus NA group; *P < 0.01 versus CIH + control siRNA group. (c) Representative bar diagrams showing quantitative relative protein levels in each group. (d) ROS was determined by staining with DCFH-DA and observed under a fluorescent microscope (magnification is ×20). The relative fluorescence intensity was quantified. (e) The rate of apoptosis in HK-2 cells transfected with NLRP3 si-RNA or control siRNA (si-NC) was measured by Annexin-V/PI staining. Similar results were obtained from three independent experiments. All data are presented as the means ± SEM. *P < 0.01 versus NA group; #P < 0.01 versus CIH + control siRNA group. ASC, apoptosis-associated speck-like protein containing a caspase recruitment domain; CIH, chronic intermittent hypoxia; DAPI, 4′,6-diamidino-2-phenylindole; DCFH-DA, 2′,7′-dichlorofluorescin diacetate ; IL-1β, interleukin-1β; NA, normal air; NC, control; NLRP3, nucleotide-binding domain like receptor protein 3; PI, propidium iodide; ROS, reactive oxygen species; SEM, standard error of the mean; siRNA, small interfering RNA [Color figure can be viewed at wileyonlinelibrary.com]
3.3 Inhibition of endogenous miR-155 reduces hypoxic NLRP3 induction
In this study, we first analyzed the expression status of miR-155 in response to hypoxia. Real-time qPCR analysis showed that in contrast with normoxia, miR-155 in HK-2 cells was dramatically elicited by IH exposure. Similarly, the levels of miR-155 in WT mice were shown to be up-regulated following the renal hypoxic injury. Importantly, we further demonstrated that NLRP3 deficiency could decrease the miR-155 alteration in response to CIH both in vivo and in vitro (Figure 4a). These results suggest that this miR-155 expression also required the NLRP3 inflammasome activation. In addition, we have found that knockdown of miR-155 by the inhibitor restored FOXO3a levels in cells, as verified by western blotting analysis. In contrast, transfection of the miR-155 inhibitor exerted an inhibitory effect on the NLRP3 and ASC expression, as well as the activated caspase-1 and IL-1β. Furthermore, when subjected to hypoxia, the expression of NLRP3 inflammasome in HK-2 cells treated with miR-155 inhibitor was revealed to be lower than that in HK-2 cells with negative control (Figure 4b).
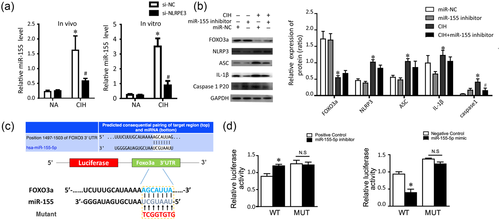
Inhibition of endogenous miR-155 reduces hypoxic NLRP3 induction. (a) Relative expression levels of miR-155 in renal tissues and HK-2 cells in response to CIH. (b) Representative immunoblotting of HK-2 cells transfected with miR-155 inhibitor in the presence or absence of CIH. Quantification densitometric analysis summarized the fold changes of protein levels normalized to GAPDH. *P < 0.01 versus miR-NC group; #p < 0.05 versus CIH group. (c) miR-155 directly targeted FOXO3a in HK-2 cells. Sequence prediction by TargetScan revealed the miR-155 target sites, and FOXO3a 3′-UTR construct was mutated at the predicted miR-155 site. (d) Luciferase reporter activities of chimeric vectors carrying the luciferase gene and a fragment of the FOXO3a 3′-UTR in the control, miR-155 mimic, and miR-155 inhibitor groups. WT is the plasmid construct that contains FOXO3a 3′-UTR wild-type. Mut is the plasmid construct that contains FOXO3a 3′-UTR mutant type. The experiment was performed in triplicate. Data are expressed as means ± SEM. *P < 0.05 versus control group. CIH, chronic intermittent hypoxia; miR-155, microRNA-155; NC, control; NLRP3, nucleotide-binding domain like receptor protein 3; NS, not significant; UTR, untranslated regions; SEM, standard error of the mean; WT, wild-type [Color figure can be viewed at wileyonlinelibrary.com]
Given that FOXO3a was the potential targeted gene of miR-155 after retrieval of TargetScan, mutated sequences were designed to verify whether miR-155 affected the predicted binding sites and luciferase activity. WT sequences and mutant sequences derived from the FOXO3a 3′UTR with a deletion in the predicted miR-155-binding sites were inserted into a luciferase reporter plasmid (Figure 4c). HK-2 cells were cotransfected with miR-155 mimics and WT (Wt-miR-155 FOXO3a) or mutant (Mut-miR-155/FOXO3a) recombinant plasmids. Our findings demonstrated that the luciferase activity of the Wt-miR-155/FOXO3a plasmid significantly decreased by 42% (P < 0.01), whereas no significant effect of the miR-155 mimics on the luciferase activity of the Mut-miR-155/FOXO3a plasmid was found (P > 0.05; Figure 4d). HK-2 cells transfected with miR-155 inhibitor and WT (Wt-miR-155/FOXO3a) or mutant (Mut-miR-155/FOXO3a) recombinant plasmids were also examined at the same time, indicative of FOXO3a as a direct target of miR-155.
3.4 MiR-155 mediates NLRP3 inflammasome signaling through targeting FOXO3a
As upregulation of miR-155 was observed in the hypoxia process, we transfected miR-155 mimics into HK-2 cells to investigate how the exogenous miR-155 affects the NLRP3 inflammasome signaling. As illustrated in Figure 5a, miR-155 mimics resulted in an increase in ROS levels, compared with control. Activation of the NLRP3 inflammasome is well known to be dependent on the generation of ROS. Thus, inhibition of NLRP3 was shown to be capable of suppressing ROS production when the HK-2 cells were pretreated with miR-155 mimics for 24 hr. Consistently, the percentage of late apoptotic cells (Annexin V+/PI+) was significantly higher in the miR-155 mimics treated group than the control. Furthermore, upon exposure of miR-155 mimics, a significant decrease of the late apoptosis in the si-NLRP3 treated cells was observed, compared with the si-NC treated cells (Figure 5b). The results indicated that the similar effects elicited by miR-155 on ROS and cell apoptosis as hypoxia stimulation, were eaccomplished by activation of the NLRP3 inflammasome.
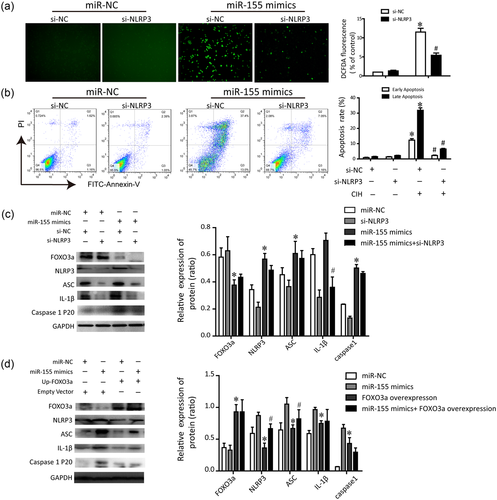
MiR-155 mediates NLRP3 inflammasome through downregulating FOXO3a. (a) miR-155 mimics elicited ROS generation was inhibited by NLRP3 deficiency. (b) Apoptosis of HK-2 cells transfected with miR-155 mimic in the presence or absence of NLRP3 was assayed by Annexin-V/PI staining. (c) Representative immunoblotting changes in the protein levels of HK-2 cells transfected with miR-155 mimic in the presence or absence of NLRP3. The quantitative densitometric ratios normalized to GAPDH were shown as histograms. The experiment was performed in triplicate. Data are expressed as means ± SEM. *P < 0.01 versus control siRNA group; #P < 0.01 versus CIH + FOXO3a overexpression group. CIH, chronic intermittent hypoxia; miR-155, microRNA-155; NLRP3, nucleotide-binding domain like receptor protein 3; PI, propidium iodide; ROS, reactive oxygen species; SEM, standard error of the mean; siRNA, small interfering RNA [Color figure can be viewed at wileyonlinelibrary.com]
Next, we attempted to elucidate the possible mechanisms underlying the link between miR-155 and NLRP3 inflammasome by western blotting. We found that miR-155 mimic positively modulated the FOXO3a expression. Similar results were obtained by analyzing changes in the levels of the activated ASC/caspase-1 and IL-1β pathways, after upregulation of miR-155 in HK-2 cells Figure 5c). Knockdown of NLRP3 by siRNA significantly inhibited these effects, whereas exerted a slight effect on FOXO3a expression. Subsequently, we retained the FOXO3a level by construction overexpression plasmid. In addition, the levels of NLRP3 inflammasome protein of different treatment groups were determined. Our results showed that overexpression of FOXO3a dampened the NLRP3 protein and enhanced the ASC/caspase-1/IL-1β, whereas pretreatment with a miR-155 mimic slightly reserved this (Figure 5d). All the data reveal that miR-155 played a key role in modulating the hypoxia-associated oxidative stress. On the other hand, miR-155 may participate in the positive regulation of NLRP3 inflammasome, at least in part by downregulating FOXO3a.
4 DISCUSSION
NLRP3 inflammasome is regarded as a general sensor of injury in response to microbial and nonmicrobial stimuli (Chen & Nunez, 2010). Many exogenous and endogenous factors, including toll-like receptor (TLR) agonists or oxidative stress, are capable of facilitating NLRP3 activation. NLRP3 inflammasome can be upregulated in both classical immune cells such as infiltrating macrophages as well as the renal tubular epithelial or endothelial cells (Wang et al., 2013). In particular, the contribution of the inflammasome to macrophages is of interest, given the pathogenic role of macrophage infiltration in intrarenal chemokine production and the inflammatory response in the progression of various renal diseases (S.M. Kim et al., 2015). CIH-induced renal injury is an inflammatory process that has been described previously with involvement of nuclear factor-kB and mitogen-activated protein kinase (MAPK) pathways. During the phase of CIH, oxygen deficiency in renal cortex or medulla accelerates the generation of local ROS, and further activates the innate immune system in kidneys. Within the cytoplasm, NLRP proteins form a complex with ASC and active caspase-1. Some research groups have identified mitochondrial function and accumulated ROS as potential triggers of the NLRP3 inflammasome (Salminen, Ojala, Kaarniranta, & Kauppinen, 2012; Zhuang et al., 2014). Mitochondrial ROS promoted the NLRP3 inflammasome complex assembly, activating the inflammatory caspase-1 and the subsequent inflammatory cytokines IL-1β release. Since excessive production of proinflammatory cytokines is harmful to the host, renal tubular inflammation infiltration preceded into the kidney, leading to tissue damage and organ dysfunction (Vilaysane et al., 2010).
Emerging evidence indicates the detrimental role of NLRP3 in a wide spectrum of kidney injuries (Chang, Ko, & Clark, 2014). Interestingly, in the current study, this extends to models of CIH, which suggests that the deleterious effect of hypoxia on the proximal tubular cells is, in part, mediated by inflammasome activation. Moreover, genetic deletion of NLRP3 displayed attenuated renal injury and protected mice from dysfunction and macrophage infiltration after 5 weeks of CIH. It is worthy to note that a marked increase of TUNEL-positive tubules was seldom observed in the genetic deletion of NLRP3, indicative of its connection with apoptosis. The altered levels of cleaved caspase-1, and IL-1β were correlated with structural and functional changes that occurred in these renal tissues. Our study confirmed that the impressive increase in serum Cr was absent in NLRP3 gene knockout mice. All of these findings were in agreement with a study of ischemia-reperfusion injury by Bakker et al (2014) who were concerned with the ameliorated kidney tubules damage and neutrophil influx in the NLRP3−/− mice. Although as a mediator of ischemic acute kidney injury (AKI), the role of NLRP3 inflammasome has not been verified by another research group in cisplatin-induced AKI (Kim et al., 2013). It was suggested that serum Cr and tubular apoptosis score were not significantly decreased in NLRP3 knockout mice compared with WT mice. Furthermore, through modulating the activity of apoptosis-associated proteins, NLRP3 deletion strikingly suppressed the apoptosis and phenotypic alternation of HK-2 cells in an in vitro model of CIH, accompanied with alterations of NLRP3 downstream cytokines. Consistent with the above results, another study revealed HK-2 cells with NLRP3 silence reversed the degradation of antiapoptotic proteins, as well as the ascendant proapoptotic protein Bax in the contrast media-induced acute kidney injury (Shen et al., 2016).
Several groups have confirmed that ROS could function as a key molecule to mediate the NLRP3 downstream signaling. As demonstrated by Ding et al (2016) in the murine model, administration of the antioxidant effectively decreased ROS production, thereby reducing the levels of NLRP3 compared with control mice. Notably, besides hypoxia, hyperoxia-induced ROS also resulted in recruitment of inflammatory cells, elevation of IL-1β, and macrophage inflammatory protein, all of which were attenuated in the NLRP3−/− mice (Fukumoto et al., 2013). Moreover, J. Wu et al (2013) observed that lung cyclic stretch-induced IL-1β release was mediated by mitochondrial ROS-dependent signaling, inhibition of which completely abolished IL-1β release following the mechanical stimulation. Our study showed that the production of ROS in NLRP3 deficiency was decreased in response to miR-155 mimics, which was partly due to the interference with NLRP3 activation. These findings together with ours suggest that CIH activates NLRP3 inflammasome partially through ROS production.
The NLRP3 inflammasome has garnered much attention as well as has broader implications in cation flux, endoplasmic reticulum (ER) stress, and mitochondrial dysfunction (Elliott & Sutterwala, 2015). However, there is a paucity of literature about the regulatory mechanism of miRs. In the present work, we have identified that miR-155 profoundly upregulated after CIH both in vitro and in vivo. Interestingly, eliminating the NLRP3 signaling outburst had a significant negative effect on the increased miR-155 level. These results indicated that hypoxia-induced miR-155 was probably driven by inflammasome activation, since inhibition of the inflammasome signaling cascade could abolish the augmented miR-155. A previous study revealed that miR-155 targeted multiple components, such as Sh2 domain containing inositol phosphatase-1 and suppressor of cytokine signaling 1 in the intracellular signaling pathways, thus mainly playing an important role in the inflammatory and immune process (Tili, Michaille, & Croce, 2013). Given that miR-155 has been proposed to target the 3′-UTRs of the FOXO3a gene, subsequently, both gain- and loss- of function techniques were used to manipulate miR-155 and investigate its regulatory effects on FOXO3a in HK-2 cells. It is well established that FOXO3a can trigger or inhibit apoptosis depending on the cell types or cellular context. Although the data from these findings are confounding, FOXO3a promoted cell survival through inducing metabolic adaptation to hypoxia, and thus protected quiescent cells from oxidative stress (Li, Zhang, Chen, Fan, & Fang, 2012).
Our data indicated that miR-155 might be a positive-regulator of NLRP3 signals by inhibiting the targeted FOXO3a gene. There are several lines of evidence to support this conclusion. First, the miR-155 inhibitor suppressed the CIH-induced NLRP3 signaling pathway such as IL-1β, which can stimulate the production of a variety of proinflammatory chemokines. Because of dysfunctional NLRP3 inflammasome activation, HK-2 cells were resistant to the oxidative stress induced by CIH. On the contrary, the presence of the miR-155 mimics resulted in a lower level of FOXO3a to coordinate with oxidative stress, accompanied by activation of NLRP3 signaling. Therefore, miR-155 mimics have also been postulated to render HK-2 cells hypersensitive to hypoxic damage such as apoptosis and ROS generation. Nevertheless, upon miR-155 mimics exposure, overexpression of the basal level of FOXO3a further inhibited the ascendant NLRP3 downstream signals. Despite these advances, the exact molecular mechanism will require further investigation. Taken together, these findings suggest that the NLRP3 inflammasome is required for miR-155 expression. In addition, miR-155 control NLRP3 inflammasome and IL-1β cytokine signaling cascade through a positive feedback loop, thus enhancing inflammatory cytokines production. Consistent with our results, another recent study has demonstrated that miR-155 is dependent on the NLRP3 inflammasome activation, for miR-155 expression could be blocked when inflammasome was inhibited (Artlett, Sassi-Gaha, Hope, Feghali-Bostwick, & Katsikis, 2017). H. Wu et al (2016) reported that overexpression of miR-155 resulted in increased levels of caspase-1, IL-1β, and IL-18, whereas knockdown of miR-155 attenuated the inflammatory cell death of HK-2 cells, indicating that anti-miR-155 could be a strategy for the prevention of renal pyroptosis. Similarly, we naturally speculated that aberrant expression of miR-155 following CIH may be of significant relevance to NLRP3-mediated oxidative stress. Thus, the attractive therapy designed to target the NLRP3 inflammasome activation or block its downstream effectors may prove useful in the treatment of progressive CKD.
In summary, CIH stimulates ROS production, which in turn assembles NLRP3, ASC and caspase-1, leading to the processing and maturation of pro-IL-1β into the active IL-1β variant in the kidney. Our study for the first time demonstrates the essential role of NLRP3 inflammasome in the CIH-induced renal injury. Activation of the NLRP3 inflammasome required integrated redox signals such as miR-155. Inhibition of the NLRP3 inflammasome ameliorated renal damage, as well as interrupted robust miR-155 release, suggestive of a positive feedback mechanism between miR-155 and NLRP3 inflammasome activation. Defining the regulatory molecules that modulate NLRP3, activation will be instrumental to understand the mechanism of inflammation initiation in response to cellular stress or oxidative damage, and further reveal attractive candidates for new therapeutic interventions.
ACKNOWLEDGMENTS
This work was financially supported grants from the National Natural Science Foundation of China to S. Li (Grant no. 81570081, 81770083), a research grant from Shanghai Tenth People’s Hospital to W.Y. Gu (Grant no. SYGZRPY2017028) and the Shanghai Three-year Plan of the key subject construction in public health infectious diseases and pathogenic microorganism in China (Grant no. 15GWZK0102).
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
W.G. conceived and designed the experiments. Shuqi Zhang and J.J. performed the experiments and analyzed the data. X.W. has contributed to manuscript preparation. S.L. finally approved the version to be submitted.