Osteogenic differentiation of preconditioned bone marrow mesenchymal stem cells with lipopolysaccharide on modified poly-l-lactic-acid nanofibers
Abstract
Tissue engineering is an interdisciplinary expertise that involves the use of nanoscaffolds for repairing, modifying, and removing tissue defects and formation of new tissues. Mesenchymal stem cells (MSCs) can differentiate into a variety of cell types, and they are attractive candidates for tissue engineering. In the current study, the electrospinning process was used for nanofiber preparation, based on a poly-l-lactic-acid (PLLA) polymer. The surface was treated with O 2 plasma to enhance hydrophilicity, cell attachment, growth, and differentiation potential. The nanoscaffolds were preconditioned with lipopolysaccharide (LPS) to enhance induction of differentiation. The nanoscaffolds were categorized by contact angle measurements and scanning electron microscopy. The MTT assay was used to analyze the rate of growth and proliferation of cells. Osteogenic differentiation of cultured MSCs was evaluated on nanofibers using common osteogenic markers, such as alkaline phosphatase activity, calcium mineral deposition, quantitative real-time polymerase chain reaction, and immunocytochemical analysis. Based on the in vitro results, primed MSCs with LPS on the PLLA nanoscaffold significantly enhanced the proliferation and osteogenesis of MSCs. Also, the combination of LPS and electrospun nanofibers can provide a new and suitable matrix to support stem cells’ differentiation for bone tissue engineering.
1 INTRODUCTION
Biomedical engineering has been applied for the development of substitutes for damaged tissues or organs and costly problems in human health care (Galbraith, Skalak, & Chien, 1998). Tissue engineering has been readily applied for the formation of a variety of connective tissues, such as bone, cartilage, ligament, or tendon, in vitro and in vivo (Vacanti & Langer, 1999). In recent years, this new field has progressed in several areas, including cell isolation, expansion, lineage specific differentiation, and transplantation (Kim et al., 2012). Research has also focused on other methods that are related to tissue engineering, including the use of bioactive matrix materials as tissue nanoscaffolds, local or systemic delivery of various hormones and growth factors or other chemical compounds, and other methods to control the culture condition (Burg, Porter, & Kellam, 2000, Petite et al., 2000, Slaughter, Khurshid, Fisher, Khademhosseini, & Peppas, 2009, Tamura et al., 2001). Careful selection of four key components is required for the development of such structures, including nanoscaffolds, growth factors, extracellular matrix (ECM), and cells (Shieh & Vacanti, 2005). There are at least two kinds of stem cells, hematopoietic stem cells and stem cells for nonhematopoietic tissues, in bone marrow that are referred to as mesenchymal stem cell (MSCs) or marrow stromal cells (Castro-Malaspina et al., 1980, Zaidi & Nixon, 2007). Stem cells are undifferentiated cells with the potential of self-renewal and differentiation into various lineages of specialized functions, so they have a special place in tissue engineering (Zaidi & Nixon, 2007). Nanofibers have been investigated for the cultivation and differentiation of MSCs due to their surface properties, porosity, and biodegradability (Fodor, 2003). Many research works have been published about nanofibers based on poly-l-lactic-acid (PLLA) as biodegradable and biocompatible polymers over synthetic polymers in vitro and in vivo (Rokkanen et al., 2000, Zhi-Hua, Jian-Ming, Zhong-Cheng, & Jian-Peng, 2007). PLLA can react with something as a carrier of supported tissue for a given length of time before its gradual biodegradation (Agrawal & Ray, 2001).
Also, in recent decades, the effects of some materials, such as various hormones and growth factors or other chemical compounds, have been assayed for differentiations of MSCs. One of these compounds is lipopolysaccharides (LPS). LPS, as the major component of the outer surface of gram-negative bacteria, differentiates into bone cells. It is often beneficial for immune modulatory properties in small amounts but causes endotoxic shocks in larger amounts. This structure shares a common architecture that strongly affects its activity. These molecules are comprised of a lipid, glycosidic, and O-chain part. The first part is called lipid A, which is considered the endotoxic component. The second part is called glycosidic, which consists of a core of approximately 10 monosaccharides and in "smooth-type" LPS. The third part consists of repetitive subunits of one to eight monosaccharides responsible for much of the immune specificity of the bacterial cell (Caroff & Karibian, 2003). A precondition exists to determine the optimal dose of LPS, which causes increased differentiation of cells in vitro and in vivo (Janzen et al., 2006).
In our previous studies, we reported that MSCs derived from murine BM cultured in the presence of H2O2 (sublethal dose) were able to uptake large amounts of the drug without significant signs of toxicity. Interestingly, H2O2-primed MSCs decreases senescence of bone marrow–derived MSCs under sublethal doses of oxidative stress (Bahmani et al., 2014).
The aim of this study is preconditioning of MSCs with LPS and cultured on PLLA. The osteogenic differentiation of MSCs was investigated after cell culture on PLLA nanoscaffolds that were loaded with LPS to enhance induction of differentiation.
2 MATERIALS AND METHODS
2.1 Materials
Dulbecco’s modified Engle’s medium (DMEM), high-glucose medium (Gibco, Germany), 10% fetal bovine serum (FBS) (Sigma-Aldrich, MI), streptomycin and penicillin antibiotics (1% S/P) (Sigma-Aldrich) were used for a normal medium cell. Trypsin/ethylenediaminetetraacetic acid (Sigma-Aldrich) and Dulbecco’s phosphate buffers saline (D-PBS) (Sigma-Aldrich) were used for passaging cells and washing, respectively. Dimethyl sulfoxide (Sigma-Aldrich) was used for freezing cells. Dexamethasone (Peprotech), ascorbic acid 2–phosphate (A.A), and β-glycerol phosphate (Merck, Japan, New Jersey) were used for adding the differentiation medium cells. Deionized distilled water (Iran) and ethanol (Merck) were used. Ethidium bromide (Merck) was used for DNA staining in electrophoresis operation. An alkaline phosphatase assay kit (Sigma-Aldrich), a calcium content assay kit (Sigma-Aldrich), and alizarin red (Sigma-Aldrich) were used to test the differentiation of bone. PLLA nanofiber (Stem Cell Technology Research Center, Iran), glutaraldehyde, paraformaldehyde (Merck), Runix2 (Cinnagen, Tehran, Iran), collagen Type 1 (Col I; Sinazhen), osteocalcin (Sinazhen), polymerase chain reaction (PCR) assay kit (Fermentas, MA), real time assay kit (Takara Bio Inc, Japan), and LPS (Sigma-Aldrich) were used. The primers were purchased from Iran.
2.2 Fabrication of PLLA nanofiber by electrospinning techniques
Nanofibers were prepared by the electrospinning techniques. The PLLA polymer was dissolved at a concentration of 6% in chloroform and dimethyl formaldehyde (ratio of 4:1). After dissolving and stabilizing, 10 ml solution was transferred to a plastic syringe for devices with an iota of stainless steel with an outer diameter of 18 gauges (270/1 mm equivalent), which were installed on a high voltage source. The setting of the device was voltage of 15 kV, feed rate of 4/0 ml/hr, distance from the nozzle to the collector (collector animated) 15 cm, and total travel speed of 400 rpm. In addition, a piece of aluminum foil is covered to clean, process and collect the produced fibers.
2.3 Scanning electron microscopy analysis
The size and morphology of the PLLA nanofiber was analyzed by a scanning electron microscope with a thin layer of sputtered gold on the sample. The coated samples were imaged at an accelerating voltage of 3 kV with a scale bar of 10 and 100 μm.
2.4 Plasma treatment
The plasma treatment was performed in order to make the surface hydrophilic, and the surface feature of the nanofiber changed for better bonding cells. The plasma process was accomplished by a generator with a low frequency of 40 kHz and 30 Watts of power into a cylindrical quartz reactor. First, the plasma process was done in a vacuum environment. Secondly, the high-purity O2 gas was blown into a chamber, and the pressure in the chamber stabilized to 4.0 mbar. In continuance, O2 was flowed into the chamber until a uniform atmospheric pressure was achieved. The plasma process was performed for 4 min. Then O2 was flowed again for 10 min into the chamber. Finally, the vacuum was broken and the samples were exposed to air.
2.5 Contact angle measurement
The contact angle of water was measured with the sticky fiber drop method using a contact angle measurement product model Cruz (from Germany) to study hydrophilic PLLA nanofiber after chemical modification at room temperature. In this method, a water drop is placed on the surface of nanofiber in this unit, and the contact angle is read after 10 s.
2.6 Cytotoxicity assay and cell viability
In this test, the MTT assay was used to compare the growth and proliferation of stem cells on nanofiber nanoscaffolds. Four wells contain precondition fibers with LPS, fibers without LPS, only LPS and control wells without any factors. The prepared sterile nanoscaffolds were cut and placed in a 96-well culture dish with 10,000 cells. The culture dish was placed in an incubator at 37°C and 5% carbon dioxide for 2 hr, and then 500 ml of culture medium was added to each well. After the primary culture in the given time of 1, 3, and 5 days, 50 ml of the MTT solution (5 mg/ml medium) was added to each well culture plate and incubated for 3 hr. After the formation of crystals inside the cells, formazan was placed on the nanoscaffold in plastic tubes and a certain amount of solvent to dissolve the crystals formazan was added to the tube. After complete dissolution of crystals, absorbance was measured at 570 nm until a purple color was attained. The control well was used without the nanoscaffold, in which cells were directly cultured on the surface of the flask. Finally, the number of the cells was determined on nanoscaffold and prepared with regard to the calibration curve.
2.7 Culturing and seeding
BM-MSCs were cultured in minimum essential medium supplemented with 10% FBS and 1% P/S. Culture flasks were incubated in an incubator at 37°C with 5% CO2. Culture media was replaced every 48 hr until the cells reached 80% confluency. Confluent cells were subcultured and used at passage 2 for this study. PLLA nanoscaffolds (0.2–0.3 mm thick & 10 mm diameter) were sterilized by exposure to UV light for 1 hr on each side and placed in 48-well culture plates. The nanoscaffolds were washed with D-PBS three times and preincubated with culture media overnight. Ten thousand cells were seeded on the nanoscaffolds for the evaluation of cell adhesion, proliferation, live/dead assay, ALP assay, while 25,000 cells were seeded to carry out real-time PCR and other staining experiments. Osteogenic induction medium was prepared in MEM growth media supplemented with 50 µl dexamethasone, 500 µl β-glycerophosphate, and 500 µl ascorbic acid-2 phosphate (Wei, Hu, Xie, Lin, & Chen, 2009). LPS was removed in between the optimal dose and added to the differentiation medium, here, some of the cells and fibers with our neighboring. Also, the normal cultured cells were compared with induction media.
2.8 Determination of optimal dose of LPS on MSCs and cell precondition with LPS
The first LPS was prepared from Sigma. After that, a different concentration was applied from stoke 1 mg/ml between 0.1 and 5 µg on the cells. The cell death was evaluated by MTT assay. After obtaining the optimal dose, the cells were preconditioned below the lethal dose. After 14 days of differentiation, the cell differentiation on PLLA nanofiber was investigated by the scanning electron microscopy (SEM) analysis.
2.9 Osteogenic differentiation
For osteogenic induction of MSCs, the basal medium was replaced with an osteogenic medium, which contained DMEM supplemented with 10% FBS, 50 mg/ml ascorbic acid 2-phosphate, 10 nM dexamethasone, and 10 mM β-glycerophosphate. The cultures were then placed in an incubator at 37°C and 5% CO2 for 2 weeks.
2.9.1 Calcium content assay
The calcium content assay measured the amount of calcium that deposited on cultured stem cell on the nanoscaffold and thermos. First, all inorganic salts precipitate was collected by normal hydrochloric acid 6/0. Then, the calcium content was measured using a commercial kit that was a mix of Krsuftalyn. The standard concentration was used for dissolved calcium in the kit, which produced a standard curve of optical density versus concentration.
2.9.2 Alkaline phosphatase assay
Alkaline phosphatase (ALP) was measured as a phenotypic marker of bone formation after 14 days of culture using an ALP substrate kit (Sethuraman et al., 2010, 2011). At the end of each time point, the media was removed, the nanoscaffolds were washed with D-PBS and cells were lysed using 1% triton X-100. Hundred microliter of the cell lysate was added to 400 µL of substrate and incubated at 37°C for 30 min. The reaction was stopped by the addition of 0.4 M sodium hydroxide solution, and the absorbance was measured at 410 nm using a multimode reader.
2.9.3 Alizarin red staining
The spent medium was removed after 14 days of culture. The cells were washed with D-PBS and fixed in 10% formalin for 15 min at room temperature. The formalin was gently removed and washed twice with double distilled water. After the formalin fixation, the nanoscaffolds were incubated in alizarin red dye (40 mM; pH 4.3) at room temperature (Wei et al., 2009). The dye was removed and washed for three to four times with double distilled water. The stained nanoscaffolds were transferred to a fresh culture dish and imaged using a phase contrast inverted microscope.
2.10 Real-time reverse transcription-PCR analysis
The expression of bone specific markers, named ALP, was related to runt-related transcription factor 2 (Runx2), Col I, and osteocalcin, which were evaluated using a real-time reverse transcription-PCR (RT-PCR). Total RNA was extracted and random hexamer primer complementary DNA (cDNA) synthesis was carried out using revert aid first strand cDNA synthesis kit cDNA and used for 40 cycles PCR in a Rotor-gene Q real-time analyzer (Ravichandran, Gandhi, Sundaramurthi, Sethuraman, & Krishnan, 2013; Sundaramurthi, Vasanthan, Kuppan, Krishnan, & Sethuraman, 2012). Real-time PCR was performed using the Maxima TM SYBR Green/ROX qPCR Master Mix followed by melting curve analysis to confirm PCR specificity. Each reaction was repeated three times, and average threshold cycle was used for data analysis by the Rotor-gene Q software (Corbett). HPRT1 was used as a housekeeping gene to calculate the change in the target gene expression. The target genes were normalized against HPRT1 and calibrated to PLLA nanoscaffolds with LPS and LPS. The genes and related specific primers are illustrated in Table 1.
| Gene | Primer sequence | Length (bp) |
|---|---|---|
| HPRT1 | 5′-CCTGGCGTCGTGATTAGTG-3′ | 125 |
| 5′-TCAGTCCTGTCCATAATTAGTCC-3′ | ||
| ALP | 5′-GCACCTGCCTTACTAACTC-3′ | 162 |
| 5′-AGACACCCATCCCATCTC-3′ | ||
| Collagen type I | 5′-TGGAGCAAGAGGCGAGAG-3′ | 122 |
| 5′-CACCAGCATCACCCTTAGC-3′ | ||
| RUNX2 | 5′-GCCTTCAAGGTGGTAGCCC-3′ | 67 |
| 5′-CGTTACCCGCCATGACAGTA-3′ | ||
| Osteocalcin | 5′-GCAAAGGTGCAGCCTTTGTG-3′ | 80 |
| 5′-GGCTCCCAGCCATTGATACAG-3′ |
- Note: ALP: alkaline phosphatase; RT-PCR: reverse transcription-polymerase chain reaction; RUNX2: runt-related transcription factor 2.
2.11 Acridine orange assay
Acridine orange (AO) is an organic compound. It is used as a nucleic-acid-selective fluorescent cationic dye and is useful for cell cycle determination. Being cell-permeable, it interacts with DNA and RNA by intercalation or electrostatic attractions, respectively. When bound to DNA, it is very similar spectrally to fluorescein, with an excitation maximum at 502 nm and an emission maximum at 525 nm (green). When it associates with RNA, the excitation maximum shifts to 460 nm (blue) and the emission maximum shifts to 650 nm (red). AO also enters acidic compartments such as lysosomes and becomes protonated and sequestered. In these low pH conditions, the dye will emit orange light when excited by blue light. Thus, AO can be used to identify engulfed apoptotic cells, because it will fluoresce upon engulfment. The dye is often used in epifluorescence microscopy (Varghese, Fischer-Hammadeh, & Hammadeh, 2011). In this test, the liquid was simply brought out and added to AO solution (1 µl) that contained 100 μg/ml AO, and 100 μg/ml EB (AO/EB) was added to each well and held for 30 min in dark. The AO was removed and washed, and photo fluorescents were taken in the final steps.
2.12 Immunocytochemistry
The cells were rinsed twice with PBS and then fixed with 4% paraformaldehyde for 20 min. The cells were permeabilized with 0.4% Triton X100 in PBS for 10 min. The fixed cells were blocked for 30 min at 37°C with 5% goat serum/PBS-tween-20 and reacted overnight at 4°C in a humid chamber with the respective primary antibodies that included osteocalcin (1:100; Santa Cruz Biotechnology, INC) and osteopontin (1:200; Santa Cruz Biotechnology, INC). At the end of the incubation time, the cells were rinsed three times with PBS-tween-20 (0.1%) and incubated with the fluorescein isothiocyanate–conjugated anti mouse IgG as the secondary antibody (1:100) at room temperature for 1 hr. After rinsing with PBS, the nuclei were counterstained with DAPI, and then the cells were analysed with a fluorescent microscope.
2.13 Statistical analysis
The two-way analysis of variance was used to evaluate the significance between the incubation days for cell proliferation, ALP activity, and gene expression. Statistical significance was evaluated at a p value less than 0.05.
3 RESULT
3.1 Nanoscaffold characterization
PLLA was fabricated using the optimized electrospinning parameters. The porous structure was shown for fabricated PLLA nanofibrous nanoscaffolds with a diameter of 2 to 10 µm. The nanofibers were illustrated with diameter of 200 nm, a smooth morphology, and length of 150 to 600 nm. The surface hydrophilicity of nanofibers was strongly increased after O2 gas surface treatment. Then, the surface of PLLA nanofiber was investigated by SEM. Moreover, the morphology or the average diameter of nanofibers was not affected after plasma treatment and contact angle (Figure 1). Surface hydrophilicity of nanofibers was strongly increased after O2 gas surface treatment, and the contact angle was decreased from 135° in PLLA nanofibers.

SEM micrograph of fabricated PLLA nanoscaffolds. PLLA: poly-l-lactic-acid; SEM: scanning electron microscopy [Color figure can be viewed at wileyonlinelibrary.com]
3.2 Cell proliferation
The biocompatibility of the coated nanoscaffolds was investigated via the MTT assay, which revealed a significant increase on the proliferation rate of MSCs cultured on PLLA nanofibrous nanoscaffolds (Figure 2).
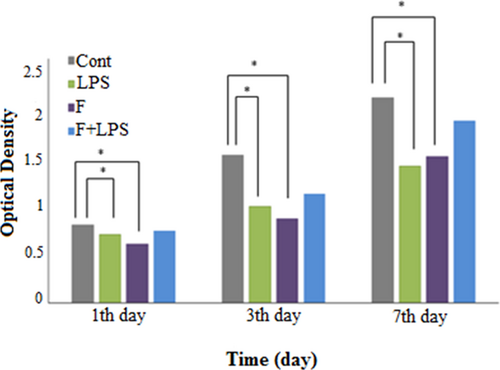
Proliferation of MSCs on fiber-LPS compared with PLLA and TCPS during a 7 day culture period. LPS: lipopolysaccharide; MSCs: mesenchymal stem cells; PLLA: poly-l-lactic-acid [Color figure can be viewed at wileyonlinelibrary.com]
3.3 AO finding test
The apoptotic cell of demonstrated by EB/AO staining and fluorescent microscopy. As illustrated in Figure 3, fiber and optimized concentration of LPS did not increase apoptosis in the MSCs.
3.4 Determination of optimal dose of lethal LPS on MSCs
The cells were preconditioned with a sublethal concentration of LPS. Then, cell viability was determined (5 µg sublethal LPS).
3.5 Osteogenic morphology
Figure 3 showed the morphology of MSCs (a) and differentiated cells (b, c, and d) by AO/EB staining. The difference was clearly specified between the morphology of differentiated cells and MSCs. In osteogenic, cell density and cell spreading were higher than MSCs.
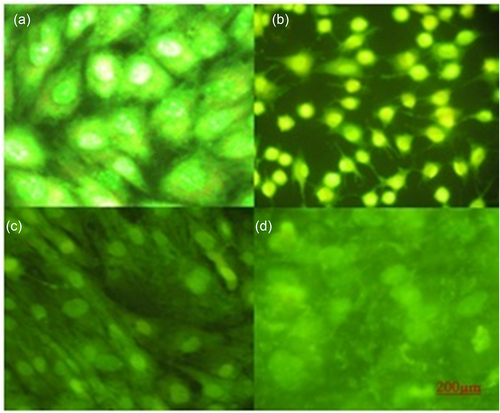
Acridine orange staining of (a) MSCs (b) differentiated cells on TCPS, (c) LPS and fiber-LPS. LPS: lipopolysaccharide; MSCs: mesenchymal stem cells [Color figure can be viewed at wileyonlinelibrary.com]
3.6 ALP expression
The pattern of ALP activity was investigated during osteogenic differentiation of MSCs on nanoscaffolds with and without LPS and TCPS on Days 7 and 14. It is worth noting that nanoscaffolds that were loaded with LPS exhibited different values. The highest ALP activity was measured on LPS-modified nanoscaffolds compared with other groups (Figure 4).
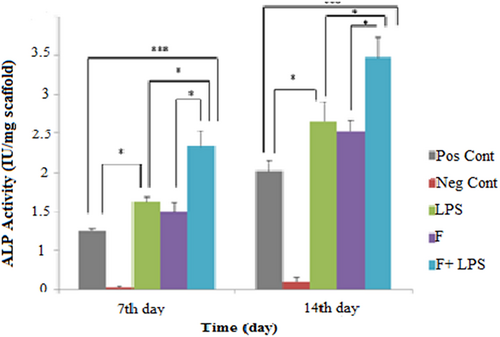
Alkaline phosphatase (ALP) activity during 2 weeks of osteogenic differentiation. The ALP activity was higher in fiber-LPS compared with the other groups. Significant levels are *p ≤ 0.05. LPS: lipopolysaccharide [Color figure can be viewed at wileyonlinelibrary.com]
3.7 Calcium content Assay
As in the late osteogenic assessment, calcium deposition was measured in differentiated MSCs on Days 7 and 14 of osteogenic induction (Figure 5). However, the nanoscaffolds that were preconditioned with LPS exhibited different values.
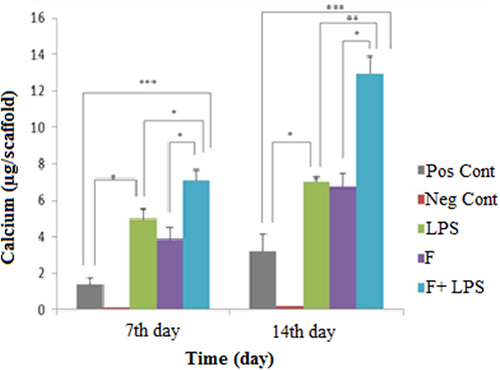
Calcium content during osteogenic differentiation. Highest mineral deposition was observed in fiber-LPS compared with other groups. Significant levels are *p ≤ 0.05. LPS: lipopolysaccharide [Color figure can be viewed at wileyonlinelibrary.com]
3.8 Alizarin red staining and mineralization
Mineralization was assessed as the late marker of differentiation toward osteogenic using alizarin red staining. Calcium deposition was significantly enhanced on fiber-LPS (Figure 6a) in comparison with fiber without LPS in 14 days (Figure 6b).

Alizarin red staining of MSCs on (a) LPS, (b) fiber and (c) fiber-LPS after a 14 days culture under osteogenic medium. LPS: lipopolysaccharide; MSCs: mesenchymal stem cells [Color figure can be viewed at wileyonlinelibrary.com]
Also, the SEM micrograph of fiber-LPS showed the huge amount of calcium that deposited in differentiated cells on preconditioning nanofibers with LPS (Figure 7a). It was compared with the other group without LPS (Figure 7b) on 14 days. There was a significant difference between the amount of mineralized calcium on these nanoscaffolds.

SEM micrograph of seeded cells on PLLA nanoscaffolds after two weeks of differentiation process. It is obviously clear that calcium mineralization on fiber-LPS (a,b) was improved in comparison to fiber without LPS (c,d). LPS: lipopolysaccharide; PLLA: poly-l-lactic-acid; SEM: scanning electron microscopy [Color figure can be viewed at wileyonlinelibrary.com]
3.9 Gene expression analysis
Gene expression was analyzed on 7 and 14 days for quantifying the difference between the messenger RNA (mRNA) levels of osteogenic markers, Runx2, Col I, ALP, and osteocalcin. The increase trend of Runx2 expression was observed on fiber-LPS during the time of induction. This gene was expressed at a higher level on Day 7 in the fiber-LPS compared with LPS and TCPS, but its expression was downregulation on 14 days. The expression of ALP was significantly increased during the differentiation in treated MSC-LPS-PLLA. In addition, osteonectin was expressed in a higher amount on 7 and 14 days in PLLA coated with LPS compared with other groups (p < 0.05). Also, in this study, the mRNA level of Col I showed a significant higher expression in comparison with pristine nanofibers and TCPS in treated PLLA with LPS on 7 and 14 days (Figure 8).
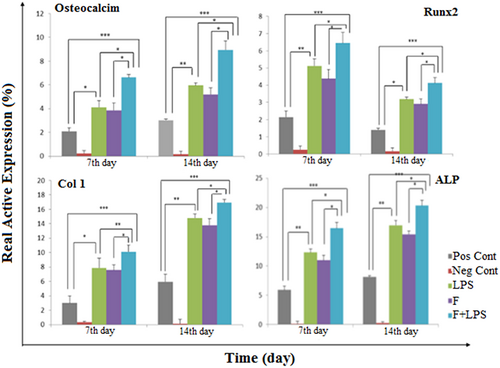
Relative expression of ALP, Col I, osteocalcin, and Runx2 on 7 and 14 days in MSCs on both nanoscaffolds during osteogenic process. The REST software was used for gene expression analysis using real-time PCR data from the rotor-gene Q. HPRT1 was used as a control for RNA sample quality. Results are presented as mean ± SD. Significant levels are *p ≤ 0.05. ALP: alkaline phosphatase; Col I: collagen Type 1; MSCs: mesenchymal stem cells; PCR: polymerase chain reaction; SD: standard deviation; Runx2: runt-related transcription factor 2 [Color figure can be viewed at wileyonlinelibrary.com]
3.10 Immunofluorescence staining for osteocalcin and osteopontin
The protein of osteogenic key genes was increased in the period of differentiation of MSCs into osteogenic lineages. Immunofluorescence staining was performed to investigate the cellular localization and expression of osteogenic markers. The expression of the transcription factors, including osteocalcin and osteopontin proteins, was assayed by immunofluorescence staining, which confirmed their presence in differentiated MSCs on TCPS, LPS and fiber-LPS after 2 weeks (Figure 9).
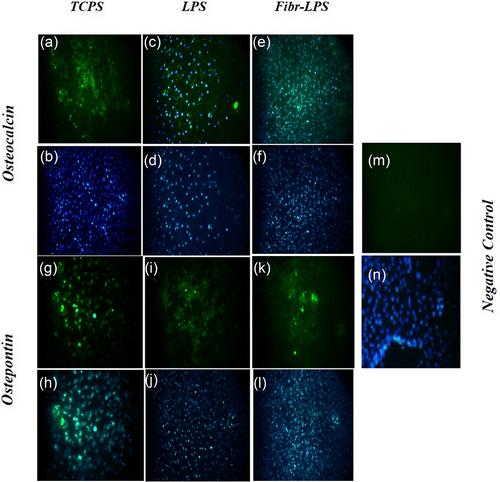
Immunofluorescence staining of differentiated MSCs on TCPS (a,b,g,h), LPS (c,d,i,j) and fiber-LPS (e,f,k,l) after 2 weeks. The cells were analyzed for the expression of osteogenic markers including osteocalcin (a-f) and osteopontin (g-l). Negative control (m,n). DAPI staining (b-f, h-l, n) with magnification × 200. LPS: lipopolysaccharide; MSCs: mesenchymal stem cells; PLLA: poly-l-lactic-acid [Color figure can be viewed at wileyonlinelibrary.com]
4 DISCUSSION
The loss or failure of an organ or tissue is one of the most frequent, devastating, and costly problems in human health care. A new field of tissue engineering applied the principles of biology and engineering to the development of functional substitutes for damaged tissue (Mikos et al., 1993). Bone tissue engineering is a rapidly developing area (X. Liu & Ma, 2004). A variety of materials have been used for the replacement and repair of damaged or traumatized bone tissues (Hench & Polak, 2002; Langer, 2000; Livingston, Ducheyne, & Garino, 2002). These materials include metals, ceramics, and polymers, including natural, synthetic, and their combinations. The mechanical and biologic properties of polymers can be tailored to different clinical applications and engineering strategies. The nanoscaffold served as a mechanical substrate for cells and bioactive factors, and it can help direct and organize the process of regeneration (Livingston et al., 2002). The ideal nanoscaffold should be biocompatible, highly porous, and biodegradable, such as PLLA nanoscaffolds. PLLA is a successful nanoscaffold for use in bone tissue engineering, which requires a high affinity for living organisms and the ability to maintain its mechanical strength until maturation of the regenerated tissue (Inui et al., 2010). The PLLA nanoscaffold plays a critical role in bone tissue engineering, and its performance could benefit from imitating the characteristics of the natural ECM (Ma, 2008) and allow the differentiation of MSCs toward osteogenic (Schofer et al., 2011). PLLA nanoscaffolds supported the highest rate of proliferation of MSCs (Schofer et al., 2011), with a tendency to higher cell densities (Badami, Kreke, Thompson, Riffle, & Goldstein, 2006; Schofer et al., 2008). So far, the experiment was performed in conjunction PLLA with the following preconditional MSCs on it and the results were shown to be successful (Janzen et al., 2006). In the electrospinning method, the majority of pore diameters were limited to the range of 25 to 100 µm. This scale of pore diameter was adequate for cell migration of most cell types, such as mesenchymal cells, whose diameters are about 10 to 15 µm (Daei-farshbaf et al., 2014). Previous investigations mentioned the relationship between pore diameter and tissue ingrowth. For vascular grafts, the effective pore diameter was between 20 and 60 µm for cell in growth while 75 to 150 µm pore diameter was required for bone cells (Recum et al., 1996). The precondition caused the latest increase in gene expression as well as an increase in viability and proliferation and delay in aging (Choudhery et al., 2012). In addition, after preconditioning MSCs with LPS, significantly increased cell viability was observed compared with untreated MSCs. Li and colleagues. investigated LPS that differentially affected the osteogenic differentiation of periodontal ligament stem cells and bone marrow MSCs (Schuster et al., 2014).
In the current study, PLLA nanofibers were made by the electrospinning method. Also, gas plasma treatment has been introduced as one of the most important methods for altering the chemical properties of surfaces. Gas was ionized by an electric discharge, and then active gas molecules were reacted with the surface and produced the active functional groups (Lai et al., 2006). O2 plasma was affected by the functional groups introduced by plasma treatment diffused from the polymer surface, and their surface concentration was optimized to minimize the free energy of the interface between the polymer and the aging medium (Murakami, Kuroda, & Osawa, 1998). O2 treatment plasma of PLLA nanoscaffolds resulted in the surface functionalization with COOH and OH groups, which enhanced hydrophilicity, growth, and cell attachment (Cheng, Lee, Komvopoulos, Yan, & Li, 2013). Our results showed the suitable porosity and very low contact angle resulted in PLLA nanofibers that had homogenous surface for attachment and proliferation. It was confirmed by the MTT assay.
LPS effects were assessed upon proliferation and differentiation of MSCs cultures using real-time PCR. LPS enhanced the proliferation of MSCs in a dose-dependent manner (Choudhery et al., 2012, Schuster et al., 2014). Candidate PLLA nanofiber and preconditioned MSCs have several properties for application in bone tissue engineering such as osteoconductivity, biocompatibility, suitable biochemical, and mechanical characteristics and should be able to enhance regeneration and reconstruction of bone defects (Daei-farshbaf et al., 2014, Marcacci et al., 2007). In this regard, LPS helped to induce signals to be further differentiated.
In this study, MSC-LPS was used on the surface of PLLA nanofibers for the enhancement of proliferation and differentiation of MSCs. Osteogenic differentiation of MSCs was analyzed by various physical and biochemical factors (Jha, Jackson, & Healy, 2014). Osteogenically secreted ALP as an ectoenzyme, degrading the inorganic pyrophosphate and causing the increase in phosphate levels, thereby activating mineralization process. Therefore, ALP activity is considered as a direct measure of the functional activity of Osteogenic (Hosseinkhani, Hosseinkhani, Tian, Kobayashi, & Tabata, 2006). In the current study, significantly higher levels of ALP activity were observed in LPS and fiber-MSC-LPS after 7 and 14 days of culture, which was confirmed. Fiber-MSC-LPS can induce the osteogenic differentiation of MSCs in the presence and absence of induction factors. In a previous study, it was shown that LPS could help in the maturation of osteogenic and thus increase the ALP levels after 7 and 14 days of culture (Ravichandran, Sundaramurthi, Gandhi, Sethuraman, & Krishnan, 2014).
In this study, the morphology and viability of the cells before and after differentiation was evaluated by AO staining. AO was used as a nucleic acid-selective fluorescent cationic dye useful for cell cycle determination (K. Liu, Liu, Liu, & Wu, 2015). On the other hand, it has been proven that differentiated cells show lower stainability (Darzynkiewicz, Traganos, Kapuscinski, Staiano-Coico, & Melamed, 1984). Thus, fiber-LPS and LPS illustrated more efficient osteogenic differentiation than TCPS and fiber wells.
MSCs differentiation in mRNA level was evaluated by the expression of osteogenic specific markers by immunofluorescence experiments as well as real-time RT-PCR analysis after 2 weeks of induction. In this study, Runx2, Col I, ALP and osteonectin, the four major bone-related genes were selected during osteogenic differentiation of the MSCs. With respect to osteogenic, it is well-established that Runx2 (formerly called Cbfa1), a member of the runt homology domain transcription factor family, plays a crucial role in osteoblast development. This gene is a member of the RUNX family of transcription factors and encodes a nuclear protein with a Runt DNA-binding domain. This protein is essential for osteoblastic differentiation and skeletal morphogenesis and acts as a nanoscaffold for nucleic acids and regulatory factors involved in skeletal gene expression (Roos et al., 2015). In our study, the data on 7 days showed the higher expression of this gene in fiber-LPS compared with other groups.
Col I is the most abundant and best studied collagen. It formed more than 90% of the organic mass of bone and is the major collagen of tendons, skin, ligaments, cornea, and many interstitial connective tissues with the exception of very few tissues such as hyaline cartilage, brain, and vitreous body (Fleischmajer, Douglas macdonald, Perlish, Burgeson, & Fisher, 1990). Col I has an important role in biomineralization and is expressed in high levels near the end of the proliferative state and during the period of matrix deposition (Fleischmajer et al., 1990). The increase in Col I expression was accompanied during the first week of differentiation by strong induction of RNA levels that were detected on 14 days. However, the expression patterns of Col I contributed to the higher expression of this gene on fiber precondition with LPS. Also, there was a different expression between bare LPS, PLLA nanoscaffold, and TCPS.
Osteogenically secreted ALP is an ectoenzyme, which degrades inorganic pyrophosphate and causes an increase in phosphate levels, thereby activating the mineralization process. Therefore, ALP activity was considered as a direct measurement of the functional activity of osteogenes (Chen et al., 2014). The results of ALP gene expression showed an increase in Athe LP mRNA level in MSCs on both fiber-LPS on 7 and 14 days in comparison with TCPS during osteogenic differentiation. But there was a significant higher level of differentiation on PLLA nanoscaffold preconditioned with LPS.
Osteonectin is a phosphorylated glycoprotein, which has a role in regulating the initiation and promotion of mineralization and crystal growth (Termine et al., 1981). In this project, it was revealed that the expression of osteonectin increased in MSCs during differentiation on all groups. This may be due to the osteoprogenitor culture conditions (Bellows, Heersche, & Aubin, 1990, Mendes et al., 2002). But there was a significant expression in MSCs on nanoscaffolds precondition with LPS on days 7 and 14. This indicates that LPS could significantly improve cell proliferation and osteogenic differentiation of MSCs in vitro.
Furthermore, immunofluorescence staining revealed the expression of osteogenic specific markers including osteocalcin. The osteopontin was higher in fiber-LPS, LPS than TCPS after 2 weeks. In this project, it achieved more than 95% expression of osteocalcin and osteopontin after osteogenic induction of MSCs. These results were in agreement with qPCR data acquired from the differentiated MSCs.
In this study, the osteogenic differentiation was demonstrated for MSCs on PLLA nanofibers than preconditional with LPS, which could be a differentiation of better osteogenesis. The results showed that the effect of preconditional with LPS of an increase in the expression of markers associated with osteoblastic lineage, which directly promoted osteoblast proliferation, mineralization and bone formation and this novel construct could be an implantable stem cell-seeded nanoscaffold to improve bone healing and also offers a promising alternative for the existing therapies (Sundaramurthi, Jaidev, Ramana, Sethuraman, & Krishnan, 2015).
5 CONCLUSION
The aim of the present study was to fabricate PLLA nanofibers and to investigate their potential to support the adhesion, proliferation, and osteogenic differentiation of BM-MSCs. PLLA nanofibers preconditioned with LPS could be used as an appropriate nanoscaffold for efficient regeneration of bone defects. In addition, preconditional with LPS showed better differentiation of MSCs. However, in vitro analysis is needed to assess the capacity of PLLA nanoscaffolds that are preconditional with LPS for increased signal in bone regeneration. We believe that this new approach may improve the design strategies that are currently used to fabricate novel nanoscaffolds for bone tissue engineering.
CONflICTS OF INTEREST
The authors declare that they have no conflicts of interest.




