Recombinant human hepatocyte growth factor provides protective effects in cerulein-induced acute pancreatitis in mice
Abstract
Acute pancreatitis is a multifactorial disease associated with profound changes of the pancreas induced by release of digestive enzymes that lead to increase in proinflammatory cytokine production, excessive tissue necrosis, edema, and bleeding. Elevated levels of hepatocyte growth factor (HGF) and its receptor c-Met have been observed in different chronic and acute pancreatic diseases including experimental models of acute pancreatitis. In the present study, we investigated the protective effects induced by the recombinant human HGF in a mouse model of cerulein-induced acute pancreatitis. Pancreatitis was induced by 8 hourly administrations of supramaximal cerulein injections (50 µg/kg, ip). HGF treatment (20 µg/kg, iv), significantly attenuated lipase content and amylase activity in serum as well as the degree inflammation and edema overall leading to less severe histologic changes such as necrosis, induced by cerulein. Protective effects of HGF were associated with activation of pro-survival pathways such as Akt, Erk1/2, and Nrf2 and increase in executor survival-related proteins and decrease in pro-apoptotic proteins. In addition, ROS content and lipid peroxidation were diminished, and glutathione synthesis increased in pancreas. Systemic protection was observed by lung histology. In conclusion, our data indicate that HGF exerts an Nrf2 and glutathione-mediated protective effect on acute pancreatitis reflected by a reduction in inflammation, edema, and oxidative stress.
1 INTRODUCTION
During the last few decades, several life style factors such as lipid rich diet and increased alcohol consumption common in the western world led to a significant increase in obesity and diabetes mellitus. The same etiological factors could also be associated with a considerable rising frequency in acute pancreatitis (AP). In consequence, AP is now the leading cause of hospital admissions for gastrointestinal disorders in many countries (Yadav & Lowenfels, 2013). Notably, AP is significantly contributing to the burden of GI diseases and, with around 150 million dollars per year, associated with high economic pressure on healthcare in United States (Peery et al., 2015).
AP is a rapid onset of inflammation of the organ, initiated by the release of digestive enzymes in the pancreatic interstitium as well as systemic circulation with increased proinflammatoy cytokine production. AP is characterized by an excessive tissue necrosis, apoptosis, edema, bleeding, among other signs (Yagci et al., 2004). Further, although the molecular mechanism is not well understood, there is a clear association to the activation cascade of digestive enzymes in the pancreas as well as increased oxidative stress. The latter has been extensively demonstrated in different experimental models as well as in humans (Bopanna et al., 2017; Xie et al., 2017).
The antioxidant and protective responses elicited by the hepatocyte growth factor (HGF) and its receptor c-Met in many liver diseases have been extensively characterized by our group (Dominguez-Perez et al., 2016; Gomez-Quiroz et al., 2008, 2016; Marquardt et al., 2012). The protective effects of the c-Met signaling is mediated by a fine regulation of the redox homeostasis, inducing repression or induction of ROS production (Clavijo-Cornejo et al., 2013). HGF/c-Met signaling exerts its function by activating key signaling pathways named PI3K/Akt, Erk1/2, Nrf2, among others, and these target antioxidants proteins, phase 2 and 3 of detoxification response. In addition, it has been reported that HGF can mediate an immunomodulatory function (Ilangumaran, Villalobos-Hernandez, Bobbala, & Ramanathan, 2016), along to the well-known anti-apoptotic and proliferative properties (Trusolino, Bertotti, & Comoglio, 2010).
Mechanistic effects of HGF signaling in pancreas are not fully understood. It has been reported that absence of HGF signaling, for example, by genetic deletion of c-Met, affects size of pancreatic islets and diminishes insulin secretion (Dai, Huh, Thorgeirsson, & Liu, 2005). In the exocrine pancreas, the expression of HGF and c-Met are dramatically increased during chronic pancreatitis and cerulein-induced AP in rats (Menke, Yamaguchi, Giehl, & Adler, 1999). Other study reports that HGF decreases damage by increasing IL-10 and concomitantly decreasing the rate of amylase and lipase in serum (Warzecha et al., 2001). In the present work, we aimed to define a potential protective role of HGF signaling in AP by exogenous supply of recombinant human HGF (rhHGF) in an AP model in mice, in order to evaluate HGF as supportive therapy during the course of the disease.
2 MATERIALS AND METHODS
rhHGF was purchased from Peprotech (Rocky Hill, NJ), primers for real time RT-PCR were obtained from Integrated DNA Technologies (IDT, Coralville, Iowa), all materials were purchased from Sigma–Aldrich, St. Louis, MO (Merck KGaA, Darmstadt, Germany) except when it is indicated.
2.1 Animal treatments
CD1 male mice (Harlan Laboratories, Mexico City) were maintained in pathogen-free housing and cared in accordance with the Universidad Autonoma Metropolitana and the National Institutes of Health guidelines for the care and use of laboratory animals. Mice were kept in the animal facility under standard conditions (22 ± 2 °C, 50 ± 5% relative humidity, 12-hr light-dark cycle) with free access to food and water throughout the study.
2.2 Experimental pancreatitis (prevention model)
Forty 12-weeks old mice were randomly separated in four groups. The first group, the cerulein (Cn)-induced pancreatitis, received 8 hourly-administrations of supramaximal cerulein injections (50 µg/kg, ip). The pretreatment with the HGF (20 µg/kg, iv, lateral tail vein) was administrated 12 hr, before the first injection of Cn, a second dose was administrated just before the first dose of Cn. Mice were euthanized 24 hr after the first dose of Cn, the graphic experimental design is shown in Supplementary Methods 1A. Similarly, the other groups received either, Cn or HGF alone as indicated. Control group (not treated, NT) received saline solution as vehicle.
2.3 Experimental pancreatitis (reversion model)
An extra cohort of animals was treated with Cn as indicated previously, after 3 hr of last Cn dose, animals received the rhHGF dose (20 µg/kg, iv, lateral tail vein), 14 hr after that animals were euthanized by exsanguination under avertin anesthesia (Supplementary Methods 1B).
Sera were collected from all animals by cardiac puncture under avertin anesthesia.
2.4 Alpha amylase activity
Sera were diluted 1:100 using physiological sterile saline solution, amylase activity was measured by automated procedure using “Pancreas-Amylase (P-AM) Reflotron Test” kit, (Roche, Inc., Basel, Switzerland), following manufacturer's instructions.
2.5 Quantitative measurement of pancreatic lipase
Serum lipase content was addressed by using ELISA Lipase kit, (Cloud-Clone Corp., Houston, TX), assay was performed following manufacturer's instructions.
2.6 Histology
Pancreas were fixed in 10% neutral formalin overnight at 4 °C. Lungs were perfused with neutral formalin and fixation continue overnight. Paraffin sections (5 µm) were stained with H&E for histology analysis.
2.7 Lipid peroxidation
Lipid peroxidation was assayed by the production of thiobarbituric acid reactive substances (TBARS) using spectrophotometry as described (Buege & Aust, 1978).
2.8 Immunofluorescence of c-Met
Immunofluorescence of c-Met was done as we previously reported (Marquardt et al., 2012) using c-Met antibody (R&D Systems, Minneapolis, MN, # AF527). DAPI was used to identify nuclei.
2.9 Western blotting
Total protein was isolated from pancreas tissue with T-Per tissue protein extraction reagent (Pierce, Inc., Lake Worth, FL), containing EDTA 1 mM, EGTA 1 mM, protease and phosphatase inhibitor cocktail (Roche, Inc.), Western blot was performed as we previously reported (Clavijo-Cornejo et al., 2014), using specific antibodies listed in Supplementary Table S1.
2.10 Electrophoretic mobility shift assay (EMSA)
Nuclear protein was isolated from fresh tissue, using the NE-PER nuclear and cytoplasmic extraction reagent kit (Thermo Fisher Scientific, Stamford, CT). The EMSA assay was performed using the LightShift kit (Thermo Fisher Scientific) following manufacturer's instructions. We used specific DNA consensus sequences for Nrf2, TTT TCT GCT GAC TCA AGG TCCG as previously we reported (Clavijo-Cornejo et al., 2013).
2.11 c-Met expression by quantitative real time RT-PCR
Total RNA was isolated using TRIzol Reagent (Thermo Fisher Scientific). One μg of total RNA was subjected to reverse transcription using iScript Advanced cDNA Synthesis kit (Bio-Rad, Inc., UK). The amplification and quantification by real time PCR was performed using SsoAdvance Universal SYBR green Supermix (Bio-Rad), reactions were run in Bio-Rad CFX96 Touch Real-Time PCR detection system (Bio-Rad). Primers for c-Met Forward: 5′-GAC TTC AGC CAT CCC AAT GT-3′; Reverse: 3′-GGT GAA CTT CTG CGT TTG C-5′; as reported (Gordin et al., 2010), As housekeeping we used 18S Forward: 5′ TGT GGT GTT GAG GAA AGC AG 3′; Reverse: 5′ TCC CAT CCT TCA CAT CCT TC 3′.
2.12 ROS in situ determination
Animals were euthanized in parallel exclusively for in situ ROS determination (Nuno-Lambarri et al., 2016). Fresh whole pancreas was rapidly sectioned, frozen in liquid nitrogen, and embedded in optimum cutting temperature reagent (OCT, Sakura Finetec). Subsequently, 8-µm frozen sections were obtained in a cryostat (Leica CM-3050S) at −20°C and the slides were immediately incubated for 15 min, in the dark, at room temperature with dihydroethidium (DHE, 5 µM) for determination of superoxide anion radical detecting ethidium fluorescence. Samples were covered and observed using a confocal microscope at excitation and emission wavelengths of 485 and 570 nm, as we previously reported (Enriquez-Cortina et al., 2013).
2.13 Signaling pathway and antioxidant proteins determination
A time-course response of HGF in mice were performed to figure out the activation of the HGF canonical signaling pathways Akt and Erk1/2, and to address the content of key antioxidants proteins such as gamma-glutamylcysteine synthetase (γGCS), glutathione peroxidase 1 and 2 (GPX1/2), and glutathione S Transferase (GSTM). Mice were treated with HGF (20 µg/kg, iv, lateral tail vein) was administrated, after that animals were euthanized at 6, 12, and 24 hr. Pancreas samples were processed for Western blot analysis.
2.14 Caspase 3 activity
Caspase 3 activity was quantified using the caspase three synthetic fluorogenic tetrapeptide substrate Ac-DEVD-AMC (BD Pharmingen, San Jose, CA) as previously we reported (Nuno-Lambarri et al., 2016) using fresh pancreatic tissue, values were normalized regarding control sample and reported as fold change.
2.15 Glutathione determination
Both reduced and oxidized glutathione (GSH and GSSG, respectively) were determined using the Glutathione assay kit (Sigma–Aldrich, Darmstadt, Germany, # CS0260) following manufactureŕs instructions. Values are reported as GSH to GSSG ratio.
2.16 Protein content determination
Protein content was determined using the bicinchoninic acid kit (Pierce, Inc), according to the manufacturer's instructions.
2.17 Statistical analysis
Data are presented as mean ± SEM of at least three independent experiments carried out by triplicate. Comparisons between groups (NT vs. Cn), were made using Student's t test. For multiple comparisons, we used ANOVA with Mann–Whitney and Tukey–Kramer test. GraphPad Prism 6 software for OSX was used to run analysis. Differences were considered significant at p ≤ 0.05.
3 RESULTS
3.1 Cn induces the increment of serum HGF content and c-Met expression
To figure out the effects of HGF/c-Met signaling in Cn-induced pancreatitis, we measured the serum levels of HGF, and mRNA of c-Met in pancreas tissue from mice treated or not with Cn, (as depicted in Supplementary Methods 1A). HGF serum levels exhibited no changes (Figure 1a) because the Cn treatment, although a tendency to the increment is observed, in contrast, the c-Met mRNA showed a significant increase (1.57-fold, p = 0.0211, Figure 1b), however, the content of the receptor c-Met, in pancreas tissue, determined by inmmunofluorescence (Figure 1c), revealed a significant decrease, probably because c-Met receptor is internalized for signal transduction and subsequent degradation, as it is extensively reported (Gaziova, Davey, & Elferink, 2015; Li, Hill, & Elferink, 2008; Menard, Parker, & Kermorgant, 2014).
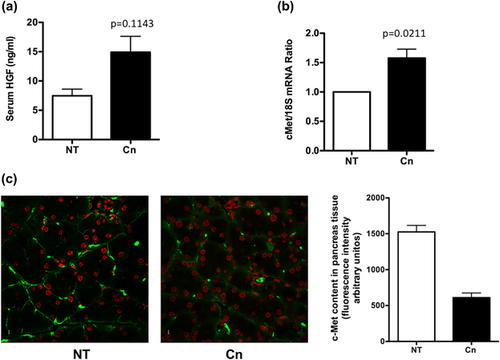
Although, c-Met expression increases because Cn treatment, there is not an efficient protection, because the well-documented effects of Cn in pancreas damage, as we proved ahead.
3.2 Exogenous rhHGF induces survival pathways activation in mice and antioxidant response
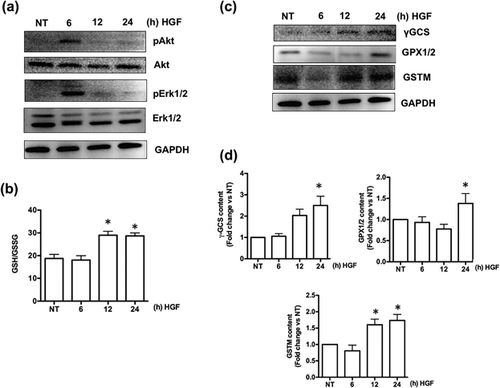
We treated mice with exogenous recombinant human HGF (rhHGF), as we previously reported in a mouse model of drug induced liver injury (Enriquez-Cortina et al., 2013). Based in the expression and membrane localization (Figure 1) of c-Met in the pancreas, we decided to explore if rhHGF alone is capable to activate a protective response in undamaged animals; first, we determined the activation of canonical survival pathways such as Akt or Erk1/2 by Western blot, Figure 2a clearly shows that rhHGF activates both signaling pathways at 6 hr post rhHGF treatment, leading to progressive increase in GSH synthesis (Figure 2b) and the expression of key protective proteins such as γGCS, GPX1/2, GSTM (Figures 2c and 2d) peaking at 24 hr of treatment.
3.3 rhHGF provides pancreas protection against acute pancreatitis
Next, we evaluated the response elicited by rhHGF in the experimental model of Cn-induced AP. rhHGF exposure led to a macroscopically visible decrease in size of the organ in HGF + Cn (Figure 3a) when comparing with Cn treatment, reflected by significant reduction pancreas to body weight ratio (Figure 3b). Consistently, histological evaluation (Figure 3c) showed typical AP signs in Cn treated mice, characterized by interstitial edema, vacuolization, and infiltration of neutrophils and mononuclear cells, whereas rhHGF treatment drastically attenuated the severity of the damage mediated by Cn.
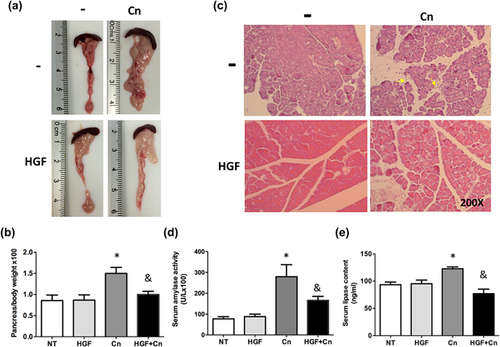
As expected, amylase activity in mice, under Cn treatment, increased threefold comparing with not treated animals, however, administration of rhHGF before the cerulein restored the activity of this enzyme to control levels (Figure 3d). Similarly, lipase serum content returned to basal levels in rhHGF treated animals, compared with the Cn treatment (Figure 3e).
Since lungs are frequently compromised in AP (Elder, Saccone, & Dixon, 2012), we next evaluated if HGF treatment induced systemic improvement in the AP model. Consistent with the less severe pancreatic phenotype, histological analysis of lung tissue revealed less pneumonic areas in HGF-treated animals, presenting a well-defined and sharp alveolar tissue compared to Cn treated mice that exhibited significant inflammatory cells (black arrows) within the alveolar tissue (Supplementary Figure S1).
To further corroborate the protective effects, we implemented another mouse model of acute pancreatitis using the essential amino acid L-Arginine (L-Arg), following the experimental design depicted in supplementary Figure S2a, according to the method reported by Chen and collaborators (Chen, Xu, Li, & Lu, 2015). Supplementary Figure S2b clearly shows that L-Arg treated animals exhibited an increase in the pancreas to body weight ratio, decreased to normal values in rhHGF pretreated animals; the serum lipase content was diminished, as well as serum amylase activity under rhHGF pretreatment (Supplementary Figures S2c and S2d), these results confirm that rhHGF exerts protective effects in AP.
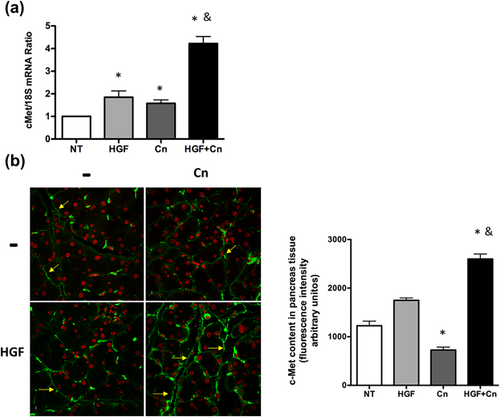
Exogenous rhHGF further increased expression of c-Met under HGF + Cn treatment, in comparison to a slight, but significantly increment in HGF or Cn treatment alone (Figure 4), suggesting that damage and HGF treatment act synergically on the expression of c-Met.
3.4 rhHGF displays antioxidant effects and decreases oxidative stress mediated damage
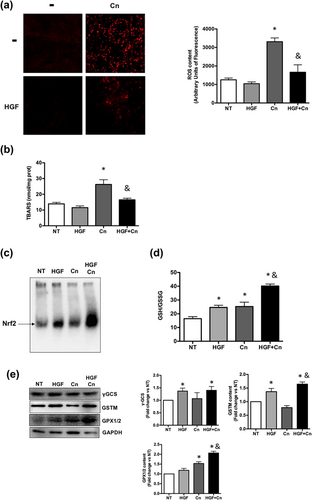
To address the effect of rhHGF in the Cn-induced AP model, we evaluated the level of ROS. In agreement with the reduction in severity of the pancreatic damage by the rhHGF a significant decrement in both, ROS generation, judged by DHE fluorescence (Figure 5a), and lipid peroxidation, was observed (Figure 5b). The protection was further associated with an activation of Nrf2 transcription factor (Figure 5c); since GSH system is driven by Nrf2 (Yang et al., 2005), content of GSH was increased in animals under rhHGF and Cn treatment (Figure 5d). We also observed an increment in GSTM and GPX1/2, two of the main enzymes that use GSH for their catalytic functions, and in γ-GCS, the limiting step enzyme in GSH synthesis (Figure 5e), suggesting an improvement of the redox system.
3.5 rhHGF treatment induces antiapoptotic protection in the AP mouse model
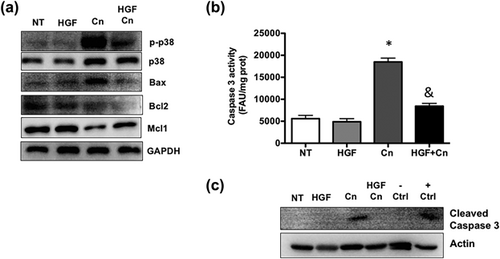
It is well known that apoptosis is a key issue in ROS-induced damage, particularly in pancreatitis (Liu et al., 2016; Xie et al., 2017). The analysis of content and activation of the main apoptosis-related proteins revealed a clear antiapoptotic response elicited by rhHGF (Figure 6a), p-p38 and Bax decrease in HGF + Cn treated pancreas tissue, interestingly, we found a null response of Blc-2, but Mcl-1 compensated this effect, in accordance with this result, the activity of caspase 3 is significantly increased in Cn treated mice, and rhHGF diminished the activation, that was corroborated by Western blot of cleaved caspase 3 (Figure 6c).
Finally, to evaluate if rhHGF can induce a healing response (reversion), we treated animals with rhHGF (20 μg/kg) 3 hr after last Cn injection (Supplementary Methods 1B) when the damage is already established; 16 hr after rhHGF treatment we proceeded to analyze some signs of pancreatitis. Interestingly, the delayed administration of rhHGF significantly decreased pancreas to body weight ratio in comparison with Cn treatment. Further, we observed improvement in macroscopic aspect of the organ, exhibiting less edema (Supplementary Figure S3b), lower serum markers of pancreas damage, lipase content and amylase activity (Supplementary Figures S2c and S2d) after Cn-induced damage. Histological analysis also showed a decrease in inflammatory infiltration and a preserved tissue architecture.
4 DISCUSSION
Acute pancreatitis is advanced to one of the major GI causes for hospital admission worldwide (Yadav & Lowenfels, 2013). This urgent need indicates the necessity to identify new options for treatment or the improvement of supportive therapies.
Cerulein-induced pancreatitis is one of the main experimental models for the study of the disease. Cn is a peptide orthologue of cholecystokinin that administrated in repeated hourly doses, can induce a severe AP (Gorelick & Lerch, 2017), resembling the AP in humans, characterized by zymogen activation, inflammation, increased values of serum amylase and lipase, and the increment in the pancreas to body weight ratio (Ulmasov, Oshima, Rodriguez, Cox, & Neuschwander-Tetri, 2013; Zhang et al., 2013), we corroborated this presentation in the present work (Figure 3).
The protective properties on damage repair and regeneration by HGF/c-Met in many organs, particularly the liver has been extensively described by our group and others (Enriquez-Cortina et al., 2013; Gomez-Quiroz et al., 2016; Marquardt et al., 2012). Some studies aimed to elucidate the effects of HGF in pancreatic diseases have been published, observing positive roles in acinar and beta-cells (Aparicio et al., 2003; Alvarez-Perez et al., 2014; Gaziova et al., 2016).
It has been proposed that HGF serum levels may be useful as severity marker in AP at the hospital admission, and may be related to organ dysfunction (Ueda et al., 1997), but it is clear that physiological and pathological circulating HGF levels are not sufficient to restore the full pancreas function.
Several studies demonstrated that the absence of c-Met signaling could worsen the damage in several organs (Gomez-Quiroz et al., 2008; Huh et al., 2004), including pancreas (Gaziova et al., 2016). In the present work, we focused on the potential beneficial effects elicited by an exogenous supply of rhHGF in AP. As it can be observed in Figure 3, HGF displays a physiological-relevant protective response when is administrated before the damage induced by Cn (preventive), such response was also observed when HGF was administrated post Cn treatment (reversion, supplementary Figure S3), demonstrating that exogenous rhHGF is also capable to induce significant protective effects after Cn.
One of the main systemic sources of HGF are lungs (Nakamura & Mizuno, 2010), which respond because of damage, particularly in epitheliums. It is fully demonstrated that lungs are principally compromised in acute pancreatitis (Elder et al., 2012; Zhang et al., 2013), in fact, in our model, we observed pneumonic areas in Cn treated animals (Supplementary Figure S1), the compromise observed in lungs in AP could be related to a poor response of the HGF/c-Met systems. Tissue damage, by itself, is enough to induce the expression and membrane localization of the basal c-Met in epithelial cells, but maybe the HGF necessary to induce the healing response is not. Cn induces a rapid internalization of c-Met (Hoffmann et al., 2006), the synthesis and localization of new c-Met could take hours, the c-Met mRNA levels, 24 hr after Cn treatment, increases 1.57-fold (p = 0.0211, Figure 1b), but the content of the receptor in plasma membrane is barely detected (Figure 1c), possible because the receptor has been used and internalized (Hoffmann et al., 2006), but the presence of rhHGF increases and sustains the c-Met expression and localization at 24 hr (Figure 4) assuring the repair or protective response as we showed in Figure 3. It is possible that the main protective mechanism of rhHGF be the induction of the expression of new c-Met receptor.
AP results in oxidative stress which, along the oxidative damage, can amplifies the inflammatory process through the recruitment and release of pro-inflammatory mediators, leading to a systemic inflammatory response (Bopanna et al., 2017; Criddle, 2016; Dabrowski, Konturek, Konturek, & Gabryelewicz, 1999), we corroborated this characteristic in the present study, Cn-treated mice exhibited increased inflammation (Figure 3c, yellow arrows), not only in the pancreas but lungs exhibited multiple pneumonic foci (Supplementary Figure S1). As expected, rhHGF induced an efficient control of ROS generation and oxidative damage judged by lipid peroxidation (Figures 5a and 5b), these were related to an increment in GSH synthesis (Figure 5d), previously we have extensively addressed the antioxidant response elicited by HGF in the liver, finding that GSH systems is the major component on such response (Gomez-Quiroz et al., 2008), this was related to a less inflammatory infiltration in pancreas and lungs, and a better histology.
In this study, we show that HGF is activating the canonical survival pathways such as Akt and Erk 1/2 and these were related to Nrf2 activation (Figure 5c) and to the increment of γ-GCS, the limiting rate enzyme in GSH synthesis, and one of the main canonical targets of Nrf2 (Yang et al., 2005), along to the Nrf2 antioxidant properties, it is known that can display regulatory effects on inflammation (Zhang, Chen, Song, Chen, & Rovin, 2005) promoting an efficient inflammatory response. The increment of the GSH-related antioxidant enzymes, and the GSH synthesis, supports the thesis that GSH system is the main mechanism of HGF-induced tissue protection, now demonstrated in the pancreas. Interestingly, we found the decrement on the content of key cytotoxic proteins, such as p38 and Bax (Figure 6a), and discrete increment of the antiapoptotic protein Mcl-1, in fact, the protective effects of HGF against apoptosis were corroborated by caspase 3 activity (Figures 6b and 6c), the control of apoptosis is another relevant issue in the pathogenesis and severity of AP and its systemic complications, being related to mortality and morbidity in severe AP (Takeyama, 2005).
Accordingly, our data demonstrate that rhHGF could be proposed as a therapeutic alternative to treat AP, because HGF could reduce importantly the inflammation not just in pancreas also, at systemic level.
In conclusion, rhHGF could be considered as a promising supportive therapy for the treatment of AP, due to the increment on antioxidant enzymes, decrease of inflammatory infiltration and a possible immunomodulation phenomena.
ACKNOWLEDGMENTS
This work was partially funded by a grant from CONACYT: CB252942, Universidad Autónoma Metropolitana. We thank CONACYT for financial support to MPD for her PhD studies. We also thank the Confocal Core Unit of the DCBS-UAM-I.
CONFLICT OF INTEREST
The authors declare no conflict of interest.