Effect of restriction vegan diet's on muscle mass, oxidative status, and myocytes differentiation: A pilot study
Abstract
This study was conceived to evaluate the effects of three different diets on body composition, metabolic parameters, and serum oxidative status. We enrolled three groups of healthy men (omnivores, vegetarians, and vegans) with similar age, weight and BMI, and we observed a significant decrease in muscle mass index and lean body mass in vegan compared to vegetarian and omnivore groups, and higher serum homocysteine levels in vegetarians and vegans compared to omnivores. We studied whether serum from omnivore, vegetarian, and vegan subjects affected oxidative stress, growth and differentiation of both cardiomyoblast cell line H9c2 and H-H9c2 (H9c2 treated with H2O2 to induce oxidative damage). We demonstrated that vegan sera treatment of both H9c2 and H-H9c2 cells induced an increase of TBARS values and cell death and a decrease of free NO2− compared to vegetarian and omnivorous sera. Afterwards, we investigated the protective effects of vegan, vegetarian, and omnivore sera on the morphological changes induced by H2O2 in H9c2 cell line. We showed that the omnivorous sera had major antioxidant and differentiation properties compared to vegetarian and vegan sera. Finally, we evaluated the influence of the three different groups of sera on MAPKs pathway and our data suggested that ERK expression increased in H-H9c2 cells treated with vegetarian and vegan sera and could promote cell death. The results obtained in this study demonstrated that restrictive vegan diet could not prevent the onset of metabolic and cardiovascular diseases nor protect by oxidative damage.
1 INTRODUCTION
Vegan and Vegetarian diets are associated with many health benefits such as a lower prevalence of hypercholesterolemia, diabetes mellitus, hypertension, and lower percentage of ischemic heart disease compared to omnivorous diets (Craig, 2009; Crowe, Appleby, Travis, & Key, 2013; Huang et al., 2012). Currently, this restrictive diets is becoming increasingly popular for various reasons ranging from the protection of animals and the risk of disease to the use of growth stimulants and antibiotics for animal rearing (Fox and Ward, 2008; Rollin, 2003; Volpe, 2005). Vegans and Vegetarians eat more fruits and vegetables, grains, and nuts than omnivores whereby they ingest large amounts of fibers and unsaturated fats and small amounts of total and saturated fatty acids. In this way, they show lower low density lipoprotein (LDL) (Chelchowska, Laskowska-Klita, & Klemarczyk, 2003; Kelly & Sabate, 2006; Mellen, Walsh, & Herrington, 2008) levels and, consequently, a decreased risk of cardiovascular diseases (Chen et al., 2008; Fraser, 2009). The vegan diet that completely excludes animal products such as meat, fish, dairy products, honey, and eggs is very common among young people. This preference may arise from the increase of dairy allergies and intolerances in addition to ethical issues (Key, Appleby, & Rosell, 2006). Vegan diet contains less cholesterol and saturated fats and a large amount of cereals, legumes, and dietary fibers compared to vegetarian diet. Therefore, vegans seem to have less proteins, saturated fats, retinol, vitamin D, calcium, and zinc (Davey et al., 2003). When calcium and protein intake is respectively below 800 mg/day and less than 1.24 g/protein/kg bodyweight, the risk of hip fracture significantly increases (Burckhardt, 2013; Munger, Cerhan, & Chiu, 1999). Although some vegetables are rich in proteins and bioavailable calcium, vegetarian and vegan diets often contain minor amounts of these nutrients, therefore they might be the cause of osteoporosis. Studies by Haddad et al. (1999) assessed the nutritional status of adult vegans compared to omnivores about vitamin B12, iron, zinc, and immune markers. They demonstrated that the protein content of vegan diet was significantly lower than the omnivorous one and that vegans had lower blood levels of saturated and monounsaturated fats as well as deficiency of iron, calcium, zinc, vitamin D, vitamin B12, and amino acids; on the other hand, they showed higher levels of fibers and most nutrients, such as ascorbic acid, folic acid, and (copper, magnesium and manganese). Moreover, vegans also showed lower blood levels of leukocytes, lymphocytes, platelets, complement factor 3, and urea nitrogen concentrations but an higher concentration of albumin. Finally, the vegans’ body mass index was significantly lower compared to non-vegetarians (Haddad, Berk, Kettering, Hubbard, & Peters, 1999). Vitamin B12 is an essential cofactor for two enzymes, methylmalonyl-CoA mutase and methionine synthase. When its deficiency occurs, increased levels of hydrolyzed methylmalonyl-CoA lead to increased concentrations of methylmalonic acid, an important marker of vitamin B12 functional deficiency (Stabler, Lindenbaum, & Allen, 1997).
Even the increase of serum homocysteine is not only a vitamin B12 but also folate deficiency marker (Snow, 1999). This phenomenon is recurrent in a large part of vegetarians and may contribute to an increase of the atherosclerotic risk in these subjects. On the other side, the intake of plant based foodstuffs with multiple antioxidants leads to a reduction of the arteriosclerosis risk, stroke, and coronary heart disease. The most likely hypothesis is that the healthier lifestyle of nonmeat-eaters could be reversed by an increase of homocysteine as result of vitamin B12 deficiency (Herrmann, Schorr, Purschwitz, Rassoul, & Richter, 2001; Mezzano et al., 1999; Rauma, Torronen, Hanninen, Verhagen, & Mykkanen, 1995). The main active antioxidants include vitamins such as α-tocopherol and ascorbic acid, flavonoids, and carotenoids such as lycopene, lutein, β-carotene, cryptoxanthin, and zeaxanthin. Their biological activity consists in reducing circulating total cholesterol levels and inhibiting the LDL cholesterol oxidation with an increase of HDL cholesterol (Grajek, 2004). However, vegetarian nutrition provides adequate antioxidants which effectively prevent free radicals generation. Somannavar and Kodliwadmath (2012) revealed that serum malondialdehyde level was significantly increased in non-vegetarians compared to vegetarians: an increased lipid peroxidation and a low antioxidant level in non-vegetarians compared to vegetarians were detected. Differences in hypertension and mean systolic and diastolic blood pressures among meat-eaters and nonmeat-eaters occur. In particular, the meat-eaters had the highest values while vegans the lowest. This could be attributed to differences in body mass index that were lower in nonmeat-eaters (Appleby, Davey, & Key, 2002). In some cases, high body mass indexes are independent of diet and can be associated to viral infections that induce alterations to adipose tissue distribution and biology. Recent studies (Rizzo, Di Domenico, Carratelli, Mazzola, & Paolillo, 2011, Rizzo, Domenico, Carratelli, & Paolillo, 2012) showed that infectious agents (such as C. Pneumoniae) induced the production of specific pro-inflammatory cytokines in the adipose tissue, with broad effects on hormone expression, lipid storage, and the composition of adipose-resident immune cell populations; these events are strictly related to the development of obesity.
The aim of this study was to establish whether omnivore, vegetarian, and vegan diets affects anthropometric, metabolic, and serum oxidative status of young men. We investigated the protective effects of vegan, vegetarian, and omnivorous sera on hydrogen peroxide-induced morphological changes in H9c2 myoblast cells. For this purpose, we treated H9c2 cell line with vegan, vegetarian, and omnivorous sera in order to prevent the effects of hydrogen peroxide (H2O2)-induced cellular damage on H9c2 differentiation.
2 MATERIALS AND METHODS
2.1 Subjects
We enrolled three groups of men (10 omnivores, 10 vegetarians, and 10 vegans) living in Italy in Campania area. According to their answers to a questionnaire about the frequency of their physical activity, these men were sedentary or moderately physically active. We excluded subjects with a history of diseases or using antibiotics. The omnivorous men consumed all major types of food, the vegetarians consumed milk products and eggs, the vegans consumed no animal products and milk derivatives and eggs. In order to be included as a regular vegetarian or vegan, the subjects were required to have followed this diet for at least 2 years. The subjects were informed of the research and gave their consensus for use of serum samples.
2.2 Anthropometry measurements
The participants height and body weight were measured with an automatic height/weight measurement system (DS-102; Jenix®, Seoul, Korea). BMI (kg/m2) was calculated. Body fat was measured using a bioelectrical impedance analyzer (Jawon Medical, Seoul, Korea).
2.3 Collection of samples
Serum samples for this study were obtained from 30 volunteers normal weight young men (age range 20–30, mean). All participants were metabolically healthy. Twelve-hour fasting blood samples were drawn from the subjects in Vacutainers (Becton-Dickinson Vacutainer Systems, Franklin Lakes, NJ) containing SST clot-activating gel. The serum was separated by centrifugation and stored at 80 °C until analysis. Weight was measured by using a scale from Pennsylvania Medical Scales (model no. 7500). Height was measured with a stadiometer from Seca, attached to the wall. BMI was calculated as weight (kg)/height (m2). Baseline clinical characteristics of the study population are summarized in Table 1. Laboratory parameters such as AST, ALT, total cholesterol, triglycerides, GGT were carried out using standard clinical chemical methods.
| Type of diet | Kcal/daily | ||
|---|---|---|---|
| Food | Omnivore | Vegetarian | Vegan |
| Beef, lamb | 312 | 0 | 0 |
| Chicken, fish, pork | 260 | 0 | 0 |
| Dairy | 338 | 208 | 0 |
| Cereals, breads | 416 | 936 | 1118 |
| Vegetables | 78 | 338 | 364 |
| Fruit | 26 | 130 | 156 |
| Oils, spreads | 702 | 624 | 598 |
| Snacks, sugar | 220 | 208 | 208 |
| Drinks | 208 | 156 | 156 |
- All the three diets have been based on an average of 2,600 Kcal of food consumed per day.
2.4 Serum evaluation of oxidative stress markers
2.4.1 DPPH—scavenging assay
The test was performed according to Brand-Williams, Cuvelier, and Berset (1995). 20 µl of serum were added to 3 ml of DPPH solution (6 × 10−5 mol/L) and the absorbance was determined at 515 nm every 5 min until the steady state.
2.4.2 Ferric reducing antioxidant power (FRAP) assay
The FRAP assay was carried out by adding 2.5 ml of acetate buffer, pH 3.6, 0.25 ml of TPTZ solution (10 mM) in 40 mM HCl, 0.25 ml of FeCl3 · 6H2O solution (12 mM), and 150 µl of sample. After 30 min incubation at room temperature the absorbance of the product (ferrous tripyridyltriazine complex) was read at 593 nm. The standard curve was linear between 20 and 800 µM of Trolox. Results were expressed as micromoles of Trolox equivalents (TE)/ml of serum.
2.4.3 Determination of total phenol content
Total phenolics were determined by Folin–Ciocalteu assay with slight modifications. Briefly, Folin–Ciocalteu's phenol reagent (62.5 µl) and dd H2O (250 µl) were added to sample (62.5 µl). After 6 min, 7% Na2CO3 solution (62.5 µl) and dd H2O (500 µl) were added to the mixture, which was incubated for 90 min and the absorbance was read at 760 nm. The standard curve was obtained in the range of 0–70 µg/ml gallic acid. Total phenolic content of serum was expressed as mg of gallic acid equivalents (GAE)/ml of serum.
2.4.4 ABTS scavenging assay
ABTS method is based on the reduction of the ABTS•+ activity by the antioxidants contained in the sample. A solution of 7.4 mM ABTS•+ (5 ml) mixed with 140 mM K2S2O8 (88 ml) was prepared, stabilized for 12 hr at 4 °C and then mixed with ethanol (1:88, v/v). Subsequently, 100 µl of diluted serum were added to 1 ml of diluted ABTS•+, incubated for 2.5 min and the absorbance was read at 734 nm. The standard curve was linear between 0 and 20 mM Trolox. Results were expressed as micromoles of TE/ml of serum.
2.5 In vitro cell culture studies
Rat cardiomyocytes (H9c2) (ATCC, Manassas, VA) cells were cultured in DMEM supplemented with 10% fetal bovine serum, 100 U/ml of penicillin, and 100 lg/ml of streptomycin in 150 cm2 tissue culture flasks at 37 °C in a humidified atmosphere of 5% CO2. The cells were fed every 2–3 days and subcultured once they reached 70–80% of confluence. After 4 hr incubation, cells were washed with 1% PBS to remove unattached dead cells and treated for 30 min with 50 mM H2O2 (H-H9c2). The H9c2 and H-H9c2 cells were then incubated for 72 hr with media containing 10% of vegan, vegetarian and omnivorous sera.
2.5.1 Cell proliferation assay
The evaluation of cell proliferation was performed on H9c2 cell line after 72 hr incubation with media containing 10% of vegan, vegetarian and omnivorous sera. The cells were seeded in 96-well plates in a number of 30 × 102 per well. The growth inhibition was assessed by MTT viability assay after 72 hr of treatment as previously described (De Maria et al., 2013). Then MTT assay was carried out by triplicate determination on at least three separate experiments. All data were expressed as mean ± SD.
2.5.2 Morphological evaluation of cardiomyocytes by confocal microscopy
After 72 hr incubation of cells with different sera, cells were fixed for 20 min with a 3% (w/v) paraformaldehyde (PFA) solution and permeabilized for 10 min with 0.1% (w/v) Triton X–100 in phosphate-buffered saline (PBS) at room temperature. To prevent nonspecific interactions of antibodies, cells were treated for 2 hr in 5% fetal bovine serum (FBS) in PBS, then cells were incubated with a specific mouse monoclonal antibody raised against vimentin (1:1,000 in blocking solution, 3% (w/w) BSA in TBS-Tween 0.1%, Sigma) for 2 hr at 37 °C. After several washes, cells were incubated with a secondary IgG goat anti-mouse antibody (Alexa Fluor 488, Life Technologies, Carlsbad, CA) diluted 1:1,000 in blocking solution for 1 hr at room temperature. The slides were mounted on microscope slides by Mowiol. The analyses were performed with a Zeiss LSM 510 microscope equipped with a plan-apochromat objective X 63 (NA 1.4) in oil immersion. Vimentin fluorescence was collected in a multi-track mode.
2.5.3 Thiobarbituric acid-reactive species (TBARS) assay
Serum samples were incubated with 0.5 ml of 20% acetic acid, pH 3.5, and 0.5 ml of 0.78% aqueous solution of thiobarbituric acid. After heating at 95 °C for 45 min, samples were centrifuged at 4000 rpm. for 5 min. The TBARS were quantified by spectrophotometry at 532 nm (Tenore, Campiglia, Stiuso, Ritieni, & Novellino, 2013). Results were expressed as TBARS µM/g of serum proteins. Each data point is the average of triplicate measurements, with each experiment performed in triplicate.
2.5.4 Nitrite levels
Nitric oxide is rapidly converted into the stable end products nitrite and nitrate. Nitrite was measured by the Griess reaction. Briefly, 10 µl of serum was mixed with an equal volume of Griess reagent (0.5% sulfanilamide, 2.5% H3PO4, and 0.05% naphthylethylene diamine in H2O) and incubated for 10 min at room temperature. Absorbance was assayed at 550 nm and compared with a standard curve obtained using sodium nitrite.
2.5.5 Western blots
Cardiac H9c2 cells were collected by centrifugation and then resuspended in ice cold 50 mM potassium phosphate buffer (pH 7.4), containing 2 mM EDTA. The cells were sonicated for 10 s, followed by centrifugation at 13,000g for 10 min at 4 °C. The resulting supernatants were collected and kept on ice for immediate measurements, as described below. Protein expression was determined by Western blot. Briefly, H9c2 cells were cultured with different sera for 72 hr, and then cell pellets were lysed with 1 ml of lysis buffer. The lysates were centrifuged at 12,000 rpm for 10 min at 4 °C. The supernatants were used to detect ERK, pERK. All Western blots were repeated for three times. Tubulin was used as internal control. To quantify the results, the relative amount of each protein was determined.
2.6 Statistical analysis
Statistical analyses were performed with GraphPad Prism version 4.0 for Windows, GraphPad Software (SanDiego, CA). The values were compared by matched-pair t-tests. All data were expressed as means ± SE unless otherwise noted. A p value of <0.05 was considered statistically significant.
3 RESULTS
3.1 Anthropometry measurements and serum metabolic parameters of vegetarian, vegan, and omnivorous men
In Table 1 was reported the distribution of food energy in the three different diets: omnivore, vegan, and vegetarian. Omnivores eat more red meat, white meat and dairy than cereals, fruit and vegetables. The Vegetarians switch away from beef and chicken to fruit and vegetables, while also reducing oils and snacks. The Vegans, compared to vegetarians, eliminate dairy and switch to cereals, fruits, and vegetables. In Table 2 were reported clinical characteristics of three volunteer groups (ages 29 ± 5) enrolled for this study: vegetarians, n = 10; vegans, n = 10, and age-matched omnivores (n = 10), both vegans and vegetarians do not have used nutritional supplements. All subjects were normal body weight men with BMI 21 ± 1.8. However, we observed significant decrease in muscle mass index and lean body mass only between vegan versus omnivorous groups (p < 0.001). The serum metabolic parameters of enrolled subjects (Table 2), were in the normal range except for the homocysteine values. In vegetarian and vegan homocysteine level was 12 ± 1 and 13 ± 0.9 micromol/L respectively, while the level in consuming traditional diet subjects was 10 ± 0.7 micromol/L. The significance of vegetarian and vegan homocysteine values vs. omnivorous ones was p < 0.0001.
| Omnivorous (n = 10) | Vegetarian (n = 10) | Vegan (n = 10) | |
|---|---|---|---|
| BMI | 23 ± 0.4 | 21 ± 2.3 | 20.5 ± 0.5 |
| FBM | 13.8 ± 1.5 | 12.5 ± 3.3 | 13.2 ± 0.35 |
| LBM | 55.4 ± 0.3 | 54.7 ± 6.3 | 44.3 ± 0.3 |
| MM | 32.1 ± 0.81 | 32.8 ± 1.4 | 27.3 ± 1.2 |
| Serum metabolic parameters | |||
| Total-C, mg/dl | 154 ± 28 | 159 ± 23 | 148 ± 10 |
| LDL-C, mg/dl | 79 ± 23 | 82 ± 21 | 81 ± 20 |
| HDL-C, mg/dl | 47 ± 11 | 52 ± 18 | 48 ± 4 |
| TG, mg/dl | 67 ± 33 | 81 ± 35 | 91 ± 23 |
| Homo, µmol/L | 10 ± 1.0 | 12 ± 0.8 | 13 ± 1 |
| AST-GOT | 19 ± 3.5 | 19 ± 2.7 | 15.5 ± 3.5 |
| ALT-GOT | 15 ± 6 | 13 ± 5 | 10 ± 1.4 |
| LDL/HDL | 1.6 ± 0.17 | 1.57 ± 0.15 | 1.7 ± 0.13 |
- BMI, body mass index; MM, muscle mass; LBM, lean body mass; FBM, fat body mass, Total-C, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; Homo, Homocysteine; TBARS, thiobarbituric acid reactive substance; NO, nitric oxide.
- All data are expressed as means ± SE (n = 10 subjects for group).
3.2 Serum oxidative status of vegetarian, vegan, and omnivorous diet men
Serum antioxidant activities of vegan, vegetarian, and omnivorous groups (Table 3) were analyzed using three methods: DPPH and ABTS assays that measured radical scavenging activity and ferric-reducing antioxidant potential (FRAP) assay. Both DPPH and ABTS values showed no significant differences among three groups, while the FRAP values were increased in vegetarians and omnivores compared to vegans but the variation was significant only in vegetarians versus vegans with a p = 0.026 values. Additionally, total phenolic content (folin assay) were also determined. Surprisingly, sera total phenolic content was significant major in vegetarians and omnivores with a p value equal to 0.0014 and 0.001, respectively, compared to vegan sera. We have also evaluated the serum oxidative status of three diet groups by nitric oxide (as NO2−) quantification and lipid peroxidation marker (TBARs) (Stiuso et al., 2014;27). We observed an increase of about twofold of NO2− in serum of vegans and vegetarians compared to omnivores, while TBARS levels increased of twofold in vegan sera with a p value <0.0001 compared to vegetarians and omnivores.
| Omnivorous (n = 10) | Vegetarians (n = 10) | Vegans (n = 10) | |
|---|---|---|---|
| Antioxidant parameters | |||
| DPPH mmol/ml | 0.0018 ± 0.0002 | 0.0016 ± 0.0003 | 0.0017 ± 0.0002 |
| Phenol mg/ml | 1.35 ± 0.02 | 1.25 ± 0.02 | 1.164 ± 0.006 |
| Abts mmol/ml | 0.027 ± 0.004 | 0.027 ± 0.003 | 0.025 ± 0.0036 |
| Frap mmol/ml | 0.00031 ± 0.00002 | 0.00037 ± 0.00003 | 0.00025 ± 0.000015 |
| Oxidative parameters | |||
| TBARS μM/μg protein | 0.0022 ± 0.00039 | 0.0028 ± 0.0004 | 0.0046 ± 0.0005 |
- All data are expressed as means ± SE (n = 10 subjectsfor group). The DPPP, Phenol, Abts, Frap value was normalized for ml of serum.
3.3 In vitro sera effects on survival and oxidative stress of H9c2 cardiomyocytes
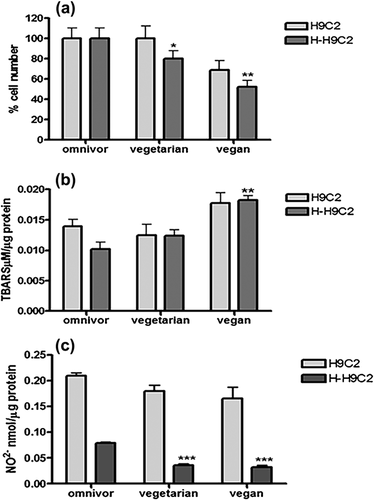
To investigate whether different diets could affect in vitro cardiomyocytes survival and oxidative stress, we cultured H9c2 and H-H9c2 (cell incubated for 30 min with 50 µM H2O2) myoblasts, an alternative cellular model for cardiomyocytes, for 72 hr with 10% of pooled sera from vegan, vegetarian, and omnivorous subjects. Both H9c2 and H-H9c2 cells number after 72 hr of treatment with sera were reported in Figure 1. The vegan sera induced a significant decrease (p = 0.0163) of cells number compared to omnivorous and vegetarian sera. Surprisingly, H-H9C2 cells number significantly decreased of twofold with vegan sera compared to omnivorous sera treated cells (p = 0.0022). The lipid peroxidation evaluated by TBARS levels significantly increased in vegan sera-treated H9c2 and H-H9c2 compared to vegetarian and omnivorous sera treated cells (p = 0.0044). Moreover we observed a significant decrease of NO2− concentration in both vegetarian and vegan sera treated H-H9c2 (p < 0.0001) compared to omnivorous sera treated cells. These results demonstrated that omnivorous sera better protected H9c2 cells from hydrogen peroxide-induced cytotoxicity compared to vegetarian and vegan sera.
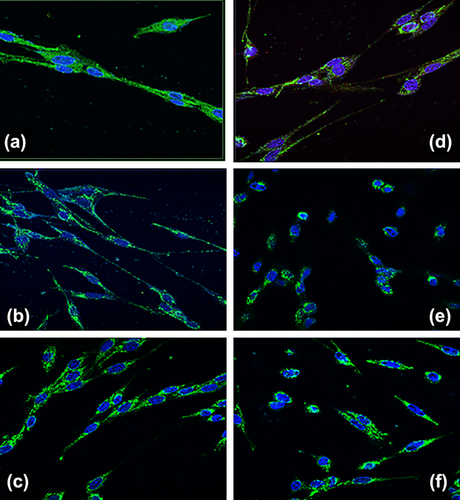
3.4 In vitro effects of omnivorous, vegetarian, and vegan sera on H9c2 cells morphology
In Figure 2 we reported a morphology change in the cytoskeleton when H9c2 and H-H9c2 cells were treated for 72 hr with vegan, vegetarian and omnivorous sera, then fixed, stained with anti-vimentin antibody and examined by confocal fluorescence microscopy. The omnivorous sera treated H9c2 cells showed a long fusiform shape and compact parallel morphology, compared to vegetarian sera treated H9c2 that had a disordered organization and a more fine form. The cellular morphology of H9c2 incubated with vegan sera showed a less elongated shape compared to omnivorous sera treated cells but a more compact organization compared to vegetarian sera treated cells. As shown in Figure 2d–f, H2O2 pre-treatment of H9c2 cells led to distinctive morphological changes in cells treated with different sera. The omnivorous sera treated H-H9c2 cells retained fine elongated shape compared to (Figure 2a) both vegetarian and vegan sera treated H-H9c2 cells that had lost cell-to-cell contacts and showed a more rounded morphological shape.
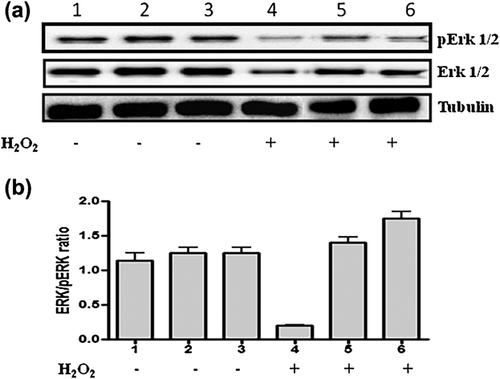
3.5 In vitro effects of omnivorous, vegetarian and vegans sera on MAP kinases pathways
In order to investigate the protective effects of omnivorous, vegetarian and vegan sera on hydrogen peroxide (H2O2)-induced cellular damage of H9c2 cells, we examined the expression of extracellular signal-regulated kinases (ERK) (Di Domenico & Giordano, 2017), ERK1/2 and pERK1/2 and Caspase-3 by Western blot analysis. The hydrogen peroxide decreased the ERK1/2 and p-ERK expression in omnivorous sera treated H-H9c2 cells compared to vegetarian and vegan sera treated H-H9c2 (Figure 3a). Caspase-3 is an executor of apoptosis and cleaved caspase-3 is a caspase-3 activation marker. Interestingly, H9c2 cells treated with vegan sera increased cleaved caspase-3 expression compared to the omnivorous and vegetarian sera treated H9c2 cells, while H2O2 pre-treatment increased the cleaved caspase-3 in all H9c2 cells treated with the three types of sera.
4 DISCUSSION
The effects of foods on human health are likely due to the synergic interactions of multiple factors, including fibers, fatty acids, proteins, and bioavailability of micronutrients (Jacobs & Tapsell, 2007). The role of a correct diet is crucial in the prevention of obesity that represents one of the main risk factors for cancer, cardiovascular, and metabolic diseases (Feola et al., 2017). Few studies have been carried out on benefits and disadvantages of omnivorous, vegetarian and vegan diets in diseases prevention (Lea, Crawford, & Worsley, 2006). In this study we have enrolled omnivorous, vegetarian, and vegan healthy men with similar age (25 ± 5), weight (65 ± 5), and BMI (21.5 ± 1) in order to evaluate the effects of three different diets on body composition, metabolic parameters, and serum oxidative status. We observed a significant decrease in muscle mass and lean body mass (p < 0.0001) only in subjects with a restrictive vegan diet compared to omnivorous and vegetarian men. Essential amino acids, such as leucine, are muscle protein synthesis activators via protein kinase mTORC1 (mammalian target of rapamycin complex 1). The minor quantity of skeletal muscle mass in vegan subjects could be due to low content of leucine in the foods. Both omnivorous and vegetarian diets include foods with high leucine content (cheese, soybeans, beef, chicken, pork, nuts, seeds, fish) compared to plus restrictive vegan diets. Mildly elevated plasmatic homocysteine levels are associated to vascular disease (Genest et al., 1991; Schnyder et al., 2001). To this purpose, we have detected a mild but significant increase of homocysteine values (Table 1) in serum of both vegan and vegetarian groups. These results may be due to dietary deficiency of folate, vitamin B12 or choline in subjects submitted to vegan or vegetarian diets. The antioxidant compounds, mainly found in fresh fruits and vegetables, should maintain low levels of serum oxidative stress of vegetarian and vegan subjects compared to omnivore subjects. We showed that both DDPH and Abts radical scavenging activity were homogeneous in sera of the three diet groups, whereas the FRAP value (total antioxidant status of plasma) was significantly lower in vegans compared to vegetarians (p = 0.026) and omnivores (p = 0.0142). Surprisingly, also the phenol serum concentration was significant higher in omnivores compared to vegetarian and vegan sera. Furthermore, lipid peroxidation evaluated by TBARS assay showed a strong increase only in vegan sera compared to vegetarian and omnivorous sera. These results could be due to the higher presence of indigestible dietary fibers, especially in vegan diet, which could have determined the low bioaccessibility and bioavailability of antioxidant molecules such as polyphenols in the small intestine, and a consequently increased of oxidative status sera only in vegan subjects (Palafox-Carlos, Ayala-Zavala, & González-Aguilar, 2011). In vitro studies have highlighted the protective activity of plant food constituents such as phenols and mixtures and their preventive effects against oxidative stress induced by cell death (D'Angelo et al., 2012; Saberi-Karimian et al., 2017). The balance between oxidation and anti-oxidation is critical in maintaining a redox-homeostasis and a consequently healthy biological system (Bouayed & Bohn, 2010).
In literature it was reported that the treatment with serum from patients with different diseases could have effects on proliferation, apoptosis, and angiogenesis of cultured cells (Pannella et al., 2016; Stiuso et al., 2014; Valgimigli et al., 2003). In this study, we have used this approach to show the effects of sera from subjects with three dietary approaches on oxidative stress, growth, and differentiation of both H9c2 and H-H9c2 cardiomyoblast cells (H9c2 treated for 30 min with 50 µM H2O2). The H9c2 cell line is used to mimic in vitro both skeletal and cardiac muscle, for its biochemical morphological, and electrical/hormonal signaling properties (Sardão, Oliveira, Holy, Oliveira, & Wallace, 2007). On the other hand, this cell line has been used as a model to investigate the protective effect of several compounds against hydrogen peroxide-induced cardiotoxicity (Kim, Kim, Shin, Kwon, & Park, 2014). Furthermore the H9c2 cell line can be inducted to differentiate when cultured in particular condition and to show an elongated shape and a parallel fashion morphology (Branco et al., 2015).
Our data demonstrated that the vegan sera treatment of both H9c2 and H-H9c2 cells induced an increase of TBARS value, markers of lipid peroxidation compared to vegetarian and omnivorous sera. Increased lipid peroxidation may be due to high doses of antioxidant molecules, vitamin C, vitamin E, and carotenoids present in the vegan diet, and may cause toxicity and also show pro-oxidant activity (Bouayed & Bohn, 2010). Moreover, we observed a significant decrease of free nitric oxide in the medium of vegan and vegetarian sera treated H-H9c2 cells with a concomitant increase of cell death compared to omnivorous sera. Under cellular oxidative stress conditions, the amount of diffusible free NO decreased because it was transformed in peroxynitrite (ONOO−), when it reacted with superoxide radicals.
The peroxynitrite damages lipids, proteins and nucleic acids by inducing apoptotic cell death (Chung, Pae, Choi, Billiar, & Kim, 2001). Studies by Levrand et al. (2007) demonstrated that homocysteine induces cell death in H9c2 cells through peroxynitrite generation. According to this report we supposed a potential involvement of the homocysteine in regulating the balance between NO and peroxynitrite, as it was increased in both vegan and vegetarian serum compared to cells treated with omnivorous serum. Moreover, we have evaluated the ability of three different sera to induce H9c2 differentiation by confocal microscopy. The image of omnivorous sera treated H9c2 cells showed an elongated morphological organization, compared to vegetarian and vegan sera treated cells with a plus fine and minus elongated form. Furthermore, the hydrogen peroxide pre-treatment of H9c2 cells induced: 1) a fine fibers organization in omnivorous sera treated cells; and 2) reduction in size and rounding up of vegan and vegetarian treated cells. These data demonstrated that the omnivorous sera have major antioxidant and differentiation properties compared to vegetarian and vegan sera. Finally, the influence of the omnivorous, vegetarian, and vegan sera on MAPKs pathway was evaluated. MAPKs are serine/threonine protein kinases involved in cellular proliferation, differentiation, and death (Di Domenico & Giordano, 2017). Activation of ERK1/2 in response to H2O2 is mediated through Ras/Raf1/Mek pathway and may promote inflammation and result in cellular necrosis (Cagnol & Chambard, 2010). The ERK expression and its active form (pERK) increased in vegetarian and vegan sera treated H-H9c2 cells. A growing number of studies suggests that several compounds present in foods consumed by vegetarians and vegans, such as resveratrol and quercetin, can promote cell death by ERK activation (Kim, Lee, Jeong, Guo, & Lee, 2008; Shih, Davis, Lin, & Davis, 2002).
Our study in vitro demonstrates the beneficial effect of omnivorous sera treatment on: 1) cellular proliferation; 2) lipid peroxidation; 3) extracellular free NO production; and 4) morphological organization of both H9c2 and H-H9c2 cells. Relying on these results, we concluded that the long-term consumption of restrictive vegan diet, if not adequately integrate with intake of necessary and protective nutrients, can't neither protect and prevent oxidative damage nor can't inhibit chronic diseases and maintain an healthy biological system.