Extracellular vesicles derived from human embryonic stem cell-MSCs ameliorate cirrhosis in thioacetamide-induced chronic liver injury
Abstract
Various somatic tissue-derived mesenchymal stromal cells (MSCs) have been considered as an attractive therapeutic tool for treatment of liver diseases in which the secretion of soluble factors or extracellular vesicles (EVs) is the most probable mechanism. The experimental application of human embryonic stem cell-derived MSC (ES-MSC) increased rapidly and showed promising results, in vitro and in vivo. However, possible therapeutic effects of human ES-MSC and their EVs on Thioacetamide (TAA)-induced chronic liver injury have not been evaluated yet. Our data indicated that human ES-MSC can significantly suppress the proliferation of peripheral blood mononuclear cells compared to bone marrow (BM)-MSC and adipose (AD)-MSC. Moreover, ES-MSC increased the secretion of anti-inflammatory cytokines (i.e., TGF-β and IL-10) and decreased IFN-γ, compared to other MSCs. ES-MSC EVs demonstrated immunomodulatory activities comparable to parental cells and ameliorated cirrhosis in TAA-induced chronic rat liver injury, that is, reduction in fibrosis and collagen density, necrosis, caspase density, portal vein diameter, and transaminitis. The gene expression analyses also showed upregulation in collagenases (MMP9 and MMP13), anti-apoptotic gene (BCL-2) and anti-inflammatory cytokines (TGF-β1 and IL-10) and down-regulation of major contributors to fibrosis (Col1α, αSMA, and TIMP1), pro-apoptotic gene (BAX) and pro-inflammatory cytokines (TNFα and IL-2) following treatment with ES-MSC and ES-MSC-EV. These results demonstrated that human ES-MSC and ES-MSC EV as an off-the-shelf product, that needs further assessment to be suggested as an allogeneic product for therapeutic applications for liver fibrosis.
Abbreviations
-
- AD
-
- adipose tissue
-
- ALT
-
- alanine aminotransferase
-
- AST
-
- aspartate aminotransferase
-
- BM
-
- bone marrow
-
- CM
-
- conditioned medium
-
- CCL4
-
- carbon tetrachloride
-
- CFSE
-
- carboxyfluorescein succinimidyl ester
-
- CLD
-
- chronic liver disease
-
- DLS
-
- dynamic light scattering
-
- EB
-
- embryoid body
-
- ESC
-
- embryonic stem cell
-
- EV
-
- extracellular vesicles
-
- GGT
-
- gamma-glutamyl transferase
-
- HCC
-
- hepatocellular carcinoma
-
- hES-MSC
-
- human embryonic stem cell-mesenchymal stromal cells
-
- iPS
-
- induced Pluripotent stem cells
-
- MMPs
-
- matrix metallopeptidase
-
- MSC
-
- mesenchymal stromal cells
-
- MV
-
- microvesicles
-
- MLR
-
- mixed lymphocyte reaction
-
- PBMCs
-
- peripheral blood mononuclear cells
-
- PDI
-
- polydispersity index
-
- PVD
-
- portal vein diameter
-
- R
-
- responder
-
- S
-
- stimulator
-
- SEM
-
- scanning electron microscopy
-
- TAA
-
- thioacetamide
-
- US
-
- ultrasound
1 INTRODUCTION
Chronic liver disease (CLD) which is often characterized by established cirrhosis accompanied by chronic inflammation, progressive destruction of liver structure, portal hypertension and liver dysfunction, is considered a life-threatening state and increases the risk of hepatocellular carcinoma (HCC) (Forbes & Newsome, 2016; Huber et al., 2015). Currently, orthotropic liver transplantation is the only effective treatment available for patients with end-stage cirrhosis. Post-operation complications and limited number of liver donors have forced researchers to find alternative therapeutic approaches.
Effectiveness of mesenchymal stromal cells (MSCs) against liver diseases has been demonstrated in experimental models (Meier et al., 2015; van Poll et al., 2008) and later in clinical trials (Mohamadnejad et al., 2007; Nicolas et al., 2016; Pan et al., 2014; Peng et al., 2011; Shi et al., 2012; Squillaro et al., 2016; Suk et al., 2016; Vosough et al., 2016). MSCs promote hepatic regeneration through hepato-protective cytokines and reduce liver inflammation with the help of anti-inflammatory and immunomodulatory properties of these cytokines (Forbes & Newsome, 2016; Than et al., 2016).
Despite the advantages of somatic tissue-derived MSCs, they show some drawbacks such as the need for a consistent source of cells and high cost of handling and maintenance. An alternative source of MSCs could be human pluripotent stem cells including embryonic stem cell (ES-MSC) or induced pluripotent stem cell (iPS-MSC) that can overcome some challenges facing clinical application of somatic tissue-derived MSCs. However, cell rejection, ectopic tissue formation, and infusion toxicity due to lodging of injected cells in the pulmonary capillaries are still problematic.
Recent evidence suggest that bilayer membranous extracellular vesicles (EVs) (i.e., exosomes (40–100 nm in diameter) and microvesicles (MVs; 0.1–1 mm in diameter) (Bruno, Deregibus, & Camussi, 2015; Rani et al., 2015) that are secreted by MSCs, might constitute a convincing alternative cell-free therapy because of their advantages over the mentioned MSCs (Lou et al., 2017).
EVs contain mRNAs, microRNAs and proteins. Previous studies demonstrated that MSC-EVs could improve acute liver injuries and increase survival rate after lethal hepatic failure (Haga, Yan, Takahashi, et al., 2017), hepatic oxidant injury (Yan et al., 2017), hepatic ischemia-reperfusion injury (Haga, Yan, Borrelli, et al., 2017; Nong et al., 2016), fulminant hepatic failure (Chen, Xiang, Wang, & Xiang, 2017). Accelerated liver regeneration (Tan et al., 2014) following MSC-EVs injection suggests that the content of these cells might be critical for reversing liver injury. Using a model of CCl4-induced acute fibrotic liver injury, it was shown that direct injection of human umbilical cord-derived MSC-EV alleviated hepatic inflammation and collagen deposition (Li, Yan, et al., 2013). However, no study has reported the application of human ES-MSC EV and their parental cells (ES-MSC) in a thioacetamide (TAA)-induced chronic liver injury model.
We evaluated the immunomodulatory effect of BM-, AD-, and ES-MSC and investigated the therapeutic potential of human ES-MSC and ES-MSC EVs. The immunomodualatory effects on peripheral blood mononuclear cells (PBMCs) were assessed in vitro and in a rat model of TAA-induced chronic liver fibrosis. The results demonstrated the therapeutic effects of human ES-MSC and ES-MSC EVs as an off-the-shelf product and an allogeneic cell source for treatment of chronic liver fibrosis.
2 MATERIALS AND METHODS
2.1 Generation and characterization of ES-MSCs
Human embryonic stem cell line, Royan H6 (Baharvand et al., 2006) (Royan Stem Cell Bank, Tehran, Iran) was cultured on matrigel-coated plates in Dulbecco's modified Eagle's medium/F12 supplemented with 20% knockout serum replacement, 2 mM GlutaMAX, 0.1 mM non-essential amino acids (NEAAs), 1% insulin-transferrin-selenium (ITS) (all from Life Technologies, Invitrogen, Carlsbad, CA), 0.1 mM β-mercaptoethanol (Sigma-Aldrich, St. Louis, MO) and 100 ng/ml basic fibroblast growth factor (bFGF, Royan Biotech, Tehran, Iran) as described before. Every other day, the medium was changed and every 5 days the colonies were splitted using collagenase IV (Sigma-Aldrich). In order to induce ESCs differentiation into MSCs, spontaneous differentiation was performed. Briefly, ESC colonies were dissociated using 0.05% trypsin/EDTA (Invitrogen) and plated on 6-cm2 non-adherent culture petri dish at 2 × 105 cells/cm2 in ESC medium supplemented with 10 μM ROCK inhibitor Y-27632 (Calbiochem, Darmstadt, Germany) in the absence of bFGF. The medium was refreshed every other day to generate embryoid bodies (EBs) after 7 days. Then, generated EBs were plated on gelatin-coated plates in Alpha Modified Eagle's Medium (α-MEM, Life technologies) supplemented with 10% fetal bovine serum (FBS, Hyclone) and 2 mM L-glutamine for 7–10 days. The outgrowing differentiated cells were collected and subsequently re-plated and cultured for additional days. Then, resulted ES-MSCs of passage 0 were passaged at 80% confluency until 16 passages. Expanded ES-MSCs of passage 3 were used for in-vitro and in-vivo experiments. The conditioned medium of ES-MSCs of passage three to seven was collected for EV purification.

The human BM- and AD-MSCs were provided by Royan Stem Cell Bank and cultured in Alpha Modified Eagle's Medium (α-MEM, Life technologies) supplemented with 10% fetal bovine serum (FBS, Hyclone) and 2 mM GlutaMAX at the same density.
2.2 Multilineage differentiation
Osteogenic and adipogenic differentiation capacity of ES-MSC were evaluated 14 and 21 days post-incubation in osteogenic and adipogenic induction medium, as described before (Lotfinia et al., 2016).
2.3 Mixed lymphocyte reaction (MLR) assay
To evaluate immunomodulatory effect of ES-MSCs, 1 × 105 cells were exposed to mitomycin-C (10 μg/ml) for 2 hr. Then, mitomycin-C-treated ES-MSCs were seeded on a 96-well plate for 24 hr. In addition, we isolated PBMCs from two HLA-mismatched donors by Ficoll-Paque™ density gradient protocol. As we described before (Pourgholaminejad et al., 2016), the proliferation of allogenic stimulator PBMCs (S), was suppressed with mitomycin-C (25 μg/ml) following 45 min treatment. Responder PBMCs (R) were incubated with 25 μM Carboxyfluorescein succinimidyl ester (CFSE) (Molecular probe, Invitrogen) for fluorescence labeling. For MLR assay, three experimental groups were considered (i.e., R, R + S, R + S + ES-MSCs). For group R, only 1 × 105 responder PBMCs were cultured as control negative. In group R + S, both stimulator and responder cells were co-cultured together and considered as positive control. In R + S + ES-MSCs group, we co-cultured R + S in the presence of pre-seeded ES-MSCs in which the proliferation of responder PBMCs was detected based on the dilution of CFSE, using flow-cytometry analysis (BD FACS caliber™ cytometer and FlowJo 7-6-1 software). In order to show the EVs-induced immunosuppression, we performed another experiment in which 10 μg of ES-MSC EVs were used instead of cells. Finally, three groups of R, R + S and R + S + ES-MSC EVs were compared to each other regarding the percentage of the proliferated PBMCs.
2.4 Purification and characterization of EVs
To isolate EVs, passage 3–7 of ES-MSCs were cultured in T150 culture flasks up to 70% cell confluency. Then, FBS-enriched medium was replaced with MSC basal medium supplemented with EV-free FBS plus 2 mM GlutaMAX. Forty-eight hours post incubation with EV-free medium, the conditioned medium (CM) was collected and stored at −80 °C. To purify EVs, CM were submitted to two light spin centrifugation (300g for 10 min and 2500g for 25 min at 4 °C) to remove debris and dead cell bodies. Then, the remaining supernatant was centrifuged at 20,000g for 25 min to isolate micro-sized vesicles. After that, the supernatant was subjected to ultracentrifugation at 10,0000g for 2 hr at 4 °C. At this point, the supernatant was discarded and the EV pellet was washed with PBS using ultracentrifugation for additional 2 hr similar to the previous step. Finally, the supernatant was removed and the pellet was re-suspended in PBS and stored at −80 °C. We used fixed angle Type 45Ti rotor and Optima™ L-100XP ultracentrifuge instrument. The protein concentration of EVs was measured using BSA protein assay kit (Pierce, ThermoFisher, Rockford, IL). To determine the size and relative intensity of ES-MSC EVs, we used dynamic light scattering (DLS) equipment (Malvern, Worcestershire, UK). The spheroid morphology of vesicles was determined using scanning electron microscopy. Moreover, the expression of general EV markers including CD81, CD63, and TSG101 were evaluated using Western blot standard protocol described by Thery et al. (2006). In order to do a qualitative assessment of targeted delivery and engraftment of EV to the liver, the EV pellet was suspended in 1 ml diluent C in a vial and 4 μl PkH-26 red florescence linker membrane lipophilic dye was added to 1 ml diluent C in another vial and mixed well to disperse. After 5 min of incubation, staining was stopped using 1%BSA and it was subjected to additional ultracentrifugation to pellet EVs. Then, PKH-labeled EVs were used for in-vivo engraftment and also for in-vitro primary hepatocyte uptake. They were observed using fluorescence microscope (IX71; Olympus) and KODAK in-vivo F series imaging system.
2.5 Histopathological analysis
Histopathology analyses performed by two blinded pathologists on four slides for each group. The liver tissue was fixed using 10% formalin, processed, and paraffin-embedded. Then, 6-μm thickness tissues were prepared using microtome and subsequently stained with hematoxylin and eosin (H&E) and masson-trichrome (MT).
Histopathology data according to histological grading and staging (Ishak's score) were classified to piecemeal necrosis (grade 0–4), confluent necrosis (grade 0–6), focal lytic necrosis (grade 0–4), portal inflammation (grade 0–4) and fibrosis (grade 0–6).
2.6 Animal models
Male wistar rats (150–200 g body weight) were kept in single cages under 12 hr light/12 hr dark cycles and controlled humidity and temperature condition in Royan animal facility. To induce cirrhosis, all animals received intraperitoneal injection of 200 mg/kg thioacetamide (TAA) twice weekly for 16 weeks. Ultrasound imaging was performed for confirmation of cirrhosis after 16 weeks. Then, cirrhotic animals were assigned to four groups including ES-MSC (n = 4), ES-MSC EVs (generated from the same lot of the cells; n = 4), fluorescence labeled ES-MSC EVs (n = 1), vehicle (received PBS; n = 4) against normal (n = 3). Next, 4 × 106 cells and 350 μg EVs were dissolved in 400 μl injection water and infused intra-splenicly under the guidance of ultrasound. To evaluate targeted delivery of vesicles, fluorescence-labeled ES-MSC EVs were injected and animals were subjected to KODAK in-vivo F series for live imaging 24 hr-post administration. Other animals were followed up for 4 weeks. After sonography imaging at week 20, animals were euthanized, the excised liver tissues were deep freezed in liquid nitrogen and animal sera were collected for additional assays.
All animal studies and procedures were already approved by the Royan Institutional Review Board and Institutional Ethics Committee of Royan Institute (No: IR.ACECR.ROYAN.REC.1394.11).
2.7 Statistical analysis
All experiments were conducted in at least three independent repeats. All data were showed as mean ± SD or mean ± SEM and one way analysis of variance (ANOVA) was used to determine differences between groups with Tukey post-hoc test and the viability with student's t test. p-values <0.05 were considered significant.
3 RESULTS
3.1 Generation and characterization of ES-MSCs
In order to produce ES-MSCs, human ES cells were cultured in ES medium in the absence of bFGF to form EBs. Then, EBs were plated in gelatin-coated plates and cultured in MSC medium. Spontaneous differentiating of EBs resulted in outgrowth of ES-MSCs. They were further passaged in order to acquire a homogenous population that had spindle-shaped morphology (Figure 1A). The resulting cells could be passaged 16 times. The procedure of MSCs isolation from ESCs is reproducible. The morphology and yield in independent experiments were similar. These cell lines were then passaged 14 times without noticeable differences in doubling time (Figure 1B) and fold changes (Figure 1C). The growth rate of the cells started to decrease at passage 14 in both lines (Figures 1B and 1C). Then, we characterized ES-MSC line 1.
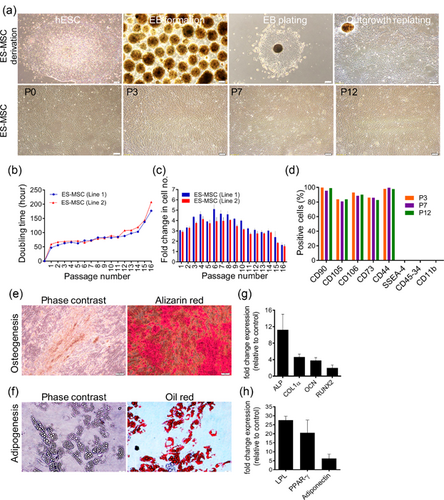
ES-MSCs at different passages were evaluated to characterize their surface antigen markers (Figure 1D and Supplementary Figure S1A). ES-MSCs expressed CD90, CD105 (endoglin, SH2), CD106, CD73 (SH3), and CD44. These cells were negative for CD34, CD45, and CD11b. This consistent antigen expression profile maintained intact in all consecutive passages. ESC markers, SSEA-4, OCT4, and Nanog did not express on ES-MSCs (Figure 1D and Supplementary Figure S1A, B).
To confirm the multipotency of ES-MSCs, we used osteogenic and adipogenic media to differentiate them. After 3 weeks, the majority of the cells were differentiated into osteoblasts (Figure 1E).
Adipogenic differentiation demonstrated the accumulation of small cytoplasmic vesicles that are lightly stained by oil red O (Figure 1F). For further characterization, the expression profile of differentiated cells were assessed using qRT-PCR. The results showed upregulation of transcription factors involved in osteogenesis and adipogenesis [bone-specific alkaline phosphatase (ALP), Collagen 1α (COL1α), Osteocalcin (OCN) and RUNX2) depicted in Figure 1G and lipoprotein lipase (LPL), PPARγ and Adiponectin depicted in Figure 1H].
ES-MSCs were also not able to stimulate PBMC response in co-culture condition (Supplementary Figure S3A). The immunophenotyping data of ES-MSCs and their similar differentiation capacity revealed that these cells presented the characteristics of MSCs.
3.2 ES-MSCs showed better immunomodulatory activity compared to BM- and AD-MSCs
MSCs should be able to modulate the activity of immune cells directly by regulating inflammatory cytokine secretion (Uccelli et al., 2008). To assess this, we analyzed the proliferation of stimulated PBMCs co-cultured with ES-MSCs as well as BM or AD-derived MSCs and detected CFSE dilution using flow cytometry following MLR assay (Figure 2A and Supplementary Figure S2A). CFSE labeled-PBMCs of the first donor were stimulated to proliferate using a second donor PBMC population according to allogenic mixed lymphocyte reaction (MLR) assay. After IL2 stimulation, in the absence of MSCs, PBMCs as responder cells were highly proliferated. However, co-culturing with MSCs from different sources resulted in inhibition of proliferation of stimulated PBMCs (R + S) (a: p < 0.05 vs. R + S; A: p < 0.0001 vs. R + S). Interestingly, ES-MSCs were able to suppress PBMC proliferation more effectively compared to BM-MSCs and AD-MSCs (p < 0.0005), suggesting that ES-MSCs have better activity in regulating immune cells rather than BM-MSCs and AD-MSCs.
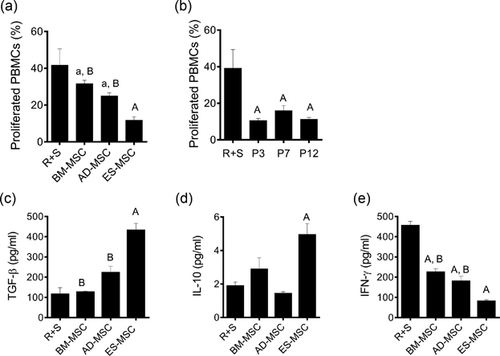
In addition, we observed that not only ES-MSCs from early passages (passage 3) exhibit immunomodulatory effect, but also cells from later passages (passages 7 and 12) showed similar suppression effect (Figure 2B, and Supplementary Figure S2B).
The crucial mechanism of immunomodulation of MSCs lies in MSC-conditioned medium containing soluble factors and EVs rather than cell-cell contacts (Caplan & Correa, 2011; Lou et al., 2017). Therefore, we evaluated the therapeutic effect of MSC-conditioned medium regarding both soluble factors and EVs.
Initially, to understand the mechanism underlying immunosuppressive properties of ES-MSCs, we analyzed one pro-inflammatory cytokine (IFN-γ) and two anti-inflammatory cytokines (TGF-β and IL-10) in the conditioned medium of each group following MLR test by ELISA. The secretion of IFN-γ from stimulated PBMCs decreased after incubation with MSCs from different sources. The secretion of TGF-β was higher in the presence of ES-MSCs (Figure 2C). In case of IL-10, a slight increase in cytokine secretion was only found as a consequence of incubation with ES-MSCs (Figure 2D). Figure 2E shows inhibitory effect of ES-MSCs on IFN-γ secretion compared to BM- and AD-MSCs. (a: p < 0.01 vs. R + S; A: p < 0.0001 vs. R + S; b: p < 0.01 vs. ES-MSC; and B: p < 0.0001 vs. ES-MSC).
The data showed that ES-MSCs were able to have impact on immune cells, suggesting that ES-MSCs have greater capability of regulating immune cell activities than BM-MSCs and AD-MSCs. Therefore, we continued our experiments using MSCs and ES-MSC-derived EVs (ES-MSC EVs).
3.3 The characterization of ES-MSC EVs
Since the therapeutic effect of MSC-conditioned medium might be also induced by EVs, in the next step, we tried to isolate EV population from 48-hr conditioned medium of ES-MSC supernatant by applying conventional ultracentrifugation procedures.
ES-MSC EVs were purified as described before (Thery et al., 2006). Scanning electron microscopy (SEM) showed uniform spheroid morphology of EVs (Figure 3A). Dynamic light scattering analysis indicated an average size of 186.43 ± 18.48 nm with a polydispersity index (PDI) of 0.39 ± 0.05, demonstrating a homogenous population of EVs (Figure 3B).
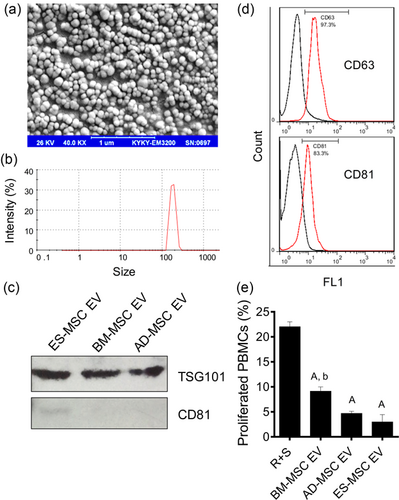
Western blot and flow cytometry results confirmed expression of EV markers, TSG101, CD81 and CD63 in ES-MSC EVs (Figures 3C and 3D). CD81 was not detected in other MSC EVs (Figure 3C). EVs from other sources did not stimulate immune cell proliferation as effective as the ES-MSCs (Figure S3B).
After allogenic stimulation, in the absence of MSCs, PBMCs highly proliferated. However, administration of EVs from different sources of MSCs inhibited the proliferation of PBMCs (Figure 3E, Supplementary Figure S2C; p < 0.0001 all vs. R + S). Additionally, ES-MSC EVs were able to inhibit PBMC proliferation more effectively compared to BM-MSCs (p < 0.005 BM- vs. ES- and AD-MSC), suggesting that ESC-MSC EVs have higher capability of regulating immune cell activities compared to those derived from BM.
3.4 ES-MSCs and ES-MSC EVs can reduce fibrosis
To test the therapeutic activity of ES-MSC EVs, they were administrated to cirrhotic rats. The model was established after intraperitoneal injection of Thioacethamide (200 mg/kg) twice/week for 16 weeks (Figure 4A). All animals survived after injection with the exception of two. The injured rats treated with ES-MSC or ES-MSC EVs and evaluated after 4 weeks. All injections were carried out under the ultrasound guide through intrasplenic route as a safe and non-invasive procedure instead of surgery.
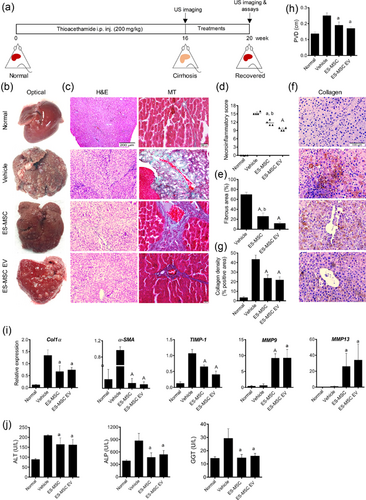
The livers treated with vehicle were pale with micro nodular features compared to the normal liver tissue which had a soft smooth surface (Figure 4B). ES-MSC or ES-MSC EV–treated livers had gross changes with less nodular morphology (Figure 4B). Histologically, the hematoxylin and eosin (H&E) staining of liver tissues for the vehicle group indicated severe hepatic necrosis and fibrosis with most areas showing periportal or periceptal interface hepatitis (piecemeal necrosis), centro-lobular necrosis with marked portal-portal and portal-central bridging (confluent necrosis) and ductal hyperplasia based on Ishak's scoring (Ishak et al., 1995). However, ES-MSC or EV-treated groups showed reduction of hepatocyte piecemeal, confluent necrosis and ductal proliferation in histological examinations.
Moreover, evaluation of H&E staining showed infiltrating of immune cells into fibrotic tissues secondary to necrosis in control group. On the other hand, infiltration of immune cells was markedly reduced in ES-MSC and EV groups compared to vehicle-treated rats.
MT staining also showed a robust fibrosis with thick bundles of collagen deposition surrounding the hepatocytes leading to a pseudo-lobules pattern. This phenomenon was observed 4 weeks after vehicle infusion (Figure 4C). On the other hand, moderate necrosis and fibrosis detected in ES-MSC or ES-MSC EV treated groups (Figure 4C). Quantitative analyses of pictures of both pathologic and Image Pro Plus software evaluations of the livers of both ES-MSC or ES-MSC EV groups showed significant improvement of necrosis and fibrosis in comparison to the vehicle-treated animals (a: p < 0.001 vs. ES MSC, and A:p < 0.0001 vs. ES MSC EV; Figures 4D and 4E). However, improvement of necrosis and fibrosis was significantly less marked in ES MSC EV compared to ES-MSC (b: p < 0.01).
Furthermore, immunostaining of collagen showed significant improvement of livers in ES-MSC and ES-MSC EV groups (p < 0.0001 for both cases; Figures 4F and 4G). There was no significant difference between EV and cell treated groups concerning the aforementioned assays.
Moreover, we tested the echogenicity of liver parenchyma and portal vein diameter (PVD) by ultrasound. Heterogeneous and extensive coarse parenchymal echogenicity with irregular surface, enlarged portal vein and splenomegaly were observed 16 weeks post-induction. However, 4 weeks after treatment with ES-MSCs and ES-MSC EVs, ultrasound examination showed homogenous echogenicity of liver parenchyma and reduction of the liver nodularity while the echogenicity and surface nodularity did not change in vehicle group (Supplementary Figure S4A–C).
Moreover, the average value of PVD in ES-MSC and EV-treated groups were significantly decreased compared to vehicle group (Figure 4H, Supplementary Figure S4, and video; p < 0.01 for both cases). Sonography findings confirmed histopathological results indicating significant regression of cirrhosis in affected animals following treatment.
Quantitative RT-PCR analysis of the livers showed up-regulation of Col1α, the major contributor to fibrosis, αSMA and TIMP1 in vehicle group. However, they were reduced in ES-MSC or ES-MSC EV-treated livers significantly (p < 0.01 and p < 0.0001, respectively; Figure 4I). We found that gene expression of collagenase enzymes (i.e., MMP9 and MMP13) which degrade fibrillar collagens, increased in ES-MSC or ES-MSC EV −treated animals compared to vehicle group (a: p < 0.01 and A: p < 0.0001 vs. vehicle, and b: p < 0.01 vs. ES-MSC EV; Figure 4I). These results indicated that both ES-MSCs and ES-MSC EVs could markedly reverse histological changes associated with cirrhosis through down-regulation of the major contributors to fibrosis namely, Col1α, αSMA, and TIMP1 and up-regulation of the key factors in the degradation of extracellular matrix namely, MMP9 and MMP13.
In addition, we measured liver enzymes in treated animals (Figure 4J). Significant reductions were observed in plasma levels of AST, ALT and GGT after 4 weeks in animals treated with ES-MSCs and ES-MSC EVs compared to the group treated with vehicle (p < 0.01).
These results indicated that transplantation of MSCs or their EVs had a therapeutic effect in liver injury.
3.5 ES-MSCs and ES-MSC EVs can improve viability and reduce hepatocyte apoptosis both in vitro and in vivo
To determine bio-distribution of EVs in cirrhotic rats, animals were treated with PKH-26-labeled EVs using ultrasound guide via intra-splenic route and subsequently subjected to fluorescence imaging. The fluorescence imaging analysis showed accumulation of EVs in the liver 24 hr post-transplantation (Figure 5A). For further confirmation, the animals were sacrificed and the spleens and livers were isolated for additional imaging. Interestingly, strong signal intensity were found in the liver; however, few signals were observed in the spleen (Figure 5B). Fluorescence microscopy also showed incorporation of PKH-26-labeled EVs into primary hepatocytes 2 hr after incubation (Figure 5C).
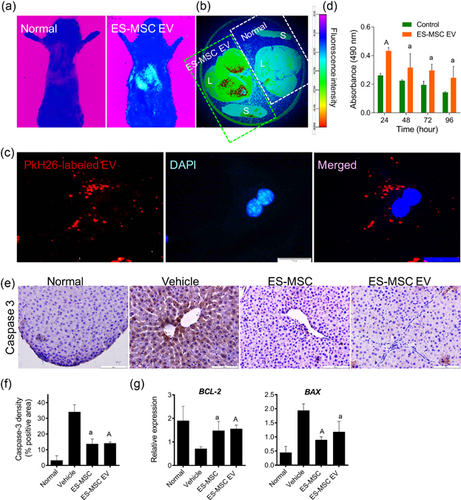
MTS analysis was used to explore the effect of EVs on the viability of primary hepatocytes. The results showed high absorbance indicating high number of viable hepatocytes even after 96 hr incubation with EVs compared to vehicle-treated ones (p < 0.01; Figure 5C).
To evaluate the anti-apoptotic effect of ES-MSCs or ES-MSC EVs in vivo, immunohistochemical evaluation showed a markedly reduction of Caspase-3 expression in liver sections post treatment (a: p < 0.0005, A: p < 0.0001, Figures 5E and 5F).
Moreover, assessment of BCL2 expression as an anti-apoptotic gene and BAX as a pro-apoptotic gene showed a significant increase in BCL2 and a significant reduction of BAX expression following incorporation of EVs and MSCs into liver cells (p < 0.01 and p < 0.001, respectively; Figure 5G). Therefore, it was confirmed that EVs could target the hepatocytes and exert anti-apoptosis effect on these cells.
3.6 ES-MSCs and ES-MSC EVs modulate pro- and anti-inflammatory cytokines in a cirrhotic model
Considering the immunomodulatory effects of ES-MSCs and their secreted EVs in MLR experiment, we investigated their anti-inflammatory capability in inhibiting immune responses, in-vivo.
Tumor necrosis factor-α (TNFα) and interleukin-2 (IL-2) pro-inflammatory cytokines are highly associated with liver fibrosis. Four weeks after treatment, qRT-PCR showed that the expression of TNFα and IL-2 were upregulated in the vehicle group. However, TNFα and IL-2 significantly declined in the ES-MSC and ES-MSC EV groups (p < 0.0001 for both cases; Figure 6A). Consistently, determination of TNFα by ELISA supported the qRT-PCR data (p < 0.01; Figure 6B).
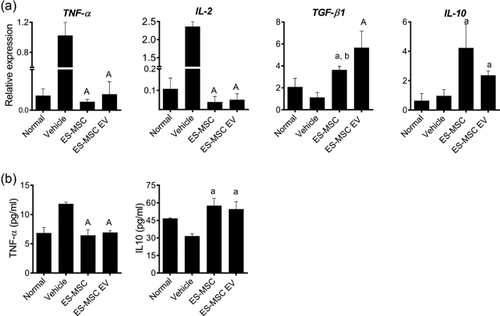
On the other hand, the expression of anti-inflammatory cytokines, TGF-β1 and IL-10 were significantly increased in ES-MSC and ES-MSC EV groups compared to vehicle group (a: p < 0.01, A: p < 0.0001 vs. vehicle, and b: p < 0.05 vs. ES-MSC EV; Figure 6A). Determination of IL-10 by ELISA confirmed the qRT-PCR data (p < 0.05; Figure 6B).
Thus, ES-MSCs and ES-MSC EVs could modulate pro- and anti-inflammatory cytokines in an AAT-injured mouse model and promote hepatic recovery.
4 DISCUSSION
In this study, initially we efficiently differentiated human ES cells into MSCs (ES-MSC) as confirmed by immunophenotyping, proliferation, and differentiation capacity in accordance with the criteria suggested by international society for cell therapy (ISCT) (Galipeau et al., 2016). Our results were in consistent with other reports (Brown, Squire, & Li, 2014; Hematti, 2011; Hynes et al., 2014; Olivier & Bouhassira, 2011). In our study, the immunomodulatory effect of ES-MSC was significantly higher than BM-MSC and AD-MSC based on MLR test results. The immunomodulatory properties of BM-MSCs (Del Fattore et al., 2015), AD-MSC (Blazquez et al., 2014) and umbilical cord-MSCs (Monguio-Tortajada et al., 2017) were previously investigated. The higher immunomodulatory activities of ES-MSC could be reflected as higher secretion of anti-inflammatory cytokines, TGF-β and IL-10 and lower production of IFN-γ compared to BM-MSC and AD-MSC (Li, Tormin, et al., 2013; Ng et al., 2016).
As an anti-inflammatory surface marker, CD106 (VCAM-1) is expressed on MSCs (Yang et al., 2013). This marker is also highly expressed on ES-MSCs compared to BM-MSCs (Lian et al., 2007). In addition, it was demonstrated that human ES-MSCs can inhibit CD83 up-regulation and IL-12p70 secretion from dendritic cells and increase T-reg cell populations after induction by IL-2 (Kimbrel et al., 2014). The therapeutic potentials of ES- and iPS-MSCs have been investigated in many animal models such as inflammatory bowel disease (Sanchez et al., 2011), fistulizing Crohn's disease (Ferrer et al., 2016), experimental autoimmune encephalitis (Wang et al., 2014), cardiac remodeling and dysfunction (Liang et al., 2017), arthritis (Gonzalo-Gil et al., 2016), lupus nephritis (Thiel et al., 2015), cardiomyopathy (Zhang et al., 2015), diabetes (Hajizadeh-Saffar et al., 2015) and pulmonary arterial hypertension (Zhang et al., 2012). We also reported the therapeutic effect of human ES-MSC on acute hepatic failure (Lotfinia et al., 2016) and lethal fulminant hepatic failure models (Moslem et al., 2013). ES-MSC exhibited higher growth rate during early in vitro expansion. The adipogenic and osteogenic differentiation capacity and colony-forming potential of these cells are similar to AM-MSC and BM-MSC (Gadkari et al., 2014; Lotfinia et al., 2016). Moreover, human ES-MSCs overcome limitations of harvesting MSCs from adult tissues, including the availability of suitable donors, invasive procedures, limited number of cells obtained during the harvesting process and restricted in vitro expansion capacity.
Most of therapeutic effects of MSCs may be related to systemically concomitant mechanisms such as inhibiting inflammation, protecting sinusoidal endothelial cells and hepatic cell death, hepatocyte regeneration promotion (Feng et al., 2011) and autophagy (Jung et al., 2013) through their secretome (Chen, Zhang, et al., 2017; Huang et al., 2016; Konala et al., 2016). Systemic injection of MSC-conditioned medium (CM) has been shown to be effective in liver experimental research (Chen et al., 2015; Du et al., 2013; Haldar et al., 2016; Lotfinia et al., 2016; Tan et al., 2014; Xagorari et al., 2013). MSC secretome includes both soluble factors and factors released from EVs, that is, exosomes (Yanez-Mo et al., 2015) and microvesicles (Bruno et al., 2015).
The therapeutic potential of MSC-derived EV (MSC-EV) was shown in several experimental conditions like damaged bone marrow, renal, pulmonary and cardiovascular tissues (for review see (Akyurekli et al., 2015; Borger et al., 2017; Crivelli et al., 2017; Quesenberry et al., 2015; Sharma et al., 2017; Wang et al., 2017)). Clinical trials using EVs are already being done on refractory graft-versus-host disease (Kordelas et al., 2014), non-small cell lung cancer (Morse et al., 2005) and colorectal cancer (Dai et al., 2008). Thus, we sought to evaluate the efficacy of human ES-MSC EV and ES-MSC on TAA-induced chronic liver injury.
In the present study, EVs were isolated in accordance to guidelines of international society for extracellular vesicles (ISEV) for conditioned media collection, isolation procedure and characterization methods (Lobb et al., 2015; Witwer et al., 2013; Yanez-Mo et al., 2015). The mean size of purified EVs was 186.43 ± 18.48 nm, as measured by DLS and electron microscopy. They also exhibited round-shaped morphology and they were positive for CD81, CD63, and TSG101, as EV markers.
MLR test demonstrated that ES-MSC EVs had a higher anti-inflammatory effect compared to EVs that were derived from BM-MSC, which could be related to higher secretion of anti-inflammatory cytokines. The immunosuppressive effect of purified MSC-EVs was reported to be similar to parent cells (Monguio-Tortajada et al., 2017).
To evaluate the hepatoprotective capacity of EVs and their parental cells, we stablished a rat liver cirrhosis model characterized by progressive fibrosis, hepatocytes degeneration, and infiltration of immune cells, by intraperitoneal injection of TAA. Periportal fibrosis with the same pattern to human, developing more regenerative nodules and persistent fibrosis (more than 2 months even in the absence of TAA induction) are considered the advantages of TAA over other liver toxic agents such as CCL4 (Delire et al., 2015). Then, we injected the cells and EVs intrasplenicly using theranostic ultrasound technique as a safe and noninvasive method for EV-based therapy.
Progressive fibrosis considered as a hallmark of cirrhosis in which liver parenchymal cells replaced with collagen fibers secondary to the activation of myofibroblasts. Both ES-MSCs and ES-MSC EVs alleviated fibrosis and collagen density. Quantitative RT-PCR analysis of the livers also showed significant down-regulation of Col1α, αSMA, and TIMP1, the major contributors to fibrosis in ES-MSC and ES-MSC EV recipient livers. This improvement in collagen density and fibrosis may be related to up-regulation of the gene expression of collagenases, MMP9 and MMP13 that are able to degrade fibrillar collagens. The fibrolytic activity of MSC in liver injury has been previously reported to be mediated through increasing the amount of matrix metalloproteinase enzymes and inhibition of enzyme inhibitors, which lead to regression of the fibrous tissue (Chen, Zhang, et al., 2017). We previously showed similar expression of these enzymes induced by BM-MSC in a model of CCL4-induced liver injury (Rabani et al., 2010). TIMP1 is expressed in hepatic myofibroblasts and plays a major role in tipping the balance of ECM turnover toward net deposition by blocking key MMPs activity. MMP9 activation results in a decrease in collagen and hydroxyproline content of the liver (Roderfeld et al., 2006). MSCs have been identified as a relevant source of MMP13. Matrix remodeling occurs quite effectively in MMP13-knockout mice (Fallowfield et al., 2007). Moreover, MSCs may contribute to matrix remodeling via up-regulation of the expression of several MMPs with collagenase activities, including MMP9 and MMP14 (Inagaki et al., 2007). Therefore, MSCs can improve liver injury by reducing collagen deposition which is possibly mediated via alteration of MMPs expression.
ES-MSCs and ES-MSC EVs also improved necrosis, caspase density, and PVD (hemodynamics of the liver) and increased liver enzymes, ALT, ALP, and GGT and upregulated the expression of BCL-2 as an anti-apoptotic gene. It was demonstrated that MSC-derived exosomes can elicit hepato-protective effects against toxicants-induced injury, mainly through inhibition of apoptosis by upregulation of Bcl-xL protein and activation of proliferative and regenerative responses (Tan et al., 2014). We also showed that ES-MSC EVs were able to modulate inflammatory microenvironment by reducing immune cell infiltration and modulating the expression and secretion of inflammatory (TGF-β1 and IL-10) and pro-inflammatory cytokines (TNFα and IL-2) which are greatly associated with liver fibrosis following treatment of animal models with ES-MSC and ES-MSC EV.
It is demonstrated that the feedback loop between MSC and activated T cells may limit the immunosuppressive effects of ES-MSCs to sites containing ongoing immunologic or inflammatory responses where activated T cells induce up-regulation of indoleamine-2,3-dioxygenase (IDO) and immunomodulatory properties of MSC (Lin et al., 2012). The immunosuppressive effects of MSC-derived exosomes have been related to reduction of recruiting macrophages and neutrophil number and TNF-α and IL-6 level in hepatic injury (Haga, Yan, Takahashi, et al., 2017; Nong et al., 2016). Protein and microRNA-mediated immunomodulation of MSC-EVs were attributed to their cargo including miR-146, TGF-β1, IL-10, IL-6, HGF, PGE2, and IDO (Haga, Yan, Takahashi, et al., 2017).
In conclusion, these findings suggest that ES-MSC and ES-MSC EV ameliorate TAA-induced rat liver fibrosis. The study provided novel evidence for ES-MSCs and ES-MSC EV-mediated tissue repair and presented an alternative source for the treatment of fibrotic liver disease. Finding the most effective content of ES-MSC EV which play a critical role in treatment of liver fibrosis, needs further investigations.
ACKNOWLEDGMENTS
This project funded by Royan Institute, Tehran University of Medical Sciences (TUMS) & Health Services, the Iran National Science Foundation (INSF) and Iran Science Elites Federation to H.B. All authors thankfully appreciate all members of the Department of Stem Cells and Developmental Biology at Royan Institute for their kind help.
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.