A novel epididymal quiescence factor inhibits sperm motility by modulating NOS activity and intracellular NO-cGMP pathway
Abstract
Mature and potentially motile spermatozoa stored in cauda epididymis in an inactive state for approximately 30 days; however, during ejaculation they regain motility. To understand the actual molecular mechanism of the sperm quiescence during caudal stay, a proteinaceous quiescence factor (QF) has been purified from caprine epididymal plasma to apparent homogeneity. In the present study complete purification, detailed characterization as well as mechanistic pathway of QF has been described. QF is purified to 215-fold with 45% activity recovery. It is a 59 kDa monomeric protein with isoelectric point 5.8 and optimally active at pH 7.5. Circular dichroism spectroscopy and atomic force microscopy study confirm its α-helical secondary structure and globular tertiary conformation. QF is a thermo-stable protein as higher temperature does not alter its helical structure. N-terminal amino acid sequencing and MALDI analysis of QF did not find 100% similarity with any available protein of the database, proved its novelty. QF at 2 μM dose inhibits sperm progressive forward motility within 10 min. This motility inhibitory activity of QF is mediated by reducing NOS enzyme activity and subsequently decreasing the intracellular NO and cGMP concentration. It does not modulate intracellular Ca++ and cAMP concentration. QF has no adverse effect on DNA integrity and morphology of spermatozoa. Motility inhibitory action of QF is reversible. Thus, the role of QF in maintaining energy saving quiescence state of mature cauda spermatozoa and its reactive nitrogen species reducing activity may lead to a new direction for storage of spermatozoa and idiopathic male infertility.
1 INTRODUCTION
Motility of spermatozoa is crucial for male fertility as individuals with poorly motile or immotile spermatozoa are usually infertile (Turner, 2006). Testicular immotile spermatozoa acquire motility and fertilizing ability during the transit through caput and corpus epididymis with a series of morphological, biochemical, and physiological changes and finally stored in cauda epididymis till ejaculation in a quiescence state to conserve energy (Jones, James, Howes, Bruckbauer, & Klenerman, 2007; Olson, NagDas, & Winfrey, 2002; Varesi, Vernocchi, Faustini, & Luvoni, 2013). Changes in expression and cellular localization of sperm proteins along with role of epididymal proteins in sperm maturation have been reported in various studies (Aitken et al., 2007; Cornwall, 2009). Most of these studies are extensively focused on the caput and corpus regions to reveal how spermatozoa become motile and fertile but, storage mechanism in cauda is not revealed adequately (Varesi et al., 2013).
Initially cauda was considered only as a storage house for mature spermatozoa, but later studies revealed its other important functions like encountering drastic ionic and non-ionic changes, protecting spermatozoa from oxidative damage and maintenance of quiescence state (Morton, Fraser, & Sagadraca, 1979; Tvrdá, Kňažická, Bárdos, Massányi, & Lukáč, 2011). Decrease in Na+, increase in K+, pH, osmolarity, and absence of glucose and fructose in cauda have been reported to be helpful for sperm quiescence (Nag, Sarkar, & Ghosh, 1975; Turner, 1979; Verma & Chinoy, 2001; Wong, Au, & Nigai, 1978). A number of studies investigated the quiescence activity of organic and inorganic constituents in rat, mouse, hamster (Hamamah & Gatti, 1998; Morton, Harrigan-Lum, Albagli, & Jooss, 1974; Peitz, 1988; Turner & Giles, 1982), and bull spermatozoa (Cascieri, Amann, & Hammerstedt, 1976). Hamilton and Olson (1976) reported that carnitine helps in sperm quiescence by reducing oxygen uptake. Seminal plasma of different species like bull, chicken, porcine, boar were also investigated for the presence of motility inhibitors (Bass, Molan, & Shannon, 1983; de Lamirande, Bardin, & Gagnon, 1983; Iwamoto et al., 1992; Jeng, Liu, & Chang, 1993; Mohan, Saini, & Joshi, 1995; Robert & Gagnon, 1996). SPMI isolated from human seminal plasma also showed sperm motility inhibitory activity (Iwamoto & Gagnon, 1988). Most of these factors are reported from seminal plasma but the quiescence mechanism of cauda epididymal spermatozoa is needed to be explored further to understand its storage strategy. Turner, D'Addario, and Howards (1978) showed that sperm immobilizing efficacy of undiluted rat cauda epididymal (CE) fluid abolished after proteases treatment, which indicated the involvement of epididymal proteins in sperm motility inhibition and these proteins presumably act on the sperm surface (Turner & Giles, 1982). Usselman and Cone (1983) demonstrated a high molecular weight glycoprotein, Immobilin, immobilizes sperm mechanically by increasing the viscoelasticity of CE fluid. Occurrence of sperm motility inhibitory factors in goat epididymal plasma and sperm plasma membrane is also reported (Das, Saha, Majumder, & Dungdung, 2010; Dungdung & Majumder, 1995, 2003). But detailed studies regarding molecules involved in quiescence of spermatozoa and their mode of action are required to understand the causes of idiopathic male infertility (Robaire, Hinton, & Orgebin-Crist, 2006).
In the present study, a sperm motility quiescence factor (QF) has been purified from caprine epididymal plasma. Its physicochemical characterization and sperm motility inhibitory mechanistic pathway has been elucidated in caprine spermatozoa. This study may contribute in fundamental ideas related to sperm quiescence and energy saving during storage in cauda epididymis and may unveil some causes of idiopathic male infertility.
2 MATERIALS AND METHODS
2.1 Material
All protein purification reagents used were of analytical grade and purchased from Sigma (St. Louis, MO) otherwise mentioned. cAMP Enzyme Immunoassay Kit (Catalog No. CA200), cGMP Enzyme Immunoassay Kit (Catalog No. CG200), Proteosilver Plus Silver Staining Kit (Catalog No. PROTSIL2) purchased from Sigma. ReadyStrip IPG Strips (Catlog no: 163-2001), ReadyPrep 2D Starer Kit (Catlog no: 163-2105) was obtained from Bio-Rad Laboratories (Hercules, CA). In-Gel Tryptic Digestion Kit was procured from Thermo Fisher Scientific (Rockford, IL). Apo-BrdU In Situ DNA fragmentation assay kit (Catalog No. K401-60) was obtained from Biovision (CA). Fluo 3/AM, 3-Amino, 4-aminomethyl-2′,7′-difluorofluorescein Diacetate (DAF-FM-DA), protease inhibitor cocktail, Nitric Oxide Synthase Assay Kit, Colorimetric (Catalog No. 482702) was purchased from Calbiochem, Darmstadt, Germany. ASTM V1 Grade Ruby Mica sheet (MICAFAB, Chennai, India).
2.2 Methods
2.2.1 Preparation of epididymal plasma (EP) and purification of sperm motility quiescence factor (QF)
EP was prepared from the goat cauda epididymis as described earlier by Das et al. (2010). Goat epididymidis were collected as a by-product of local slaughter house, so, no ethical clearance was required for it. Sperm suspension in modified Ringer's solution (RPS) free of Ca++ (119 mM NaCl, 5 mM KCl, 1.2 mM MgSO4, 16.3 mM potassium phosphate buffer, 10 mM glucose, 50 units/ml penicillin [pH-6.9]) was centrifuged at 800g for 10 min to remove the pelleted spermatozoa. Resultant supernatant was again centrifuged at 14,000g for 30 min to obtain cell-free clear EP and kept at −20°C for further use.
To purify QF, goat EP was first heated at 100°C for 2 min in a water bath. EP was then centrifuged at 10,000g for 10 min and the supernatant was concentrated and dialyzed overnight against 10 mM KPO4 buffer. Dialyzed EP was then electrophoresed in a 10% nondenaturing poly acrylamide gel electrophoresis (PAGE) in multiple lanes. After completion of run, one lane was sliced off and stained with coomassie blue and the unstained portion of the lanes parallel to active band of approximately 60 kDa of stained section, was sliced off, smashed into small pieces and then dissolved in 2% (v/v) tri ethyl amine (TEA) solution in 10 mM KPO4 buffer for 1 hr Protein was eluted from smashed gel as supernatant after centrifugation at 10,000g for 10 min. This step was repeated twice and collected elute was dialyzed, concentrated and subjected to diethylaminoethyl (DEAE) cellulose anion exchanger chromatography, pre-equilibrated with 10 mM KPO4 buffer. The column was then eluted with 25, 50, 100, and 250 mM KPO4 buffer, at pH 7.4. The active fraction was eluted by 50 mM KPO4 buffer, then dialyzed and concentrated. The active fraction further subjected to molecular sieving high-performance liquid chromatography (HPLC; using waters protein-pack 125A 7.8 × 300 mm column, flow rate 0.8 ml/min). The active peak at the retention time of approximately 12.7 min was eluted with 10 mM KPO4 buffer containing 150 mM NaCl was dialyzed, concentrated and stored in −20°C for further use. All the purification steps were carried out at 4°C unless otherwise specified. The protein purified by HPLC was again subjected to same HPLC column maintaining same parameters as in the purification procedure to confirm its purity. Homogeneity of QF was further checked by fast performance liquid chromatography (FPLC) in Mono-Q 5/50 GL Tricorn column using 10 mM KPO4 buffer with 0.7 ml/min flow rate.
2.2.2 Determination of molecular weight
Molecular weight of purified QF protein was determined by HPLC and sephacryl S-200 gel filtration chromatography. For HPLC, 100 μg QF was applied to HPLC using same column and buffer as purification step with 0.8 ml/min flow rate. Known molecular weight proteins were used as marker and passed through HPLC with same parameters. Molecular weight of QF was calculated from molecular weight versus retention time values of known marker proteins. For sephacryl S-200 column, 300 μl (100 μg) purified QF was loaded on the equilibrated sephacryl S-200 column (1 × 30 cm) with 10 mM KPO4 buffer, pH 7.4. Each 1 ml fractions were collected and protein was monitored at 280 nm absorbance to track the eluted protein. Molecular weight of QF was calculated from the plot of log molecular weight versus Ve/V0 value of known marker proteins. V0 was obtained by running blue dextran (2,000 kDa).
2.2.3 Detection of subunit composition of QF
The subunit structure of QF was determined by denaturing 10% (w/v) SDS–PAGE (Laemmli, 1970). Mixture of known molecular weight markers was run in adjacent lane. Gel was stained using PROTSIL2 silver staining kit (Sigma).
2.2.4 Isoelectric focusing (IEF) and two dimensional gel electrophoresis (2DE) of QF
IEF and 2DE studies were carried out using Bio-Rad's 2D kit. Purified QF (30 μg) was rehydrated overnight on 7 cm immobilized pH gradient (IPG) strip (pH 4–7 linear) and separated by IEF at 4,000 V and 10,000 Vhr for 4.5 hr One strip was silver stained for calculating isoelectric point (PI) and another strip was then equilibrated and resolved for protein separation in the second dimension gel (12% [w/v] SDS–PAGE) and visualized by using PROTSIL2 silver staining kit.
2.2.5 N-terminal amino acid sequencing
N-terminal amino acid sequencing of QF was done by Edman degradation method on Simadzu Biotech protein sequencer (Model: PPSQ-31A). Protein was run in 10% SDS–PAGE and electro transferred on a PVDF membrane. The transfer buffer used was 10 mM 3-cyclohexylamino-l-propanesulfonic acid, pH 11, containing 10% methanol. The transferred band was visualized by Ponceau-S staining method, destained by washing with water and a portion was cut and loaded on the sample cartridge. The N-terminal sequence was matched with available sequences in the BLAST homology search program database (NCBI, NIH, USA). Phylogenetic tree was constructed using Molecular Evolutionary Genetics Analysis 6.0 (MEGA6) software and clustered using minimum evolution module in MEGA6 and distance tree generation.
2.2.6 Secondary structure analysis
The secondary structure of QF was analyzed by circular dichroic spectra of protein (1.4 μM QF in 10 mM phosphate buffer, pH 7.4) in the far-UV wavelength (190–250 nm) at 25°C temperature in a JASCO J-720 (JASCO Corporation, Tokyo, Japan) spectropolarimeter. Spectra were recorded in a continuous mode with following scan parameters: bandwidth 1 nm, scanning speed 50 nm/min with average sensitivity and path length of quartz cuvette was 0.1 cm. Spectra at different temperatures (25–75°C) were recorded using the same parameters to determine the effect of increased temperature on the secondary structure of QF. Protein-free buffer (10 mM phosphate buffer, pH 7.4) was used as blank and the acquired spectra were corrected by subtracting the blank spectra. Data were plotted using OriginLab software and analysis was done using Dichroweb software (Department of Crystallography, Institute of Structural and Molecular Biology, Birkbeck College, University of London, UK) using Selecon3 program, with Reference set 7 (Whitmore & Wallace, 2008).
2.2.7 Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF/MS) analysis based on peptide mass fingerprinting
For MALDI-TOF/MS assay, purified QF protein was first electrophoresed in 10% (w/v) SDS–PAGE followed by silver staining using PROTSIL2 kit. The sample was then destained and tripsinized using In-gel tryptic digestion kit for mass spectrometry as per manufacturer's protocol. This trypsin digested protein was then concentrated, desalted using C18 zip tip and analyzed using a standard solution of matrix CHCA (α cyano—4 hydroxy cinnamic acid) in 50% Acetonitrile (ACN) and 0.1 % Trifluoroacetic acid (TFA) in a 4700 MALDI TOF/TOF (Applied Biosystems, CA) instrument. The peaks corresponding to the most abundant peptides generated after trypsin digestion were searched against available NCBInr database using Mascot (Matrix Science Ltd, London, UK; http://www.matrixscience.com) search program with fixed and variable modifications: carbamidomethyl (C) and oxidation (M), respectively.
2.2.8 Microscopic sperm motility assay
Microscopic sperm motility was determined following the method described in Ghosh et al. (2018). EP also contains anti-sticking property thus a specific amount of EP (0.6 mg protein/ml) was used in the assay to prevent the adhesion of sperm cells on the glass surface (Roy & Majumder, 1989). A total of 1 × 106 sperm cells and EP were incubated in 0.5 ml RPS medium with or without test sample (QF treated) at room temperature for 1 min. Then 5 μl of the control or treated samples were placed on a hemocytometer, covered with coverslip and the number of spermatozoa that showed well-defined progressive forward motility (PFM), other types of motility and no motility were counted under a phase contrast microscope at 400 × magnification. Percentage of progressive forward motile spermatozoa was calculated as ([forward motile spermatozoa/total spermatozoa] × 100).
For motility retrieval assay, prepared spermatozoa were treated with 2 μM QF for 10 min and motility of spermatozoa in control and treated group was assessed by microscopic method as mentioned above. QF was then washed off by centrifugation (1,000g for 3 min) and resuspended in assay medium for motility estimation at different time interval.
Effect of glycosidase enzymes on QF was studied after incubation of 2 μM QF with α-mannosidase (27 units/ml), β-galactosidase (5 units/ml), and neuraminidase (5 units/ml) for 10 min, centrifuged and supernatant was used for microscopic sperm motility assay. A total of 2 μM QF without enzyme treatment was used as control.
Effect of different pH on QF was evaluated by changing the pH of RPS medium however other assay conditions were same as microscopic sperm motility assay.
2.2.9 Preparation of sperm sample and determination of vertical velocity by SPERMA
A total of 400 μl of sperm suspension (200 × 106 cells/ml) in RPS medium was mixed with 100 μl of 10% ficoll-400. This 2% (final concentration) ficoll-400 has no adverse effect on sperm motility and from this ficoll-sperm mixture only motile spermatozoa can swim-up. The dead or less motile cells were unable to swim-up against density formed by ficoll (Saha et al., 2013). Average vertical velocity of motile cells was determined by SPERMA, a unique computer-based spectrophotometric system, as mentioned in Saha, Paul, Mukherjee, Banerjee, and Majumder (2007). In this instrument absorbance can be recorded from four different heights of the cuvette. Cuvette was filled with 1.5 ml of modified RPS medium and placed in the cuvette holder. A total of 50 μl of prepared sample (with or without QF) was layered slowly at the bottom of the cuvette with the help of a Hamilton Syringe. During the scanning cuvette was moved from one height to the adjacent one and absorbance were measured at 545 nm for 10 min. Absorbance versus time data was acquired at four different heights of the cuvette during each cycle of time scanning and average vertical velocity was estimated by the software attached with the instrument.
2.2.10 Determination of intracellular cAMP and cGMP
cAMP and cGMP level in goat spermatozoa were determined by using “cAMP enzyme Immunoassay Kit, Direct” and “cGMP enzyme Immunoassay Kit, Direct” and the method followed as described earlier in Das et al. (2010). Both the enzyme immune assay direct cAMP and cGMP kits quantitatively determined the cAMP and cGMP concentrations in samples. Different concentration of spermatozoa (30 × 106 cells/ml [for cAMP] and 100 × 106 cells/ml [for cGMP]) were treated separately with PBS (control), 1 and 2 μM QF for 15 min. Then the samples were treated with HCl followed by acetylation to increase the sensitivity of the both assays. The experiments were done as per manufacturer's protocol and OD was recorded at 405 nm. The concentrations of cAMP and cGMP in standard, control and treated samples were calculated as described in the manufacturer's protocol.
2.2.11 Estimation of intracellular calcium
Changes in the intracellular calcium level was monitored in the fluorimeter with the fluorescent probe fluo 3AM, a high affinity calcium indicator. Cell permeable fluo 3AM cleaved by esterase and emits fluorescence following reaction with intracellular calcium. A total of 2 × 106 goat sperm cells treated separately with PBS (control), 1 and 2 μM QF and 50 μM EGTA (negative control) for 15 min. Then the cells were incubated with 4 μM fluo 3AM containing 0.1% pluoronic acid F-127 in a dark humidified incubator (37°C, 5% [v/v] CO2 in air) for 30 min. Relative fluorescence was measured in a Perkin-Elmer LS50B Spectrofluorometer at an excitation wavelength of 488 nm and an emission wavelength of 522 nm. The data were expressed as fluorescence intensity observed.
2.2.12 Estimation of nitric oxide synthase (NOS) enzyme activity
NOS enzyme activity was measured by colorimetric method using Nitric Oxide Synthase assay kit (Calbiochem) which is based on a modified Griess method that quantifies the total level of nitrite and nitrate (stable NO metabolites) as an indicator of NOS activity. Briefly, 2 × 106 prepared sperm cells were incubated with PBS (Control), 1 and 2 µM QF for 15 min. Reaction was then stopped by heat inactivation after placing in a boiling water bath for 30 s followed by homogenization. Cells were then centrifuged at 10,000g for 5 min and the supernatant was used for the NOS activity assay as per manufacturer's protocol. Excess NADPH in the reaction mixture was degraded using lactate dehydrogenase as it interferes with Griess reagents. After addition of Griess reagents the purple azo dye formed by its interaction of nitrite. The optical density of this dye was measured at 540 nm using Bio-Rad iMark Microplate Reader (Bio-Rad Laboratories, Hercules, CA). Amount of nitrite in control and treated samples were calculated from nitrate standard curve as mentioned in manufacturer's protocol. The values were normalized and control was expressed as a value of 100% NOS activity.
2.2.13 Estimation of intracellular nitric oxide (NO)
Intracellular NO was estimated using cell-permeant nonfluorescent probe DAF-FM-DA, which after entering into the cell get deacetylated by the intracellular esterases and upon reacting with NO emits fluorescence. Briefly, 2 × 106 goat sperm cells were incubated with PBS (control), 1 μM, 2 μM QF and 1 mM N(G)-Nitro-l-arginine methyl ester (L-NAME; negative control) for 15 min and then cell-permeant probe DAF-FM-DA (4 μM) was added. After 30 min incubation at 30°C in the dark, relative fluorescence was measured in a Perkin-Elmer LS50B Spectrofluorometer at an excitation wavelength of 495 nm and an emission wavelength of 515 nm. The data were normalized to normal values, and the control was expressed as a value of 100%.
2.2.14 DNA fragmentation assay
Detection of DNA fragmentation was done by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using a standard APO-BrdU in situ DNA fragmentation assay kit (Biovision) as per manufacturer's protocol and as described in Ghosh et al. (2018). BrdU-FITC positive cells are DNA fragmented cells and the increase in fluorescence intensity indicates higher DNA fragmentation. In brief, 2 × 106 prepared sperm cells were treated with PBS (control), 1 and 2 μM QF for 15 min. Then the cells were pellet down, fixed with 1% paraformaldehyde in PBS for 15 min and resuspended in 70% ice cold ethanol for 30 min. After removing ethanol, control and treated sperm cells were incubated for 1 hr with DNA labeling solution containing Br-dUTP. Then the cells were washed with Rinse Buffer and the pellet was resuspended in Anti-BrdU-FITC antibody solution for 30 min in dark at room temp. Flow cytometer analysis was performed using BD LSR Fortessa fitted with a 488 nm laser with an excitation wavelength of 488 nm and emission 520 nm. The cell population was separated into four groups, upper left quadrant (Q1) represented necrotic cells, upper right quadrant (Q2) and lower right quadrant (Q4) both represented DNA fragmented cells and lower left quadrant (Q3) represented viable live cells. Percentage of DNA fragmented cells were counted by adding the percentage of cells in Q2 and Q4 quadrant. All tests were done with four different sperm samples. For confocal microscopy, control and 2 μM QF treated cells (15 μl) were spread on the glass slides and TUNEL assay was performed as per manufacturer protocol. Then the slides were mounted with cover slip using mounting medium. The fluorescence was visualized using fluorescein isothiocyanate (FITC) and rhodamine filter through Andor Spinning Disc Confocal Microscope (Ireland, UK) with Andor ixon 897 EMCCD camera attached. The software used for analysis was Andor iQ 2.4.7.
2.2.15 Analysis of sperm morphology and tertiary structure of protein by atomic force microscopy (AFM)
The AFM observations of both spermatozoa and protein (QF) were performed with an Agilent Technologies (CA) 5500 ILM Pico plus AFM system with a piezo scanner maximum range of 100 μm (for spermatozoa) and 9 μm (for protein). Imaging of spermatozoa was carried out as described in Ghosh et al. (2018). A total of 1 × 106 sperm cells were treated with PBS (control) and 2 μM of QF for 15 min and fixed with 4% paraformaldehyde. A total of 10 μl of the diluted sample (1:10 [v/v] with milli Q water) was then placed on mica sheet, air dried, gently washed with 0.5 ml milli-Q water and again kept for drying. For protein sample AFM experiment was prepared by placing 10 μl QF (100 nM) on a freshly cleaved muscovite ruby mica sheet and kept 20 min for air dry. After proper drying the samples were used for AFM study. Images were captured in contact mode (spermatozoa) and tapping mode (protein) with scan speed 0.5 line/s. Micro fabricated silicon cantilevers of 450 and 225 μm in length with a nominal spring force constant of 0.02–0.77 and 21–98 N/m for spermatozoa and protein respectively from Nano sensors, USA. Cantilever oscillation frequency was turned into resonance frequency (6–21 and 150–350 kHz for spermatozoa and protein, respectively). Images were processed by Pico view 1.12 version software (Agilent Technologies).
2.2.16 Protein estimation
Microgram amount of protein were measured by the rapid and sensitive method developed by Bradford (1976).
2.2.17 Statistical analysis
For Statistical analysis Graph pad Instat software (La Jolla, CA) was used. All experiments were repeated at least three times with different samples. Data are represented as the mean ± SEM. Experiments were compared using one-way ANOVA; post hoc tests were done with Dunnett's multiple comparison tests to reveal difference in groups. Value of p < 0.05 was considered significance.
3 RESULTS
3.1 Purification of QF and its homogeneity check
Summary of the purification of QF has been shown in Table 1. QF was purified by heat precipitation, gel elution, DEAE anion exchange chromatography, and HPLC. As QF is heat stable so, first suitable purification step undertaken was the heat precipitation of unwanted heat labile proteins. Native gel eluted QF was purified up to 30-fold with 70% of activity recovery. QF further binds to DEAE cellulose resin and the bound fraction was eluted with 50 mM KPO4 buffer. By this step QF was purified up to 106-fold with 64% activity recovery. After HPLC separation of DEAE eluted fraction, QF purification was further increased to 215-fold with 45% activity recovery (Table 1). Purity of QF was checked by HPLC (Figure 1a) as well as FPLC (Figure S1) and further confirmed by two-dimensional electrophoresis (Figure 1bb2). When QF was subjected to HPLC column a single peak at around 12.7 min was observed. The same protein when resolved in the FPLC column, a single peak was found at around 17.35 min. As both HPLC and FPLC showed single peak, hence confirmed that QF has been purified up to apparent homogeneity. Purity of QF was further confirmed by 2DE experiment and after silver staining of second dimensional gel a single spot was observed (Figure 1bb2) which proved the homogeneity of the purified QF.
| Fractions | Total activity (Units) | Total protein (mg) | Specific activity (Units/mg) | Activity recovery (%) | Fold purification |
|---|---|---|---|---|---|
| Epididymal plasma | 3,870 | 500.0 | 7.7 | 100.0 | 1.0 |
| Gel elute | 2,733 | 11.7 | 233.6 | 70.6 | 30.2 |
| DEAE—ion exchange chromatography | 2,475 | 3.0 | 825.0 | 64.0 | 106.6 |
| HPLC | 1,664 | 1 | 1,664 | 45.2 | 215.0 |
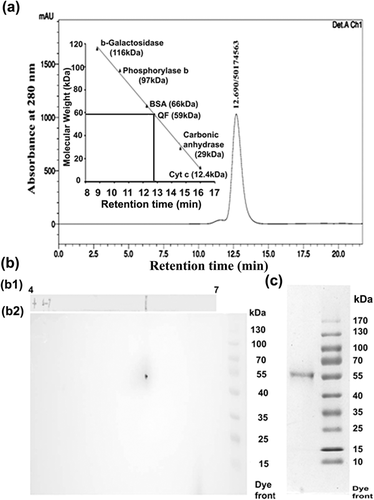
3.2 Biochemical characterization of QF
3.2.1 Effect of trypsin on QF activity
Trypsin is widely known for its protein cleavage activity. Spermatozoa after treated with 2 μM QF, their progressive forward motility was initially decreased and later completely stopped with advancement of time. But trypsin treated QF completely lost its activity and progressive forward motility was observed as good as control cells. This study proved that QF is a protein and lost its activity due to peptide bond cleavage by trypsin (Figure S4).
3.2.2 Effect of pH on QF activity
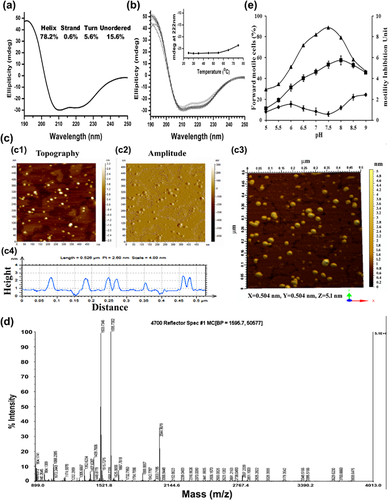
pH is an important parameter for proper protein structure and its activity. Progressive forward motility inhibiting activity of QF in different pH was studied by altering the pH of assay media and results showed QF was active at a range of pH 6.5–8 and optimally active at pH 7.5 where it showed maximum progressive forward motility inhibiting activity (Figure 2e).
3.2.3 Effect of different glycosidase enzymes on QF activity
Specific glycosidase enzymes hydrolyze a definite glycosidic linkage which causes degradation of oligosaccharides and glycoconjugates and ultimately loss of functional activity occur. After treatment with different glycosidase enzymes namely β-galactosidase, α-mannosidase, neuraminidase, QF did not lose its sperm motility inhibitory activity, which confirmed that QF did not possess galactose, mannose, or neuraminic acid as glycoconjugate in it (Figure S5).
3.3 Biophysical characterization of QF
3.3.1 Determination of molecular weight, subunit structure, and PI value
Molecular weight of QF was determined by HPLC (Figure 1a) and further confirmed by sephacryl S-200 gel filtration chromatography (Figure S2). Molecular weight of QF was found approximately 59 kDa in both HPLC and sephacryl S-200 gel filtration chromatography. Subunit composition of QF was determined by electrophoretic separation of QF in 10% (w/v) SDS–PAGE. Result depicted a single band after purified QF resolved in SDS–PAGE, which proved its monomeric nature (Figure 1c). PI value of QF was determined by isoelectric focusing (IEF) of QF on a linear grade IPG strip. After silver staining of strip a single band was observed on the strip which denotes the PI value of the protein calculated as 5.8 (Figure 1bb1).
3.3.2 Secondary structure detection and effect of temperature on secondary structure
Circular dichroism (CD) spectroscopy is a well established technique to determine the secondary structure of protein hence in this study this technique was used to identify the secondary structure of QF. Results depicted well defined peak at 208 and 222 nm those are the characteristics of helical protein. Thus QF found to be a helical protein with 78% α-helical structure at 25°C (Figure 2a) as calculated using Selecon3 program, Reference set 7 in Dichroweb software. In subsequent experiment alteration of secondary structure with increased temperature was studied. Result showed that the ellipticity change at 222 nm was only 1 mdeg at 55°C and 4 mdeg when the temperature rose to 75°C as compared to 25°C which proved QF was able to maintain its secondary structural stability even at high temperature (Figure 2b).
3.3.3 Tertiary structure detection of QF
AFM imaging study was used to identify the tertiary structure of QF. Both topography (Figure 2cc1), amplitude (Figure 2cc2) images of AFM and software constructed three dimensional image (Figure 2cc3) clearly depicted the tertiary structure of QF as a globular protein. Diameter of QF was measured from topography image and median value was found 11.16 nm and the range was 10–13 nm whereas, height of QF molecules was found at a range of 1.2–1.5 nm (Figure 2cc4).
3.3.4 N-terminal amino acid sequencing of QF
N-terminal sequencing of QF was done by Edman degradation method and first 19 N-terminal amino acid sequences were found Asp-Thr-Gly-Asp-Ile-Ala-Ile-Ala-Arg-Trp-Phe-Asn-Asp-Leu-Gly-Ala-Phe-Ile-Phe. This sequence was used to find match with available protein sequences using NCBI BLAST 2.6.0 program with whole organism database. None of the protein in the database showed 100% match with N-terminal sequence of QF. Maximum 68% query coverage with 54% identity was seen with hypothetical protein (accession no: WP051555878.1) and MFS transporter (accession no: WP020399734.1) of Kordiimonas gwangyangensis (Table 2). When the search was restricted to Capra hircus (source of the QF) database, QF showed sequence similarity with axonemal dynein heavy chain 11 (86% identity), full serum albumin and albumin precursor (78% identity in both) but total score was too low (maximum score 24.0) and E-value was very high (minimum E-value 6.2) (Table 3). Hence, none of the available proteins in the database showed 100% sequence similarity with N-terminal amino acid sequence of QF. Phylogenetic tree was constructed from the first 10 protein showed similarity with QF in whole organism NCBI BLASTP search. They were aligned with MEGA6 software and clustered using minimum evolution module in MEGA6 and generated a distance tree for all 11 protein sequences. QF was found closely related to Myosin C of Phaeodactylum tricornium and Sugar ABC transporter substrate binding protein proteins of Psuedomonus sp. (Figure S3).
| Protein name | Organism | Max score | Query cover (%) | E-value | Ident | Accession No. |
|---|---|---|---|---|---|---|
| Hypothetical protein | K. gwangyangensis | 35.4 | 68 | 0.90 | 54% | WP051555878.1 |
| MFS transporter | K. gwangyangensis | 35.4 | 68 | 0.90 | 54% | WP020399734.1 |
| Esterase/lipase-like protein | Paenibacillus sp. Aloe-11 | 34.1 | 47 | 2.5 | 100% | EHS56900.1 |
| Esterase | Spirosoma sp. 48–14 | 34.1 | 63 | 2.5 | 71% | OJW78836.1 |
| Myosin C | Phaeodactylum tricornutum | 34.1 | 63 | 2.5 | 71% | OJW78836.1 |
- Parameters used non redundant and all organism database. Proteins showed maximum score ≥ 34 and E-value ≤ 2.5 have been tabulated.
| Protein name | Max score | Query cover (%) | E-value | Ident (%) | Accession no. |
|---|---|---|---|---|---|
| Dynein heavy chain 11, axonemal | 24.0 | 36 | 6.2 | 86 | XP017902825.1 |
| Full serum albumin | 23.5 | 47 | 8.3 | 78 | P85295.2 |
| Albumin precursor | 23.5 | 47 | 8.7 | 78 | ACF10391.1 |
| F-box only protein 27-like | 23.1 | 68 | 12 | 47 | XP_017918656.1 |
| MAM and LDL-receptor class A domain-containing protein 1 | 23.1 | 68 | 12 | 62 | XP_005687969.3 |
- Parameters used non redundant and C. hircus (goat) organism database. Proteins showed maximum score ≥ 23 and E-value ≤ 12 have been tabulated.
3.3.5 Protein identification by MALDI-TOF/MS study
MALDI-TOF/MS spectrum of QF was analyzed for its identification. 46 peptides identified after in-gel trypsin digestion of the QF protein followed by MALDI-TOF/MS analysis (Figure 2d). These fragments were searched in MASCOT software but none of the available protein in the database showed 100% sequence coverage similarity with QF. Protein Mascot score greater than 79 (p < 0.05) were taken as significant search and description of matched peptides in terms of protein accession number, peptide mass, PI value, Mascot score, and sequence coverage are provided in Table 4. Maximum sequence coverage was found 55% with full serum albumin of C. hircus thus indicating its novelty.
| Protein name | Taxonomy | Accession no. | MW | PI | score | Sequence coverage (%) | Expect |
|---|---|---|---|---|---|---|---|
| Albumin precursor | C. hircus | gi|193085052 | 68266 | 5.58 | 303 | 19 | 2e-024 |
| Pre-pro serum albumin | Ovis aries | gi|57164373 | 71139 | 5.80 | 292 | 16 | 2.5e-023 |
| Full serum albumin | C. hircus | gi|205371736 | 10048 | 9.16 | 142 | 55 | 2.5e-008 |
| Albumin | Bos taurus | gi|30794280 | 71274 | 5.82 | 79 | 10 | 0.05 |
| Full serum albumin | B. taurus | gi|1351907 | 71244 | 5.82 | 79 | 10 | 0.05 |
3.4 Functional characterization of QF
3.4.1 Effect of QF on sperm motility
Sperm PFM inhibitory activity of QF was evaluated by both microscopic assay and SPERMA at different time scale (1–10 min). Mature Spermatozoa when treated with 1 μM QF, it reduced sperm PFM up to 59% within 1 min, percentage of inhibition was gradually increased 73–81% at 5 and 10 min, respectively, as compared to control. When higher dose of QF (2 μM) was applied, 80% and 92% reduction in sperm PFM was observed at 1 and 5 min, respectively, and completely stopped PFM at 10 min as observed in microscopic assay (Figure 3a). Upward movement of spermatozoa against gravity was measured by SPERMA. Control sperm cells showed well defined vertical velocity (168 μM/s) at 1 min but treatment with 1 and 2 μM QF, reduced the vertical velocity to 78 and 50 μM/s (53% and 70% reduction) respectively at 1 min. With advancement of time reduction of vertical velocity also increased. At 10 min control cells showed 101 μM/s vertical velocity, however, 1 μM QF treated cells showed a significant reduction of vertical velocity to 20 μM/s (80% decrease) and in the 2μM QF dose vertical velocity completely stopped (100% decrease) (Figure 3b). The suboptimum (1 μM) and optimum dose (2 μM) of QF was further used to elucidate mechanistic pathways involved in sperm motility quiescence.
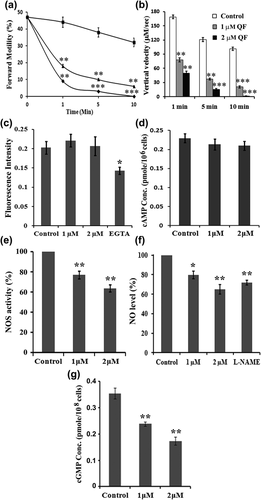
3.4.2 Mechanism of action of sperm motility inhibitory effect of QF
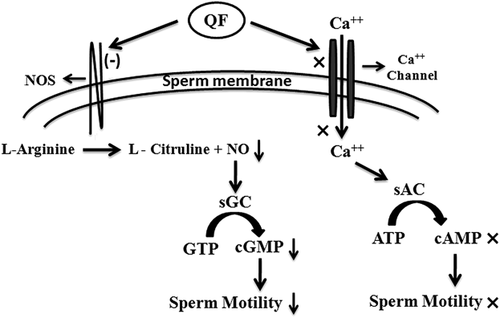
Effect of QF on different sperm motility regulatory molecules has been studied to find out its mechanistic pathway. Several studies have reported intracellular Ca++ and cAMP as two key molecules responsible for motility regulation in mature spermatozoa. When the effect of suboptimum and optimum dose (1 and 2 μM) of QF on intracellular Ca++ concentration was assessed it showed approximately similar intracellular Ca++ concentration as PBS treated control but EGTA (Ca++ chelator) treated spermatozoa showed significant decreased (30%) in intracellular Ca++ concentration (Figure 3c). Subsequently same doses of QF (1 and 2 μM) also showed no significant change in intracellular cAMP concentration (Figure 3d). Hence, to unveil mechanistic pathway involved in sperm PFM inhibitory activity of QF, its effect on other reported sperm motility regulatory molecules, that is, NO and cGMP were observed. All the three isoform of NOS enzyme are located on the sperm membrane and produce NO from L-arginine. Suboptimum and optimum doses of QF (1 and 2 μM) on the NOS enzyme activity in spermatozoa was studied by colorimetric method which showed a dose-dependent gradual decrease (24% and 36%, respectively) in NOS enzyme activity when compared to control (Figure 3e). As QF showed significant decrease in NOS enzyme activity so, further studies were focused to investigate its effect on intracellular NO and cGMP concentration. Spermatozoa when treated with 1 μM and 2 μM of QF, intracellular NO level significantly decreased to around 80% and 65%, respectively, compared to control (100%). In L-NAME (inhibitor of NO production; negative control) treated spermatozoa intracellular NO concentration significantly decreased to 70% compared to control (Figure 3f). In sperm motility regulatory pathway, cGMP is downstream effector molecule of NO. Intracellular cGMP level markedly decreased 33% and 50%, respectively, after treatment with 1 and 2 μM QF compared to control (Figure 3g). Thus QF acts by decreasing NOS enzyme activity and subsequently reducing the concentration of intracellular NO and its effector molecule intracellular cGMP in course of its sperm motility inhibitory activity.
3.5 Effect of QF on DNA integrity and morphology of mature spermatozoa
TUNEL assay was performed to study the effect of QF on sperm DNA integrity. In PBS treated control group 95% viable cells with intact DNA were assessed and after treatment with 1 and 2 μM QF, integrity of DNA was maintained as good as PBS treated control cells (94% and 93% live cells, respectively) (Figure 4a). This was further confirmed by confocal microscopy imaging after treatment with PBS (control) and 2 μM QF in mature sperm cells (Figure 4b). In both the groups green fluorescence of FITC tagged anti-BrdU antibody (indicator of damaged DNA) was absent which signify intactness of DNA. PI was used as counter stain and visualized in both control and treaded cells. Morphological changes in mature spermatozoa were studied by high resolution AFM imaging. Results depicted that after 2 μM QF treatment sperm membrane integrity was maintained like PBS treated control cells (Figure 4c).

3.6 Retrieval of sperm motility after QF treatment
As QF had no adverse effect on the morphology and DNA integrity of spermatozoa hence, the sperm motility retrieval efficacy was studied after washing off QF. Result confirmed that sperm PFM inhibitory activity of QF was not permanent. After washing off QF, motility of spermatozoa was gradually increased with time and within 10 min sperm motility in treated group was restored as good as control spermatozoa (Figure S6).
4 DISCUSSION
Mammalian spermatozoa are maintained in a quiescent state in the epididymis until ejaculation. Various factors released in cauda epididymis and some intrinsic features are reported for keeping sperm in quiescence (Aitken, 2000). Although earlier researchers have reported the occurrence of undefined quiescent factors and motility inhibitors in various mammalian cauda epididymis, none of these factors are adequately characterized and also their mechanism of quiescence have not been defined. To our knowledge, in this report for the very first time a novel sperm motility quiescence factor (QF) has been purified, characterized and its mode of action to quiescence has been fully elucidated. QF was purified to apparent homogeneity from caprine cauda EP by conventional protein purification techniques namely heat precipitation, gel elution, DEAE-anion exchange chromatography, and gel filtration chromatography which is different from the purification techniques (gel adsorption chromatography, DEAE-anion exchange chromatography, and chromatofocusing) used to purify earlier reported sperm motility inhibiting factor (MIF-II) (Das et al., 2010). It is a highly thermo stable protein (Figure 2b) therefore other unwanted thermo labile proteins were removed by initial heat treatment. Incubation of QF with glycosidase enzymes did not affect its activity thus confirmed that QF did not contain functional glycoconjugate in it but loosing activity after trypsinization proved its proteinaceous nature (Figures S4 and S5). QF at the optimum concentration of 2 μM completely stopped PFM and vertical velocity of mature spermatozoa within 10 min (Figures 3a and 3b). Sperm motility inhibitory activity of QF was found pH dependent. Unlike a previous report that quiescence of bovine caudal sperm is maintained by an unidentified factor which is active in pH 5.5 but not active at pH 7.6 (Acott & Carr, 1984), here we found QF was active at a range of pH 6.5–8 and maximally active at pH 7.5 (Figure 2e). In further studies, biophysical characterization of QF was evaluated. Determinations of molecular weight, subunit structure, isoelectric point (PI), secondary and tertiary structure are important tools for protein identification. It is a 59 kDa monomeric protein (Figures 1a and S2). The QF is an acidic protein as indicated by its isoelectric point: PI 5.8 (Figure 1bb1). These biophysical properties of QF were found quite different from earlier reported sperm motility inhibitory proteins of epididymal plasma (MIF-II, Immobilin) or sperm membrane (MIF) of cauda region. MIF-II was reported as a 160 kDa, dimeric, heat-labile protein with PI value 5.1 whereas MIF was a 100 kDa monomeric, heat-stable protein. Immobilin was reported as high molecular weight protein and decrease sperm motility by increasing viscoelastic drag. But detailed mechanism of action of these proteins still remains unclear (Das et al., 2010; Dungdung & Majumder, 2003; Usselman & Cone, 1983). Hence, QF is different from other reported sperm motility inhibitory molecules from cauda region. Function of a protein completely depends on its structure therefore; CD spectroscopy and AFM imaging were used to reveal secondary and tertiary structure of QF respectively. Secondary structure of QF was found to be a predominantly α-helical (78%) at 25°C and with increased temperature (up to 75°C) its helical structure did not alter much (Figures 2a and 2b), which proved its structural stability at higher temperature. Hence, QF may be considered as a highly thermo-stable protein. AFM imaging identified the tertiary structure of QF as a globular protein with median diameter of 11.16 nm (Figure 3c). In subsequent studies of protein identification, N-terminal amino acid sequencing and MALDI-TOF/MS analysis were carried out to find similar proteins available in the protein databases. QF showed only partial similarity with axonemal dynein heavy chain protein (crucial for sperm motility), albumin precursor and its derivatives but these proteins were not identical to QF with 100% similarity (Tables 3 and 4). These results also confirmed the novelty of QF. Moreover these detailed characterizations of the earlier reported sperm motility inhibitory molecules are not available till date. From evolutionary aspect, QF showed close relation with myosin C (popularly known for its muscle contraction ability) as shown in distant phylogenetic tree (Figure S3).
Subsequent studies were done to elucidate the molecular mechanism of sperm motility inhibitory activity of QF. Ca++ and cAMP are two extensively studied molecules and confirmed to have sperm motility regulatory effect (Brokaw, 1987; Lasko, Schlingmann, Klocke, Mengel, & Turner, 2012; Li et al., 2016). Researchers showed the requirement of Ca++ for hyper activation of spermatozoa (Quill et al., 2003; Suarez, Varosi, & Dai, 1993). Bhoumik, Saha, Majumder, and Dungdung (2014) reported that Ca++ enhanced sperm motility in a dose-dependent manner and at higher dose it showed motility inhibitory effect. However, the presently studied molecule QF did not show any significant alteration in intracellular Ca++ conc (Figure 3c). Esposito et al. (2004) showed that soluble adenylyl cyclase (sAC) deficient mice were not able to produce cAMP, thus infertile due to severe sperm-motility defect. Das et al. (2010) reported MIF-II reduced intracellular cAMP level of spermatozoa thus inhibited motility but detailed mechanism of action was not reported. However, in QF treated mature cauda spermatozoa no significant changes in intracellular cAMP concentration were observed (Figure 3d), which revealed the sperm motility inhibitory activity of QF was not mediated by modulating intracellular Ca++ as well as cAMP concentration.
Among other molecules, involvement of NO and cGMP are also reported for sperm motility regulation. Expression of all three isoforms of NOS (eNOS, nNOS, and iNOS), the NO producing enzyme, on the sperm membrane has been reported. A positive correlation between NOS enzyme activity and sperm motility is also established as inhibition of NOS enzyme either by L-NAME or methylene blue decrease sperm motility (Donnelly, Lewis, Thompson, & Chakravarthy, 1997; Lewis et al., 1996; Salvolini et al., 2012). Human seminal plasma has been reported for sperm motility inhibitory as well as NOS activity reducing efficacy (de Lamirande, Belles-Isles, & Gagnon, 1984; Schaad, Zhang, Campana, & Schorderet-Slatkine, 1996). Interestingly, the presently studied sperm PFM inhibitory molecule, QF, also showed NOS enzyme activity reducing efficacy (Figure 3e). Researchers also showed that externally added NO plays a bimodal role in sperm motility regulation. Sodium nitropruside a NO releasing substrate, at low concentration (25–100 nM) stimulates sperm motility but at higher concentration (0.25–2.5 mM) it exerts opposite effect (Hellstrom, Bell, Wang, & Sikka, 1994; Rosselli, Dubey, Imthurn, Macas, & Keller, 1995; Weinberg, Doty, Bonaventura, & Haney, 1995) whereas, NO synthesis inhibitor, L-NAME, treatment shows decrease in sperm motility (Leal, Caldas-Bussiere, Carvalho, Viana, & Quirino, 2009, Nagaoka, Asagoshi, Kato, & Takata, 2017). When mature spermatozoa were treated with QF a significant decrease in NO production was observed as found in L-NAME treated negative control cells (Figure 3f). cGMP is the downstream effector molecule of NO and positively correlated with sperm motility. Increase in cGMP conc. either by activation of NO and soluble guanylyl cyclase (sGC) pathway or by inhibition of phosphodiesterase-5 (cGMP degrading molecule) leads to increase in sperm motility (Lefièvre, De Lamirande, & Gagnon, 2000; Miraglia et al., 2011). In this study, a significant decrease in cGMP production was observed in a dose-dependent manner after treatment with QF (Figure 3g). Hence, the sperm motility inhibiting mode of action of QF was mediated by reducing the NOS enzyme activity and subsequently reducing the intracellular concentration of NO and its downstream effector molecule cGMP (Figure 5). This study also confirmed that optimum dose of QF treatment did not damage DNA integrity or morphology of spermatozoa (Figure 4). As well removal of QF after RPS buffer wash showed restoration of sperm forward motility ascertains QF was not harmful to spermatozoa (Figure S6). As sperm PFM inhibitory activity of QF was transient hence, during ejaculation QF may be diluted with other accessory glands secretion and spermatozoa may regain motility in natural way.
Finally it is important to discuss the significance of the presence of QF in cauda region. QF plays two important physiological roles during storage of spermatozoa in cauda epididymis. Primarily it helps to maintain spermatozoa in quiescence state and thus conserve energy require during their travel through female reproductive tract. It also decreases NO production in mature spermatozoa by reducing NOS enzyme activity thus may protect them from reactive nitrogen species related damage and may help spermatozoa to store in better condition. Hence, these functions of QF may lead to a new direction toward energy saving during caudal storage of spermatozoa and may unveil some causes of motility related idiopathic male infertility. Its reactive nitrogen species reducing property may also helpful in sperm cryopreservation in infertility clinics and animal breeding centers.
ACKNOWLEDGMENTS
Authors want to acknowledge Council of Scientific and Industrial Research (CSIR), INDIA and CSIR networking project PROGRAM (BSC-0101) for providing fellowship and funding the project. Authors are thankful to Mr. T. Muruganandan of CSIR-Indian Institute of Chemical Biology for assisting in AFM study. Authors are also grateful to Director of CSIR-Indian Institute of Chemical Biology for his continuous support.
CONFLICTS OF INTEREST
Authors declare that there is no conflicts of interest for this study.




