Generation of insulin-producing cells from human adipose-derived mesenchymal stem cells on PVA scaffold by optimized differentiation protocol
Abstract
The studies have been done on patient-specific human adipose-derived from mesenchymal stem cells (hADSCs) like a series of autologous growth factors and nanofibrous scaffolds (3D culture) will probably have many benefits for regenerative medicine in type 1 diabetes mellitus (TIDM) patients in the future. For this purpose, we established a polyvinyl alcohol (PVA) scaffold and a differentiation protocol by adding platelet-rich plasma (PRP) that induces the hADSCs into insulin-producing cells (IPCs). The characteristics of the derived IPCs in 3D culture were compared with conventional culture (2D) groups evaluated at the mRNA and protein levels. The viability of induced pancreatic cells was 14 days. The in vitro studies showed that the treatment of hADSCs in the 3D culture resulted in differentiated cells with strong characteristics of IPCs including pancreatic-like cells, the expression of the islet-associated genes at the mRNA and protein levels in comparison of 2D culture group. Furthermore, the immunoassay tests showed that these differentiated cells in these two groups are functional and secreted C-peptide and insulin in a glucose stimulation challenge. The results of our study for the first time demonstrated that the PVA nanofibrous scaffolds along with the optimized differentiation protocol with PRP can enhance the differentiation of IPCs from hADSCs. In conclusion, this study provides a new approach to the future pancreatic tissue engineering and beta cell replacement therapies for T1DM.
1 INTRODUCTION
Diabetes mellitus is one of the most common endocrine diseases which spread, significantly in all countries including Iran. According to the World Health Organization (WHO), diabetes is increasing (American Diabetes Association, 2016; Rathmann & Giani, 2004). Diabetes is one of the main reasons of blindness, kidney failure, and in many cases, it leads to lower limb amputation. It is mostly considered as a mortality factor in heart diseases and strokes (Chapman, 2011). Type I diabetes mellitus (T1DM) is due to autoimmune destruction of the pancreatic islet insulin-producing beta-cells and insulin-dependent feature. Losing pancreatic beta cells and insulin deficiency are the main causes of the emersion of diabetic complications. The type I diabetes include 5–10% of diabetics. Daily injection to adjust the optimal insulin dosage is really annoying for the patients and it can also impose huge costs on the society. Yet, there is no exact reason why people get into this disease. It is probable that some factors such as hereditary or genetic and environmental factors have an important role. In the past two decades, the ultimate cure for T1DM was the transplantation of intact pancreases or isolated islets which had been severely difficult by the scarcity of the islets cells of cadaver (Yin et al., 2016). At the moment, stem cells that generate beta cells are new promising tools on which many studies have been concentrated to expand renewable sources of islet-replacement cells (Enderami et al., 2016; Piran et al., 2017; Rajaei et al., 2016). The stem cells are divided into two major categories, embryonic stem cells and adult stem cells. Mesenchymal stem cells (MSCs) are the most important members in adult stem cells family and are considerable as a very proper cell source for the treatment of pancreatic diseases among researchers (Dave, 2014). The MSCs have an ability to differentiate into multiple lineage cells in vitro. MSCs are the best candidates for cell-based therapies due to their convenient proliferation and being autologous (Hashemian, Kouhnavard, & Nasli-Esfahani, 2015). Stem cells can be cultured outside the body by using growth and transcriptional factors. It can be differentiated into insulin-producing cells (IPCs). In recent decades, the researchers have studied the use of cell-based therapy for the treatment of diabetes mellitus with a variety of different gene transfer vector and differentiated media (Caplan, 2015; Li et al., 2007; Okere, Lucaccioni, Dominici, & Iughetti, 2016). The performing differentiation protocols are expensive and time consuming. Thus, new strategies should be applied to make these protocols inexpensive and more efficient. Previous studies showed the use of platelet-rich plasma (PRP) and three-dimensional (3D) culture can solve the restrictions of pancreatic differentiation protocols (Aloysious & Nair, 2014; Enderami et al., 2016; Hoveizi, Nabiuni, Parivar, Ai, & Massumi, 2014). PRP is a portion of the whole blood that can be extracted with centrifugation. The achieved sediment includes a high concentration of platelets and fibrin. It has been shown that these sediments have a potent effect on stimulating the differentiation of stem cells into blood vessels, tendon, bone, beta cells, and cartilage cells (Enderami et al., 2016; Teixeira et al., 2012; Xu et al., 2015; Zhang & Wang, 2010). Due to the autologous nature of these growth factors, PRP may also have other useful applications such as improving differentiation protocols. Moreover, it is a cost-effective and readily available material. In stem cell biology, using 3D culture for various cell types differentiation into IPCs is much better than two-dimensional (2D) culture for the conventional cells differentiation (Aloysious & Nair, 2014; Hoveizi et al., 2014). A 3D culture system is effective in mimicking the in vivo microenvironment by enhancing cell-cell and cell-matrix interactions and following cell signaling. Three-dimensional cell culture is crucial for maintaining its spherical morphology and examining some processes such as tissue and biochemical factors. In fact, in this study was tried to create an artificial niche based on nanofiberous scaffold, using the fabric of polyvinyl alcohol (PVA)-based scaffold that was hydrophilic, flexible, elastic, biocompatible, and biodegradable (Kanimozhi, Basha, & Kumari, 2016; Vashisth & Pruthi, 2016). The extracellular matrix-modified PVA polymers may display biological properties and represent a suitable platform for the promotion of islet organization and proliferation. Therefore, in this study we aimed to evaluate the effect of the PVA scaffold and use a stepwise differentiation protocol optimized with PRP on human adipose-derived stem cells (hADSCs) differentiation into IPCs.
2 MATERIALS AND METHODS
2.1 Stem cell isolation and characterization
In this study, hADSCs were extracted from adipose tissue samples collected at liposuctions from four donors (mean age 40 ± 5, Baqiyatallah Hospital, Tehran, Iran) after informed consent according to guidelines of the Medical Ethics Committee, Baqiyatallah University of Medical Sciences and Health services (Tehran, Iran). Abdominal Lipo-aspirations were divided into 50 ml falcons and rinsed by PBS including 3% pen/strep (Sigma–Aldrich, St. Louis, MO) three times. Samples were centrifuged for 5 min at 1,200 RPM. Fat remained on tap and underneath solution containing white and red blood cells was discarded. This stage was done several times. Then the sample was transferred to DMEM high glucose supplemented with 10 mg collagenase type I (Invitrogen, Carlsbad, CA) and incubated at 37°C for 45 min, 95% O2, and 5% CO2 on shaker. After that the sample was centrifuged at 1,500 RPM for 15 min. Erythrocyte lysing buffer was added to the cell pellet at room temperature for 5 min. Then PBS was added and centrifuged at 1,200 RPM for 5 min. The cells were suspended by DMEM high glucose supplemented with Fungizone and 15% FBS (all from Sigma–Aldrich) and divided into the several cell culture flasks. Finally, the flasks were placed in a 37°C incubator with 5% CO2. The culture media was changed daily and passaged using trypsin-EDTA.
2.2 Flow cytometry analysis
The hADSCs were detached after passage 3 with trypsin-EDTA and about 4 × 105 single cells were divided into aliquots and centrifuged for 5 min at 1,200 RPM. The pellet was resuspended in human serum and incubated on ice for 30 min. After centrifugation for 5 min at 1,200 RPM, the pellet was resuspended in 3% (v/v) goat serum in PBS and incubated with fluorochrome-conjugated antibodies including Phycoerythrin (PE)-conjugated CD106, CD34, CD44, and CD10 5, fluorescent isothiocyanate (FITC)-conjugated mouse anti-human CD45 (Amersham Biosciences, Piscataway, NJ) for 1 hr on ice. Then it was washed twice in PBS and centrifuged for 5 min at 1,200 RPM. The cells were resuspended in 200 µl of PBS and analyzed using flow cytometry (Acoustic Focusing Cytometer, Thermo Fisher Scientific, MA). For each sample, the cells were analyzed using FlowJo® software.
2.3 Adipogenic and osteogenic differentiation
For osteocyte differentiation, 2 × 103 cells at passage 3 were cultured per well at a four-well plate and 0.5 ml specific DMEM media supplemented with 0.1 μmol/L dexamethasone, 10 mmol/L beta glycerol phosphate and 60 mmol/L ascorbate (all from Sigma–Aldrich) was added to each well. Two wells were exposed to DMEM supplemented with 10% FBS as control group and the other two wells were exposed to differentiation medium. The medium was changed daily. After 14 days, the differentiated cells were fixed with Paraformaldehyde 4% at 4°C for 20 min and then rinsed by PBS and stained with alizarin red (Behnogen, Tehran, Iran) for 5 min. For adipogenic differentiation 2 × 103 cells at passage 3 were cultured per well at a four-well plate and 0.5 ml specific DMEM media supplemented with 1 μmol/Ldexamethasone, 60 μmol/L indomethacin, 5 μl insulin 1.7 μm, and 450 μl isobutylmethylxanthine (all from Sigma–Aldrich) was added to the wells. Two wells were exposed to DMEM supplemented with 10% FBS as control group and the other two other wells exposed to differentiation medium. The medium was changed daily. After 14 days, the differentiated cells were fixed with Paraformaldehyde 4% at 4°C for 20 min and rinsed by PBS and stained with Oil red (Merck, Germany) for 5 min (Bayati, Hashemitabar, Gazor, Nejatbakhsh, & Bijannejad, 2013).
2.4 Preparation of platelet-rich plasma
Platelet-rich plasma was prepared with a two-step centrifugation (Enderami et al., 2016; Tang et al., 2015). Briefly, 10 ml human fresh total blood was collected into falcons containing 1 ml acid-citrate-dextrose reagent as an anticoagulant. Then the citrated blood was centrifuged in a laboratory centrifuge at 250g for 10 min. Subsequently, the plasma with the buffy coat fraction was collected, and a second centrifugation was done at 1,000g for 10 min. The platelet pellet accumulated at the bottom of the centrifuge falcon was collected using 2 ml of the platelet-poor plasma to yield PRP. The plasma supernatant was applied as platelet-poor plasma. The PRP solution was stored at −70°C to be used in the future.
2.5 Scaffold fabrication
In order to obtain a homogeneous PVA solution for electrospinning, 1.6 g PVA was dissolved in 20 ml deionized water by magnetic stirring for 12 hr at 80°C. Afterwards, The solution was loaded into two 10 ml plastic syringe and placed across the collector at fixed distance of 18 cm. Electrospinning was performed at flow rate of 1 ml/hr, under a positive voltage of 23 kV between needle and collector.
2.6 Surface modification
After scaffold fabrication, to optimize the PVA scaffolds they were treated under oxygen plasma, with plasma technique. The frequency of plasma generator (Diener Eletronics, Baden-wurttemberg, Germany) was set at 40 kHz with a cylindrical quartz reactor for 5 min by introducing pure oxygen. The nanofibrous scaffolds were cut to the desired size and sterilized under ultraviolet light for 20 min. Then the scaffolds were washed with PBS and soaked overnight in the DMEM medium supplemented with 10% serum at 37°C.
2.7 Scanning electron microscopy
Unseeded and seeded PVA scaffolds were immersed in a fixation solution containing 2.5% glutaraldehyde buffer in PBS (45 min). After the fixation, the samples were, respectively, incubated for 10 min in 50% ethanol, 10 min in 75% ethanol, 10 min in 90% ethanol, and then 10 min in 100% ethanol. The scaffolds were dried with a dryer. The dried scaffolds were coated with gold and SEM images were visualized using a scanning electron microscope (SEM; Hitachi S-4500, Tokyo, Japan) at an accelerating voltage of 5 kV.
2.8 Cell seeding on scaffolds
The scaffolds for decontamination were exposed to ultraviolet radiation for 1 hr, rinsed with 70% ethanol and then incubated in DMEM supplemented with 10% FBS for overnight before cell seeding. When the hADSCs reached 85–90% confluency, they were detached by 0.25% trypsin containing 0.1% EDTA to make a single cell suspension. Then, 5 × 104 hADSCs were seeded onto the top of the scaffold and incubated in DMEM supplemented with 10% FBS for 2 hr to allow hADSCs to attach on the surface of the scaffold. Finally, fresh medium was added for further incubation.
2.9 Experimental design
After characterization and analysis at passages 3, the hADSCs were used in this study. Four groups including two experimental and two control groups were formed. The hADSCs were cultured in DMEM media as the 2D control group. The seeded hADSCs onto the PVA scaffolds were cultured in DMEM media and used as the 3D control group. The cultured hADSCs in IPC differentiation media were used as the 2D experimental group. The seeded hADSCs onto the PVA scaffolds were cultured in IPC differentiation media and applied as the 3D experimental group. A three-stage differentiation protocol was used to induce hADSCs into IPC. In stage 1: the hADSCs were cultured (37°C, 5% CO2) in the medium I containing serum-free high glucose DMEM (25 mmol/L) with 0.5 mmol/L beta-mercaptoethanol (Invitrogen) and 5% PRP for 2 days. In the second stage the ADSCs were then cultured in the differentiation medium II containing 1% nonessential amino acids (Invitrogen), 20 ng/ml epidermal growth factor (EGF, Sigma–Aldrich), 20 ng/ml basic fibroblast growth factor (bFGF, Sigma–Aldrich), 2 mmol/L Lglutamine (Sigma–Aldrich), 2% B27 (Invitrogen), 10 ng/ml exendine-4 (Sigma–Aldrich), and 2% PRP for 6 days. In stage 3, the hADSCs were cultured for an additional 6 days in differentiation medium III containing 10 ng/ml betacellulin (Sigma–Aldrich), 2% B27, 10 mmol/ L nicotinamide (Sigma–Aldrich), 10 ng/ml activin A (Sigma–Aldrich), 10 ng/ml exendine-4, and 1% PRP (Enderami et al., 2016). In the second and third stages, the differential media was replaced with fresh medium every 2 days.
2.10 MTT assay
The attachment, viability, and proliferation ability of hADSCs on PVA nanofibers were determined via MTT assay during 7 days. To do that, sterilized scaffolds were placed in cell culture plate, seeded with as many as 4 × 103 cells per cm2 and incubated at 37°C, 5% CO2 (Enderami et al., 2016). At 1, 3, 5, and 7 days after seeding hADSCs, 50 μl MTT solution (3-[4,5-dimethyl-thiazolyl-2]-2,5-diphenoltetrazolium bromide) with a final concentration of 5 mg/ml in DMEM without FBS was added to each well. After 3 hr incubation, the supernatant was aspirated and the formazan crystals were solubilized with adding dimethylsulfoxide (DMSO). The optical density of the converted dye was quantified by absorbance measurement at 570 nm in a micro-plate reader. The results were obtained as mean values of three repeats in 2D and 3D culture groups.
2.11 RNA extraction and quantitative real-time PCR
The MSCs seeded in six-well plates at 2.0 × 105 cells/well were grown until the cell density reached 80%. Total RNA was isolated from differentiated hADSCs and control cells at the end of the induction process with the RNeasy Mini Kit (Qiagen, Hilden, Germany) following the manufacturer's protocol. Then, 1 µg of total RNA was reverse transcribed by the random hexamer primer to complementary DNA (cDNA) using a M-MuLV Reverse Transcriptase kit (Fermentas, Waltham, MA) at 42°C for 60 min and 70°C for 5 min in a total reaction volume of 15 µl. Quantitative real-time PCR was performed using a SYBR premix ExTaq (Takara, Korea) according to the manufacturer's protocol and with the ABI Light Cycler (ABI step one) in a total reaction volume of 10 µl in 96-well optical reaction plates. The primer sequences were designed using AlleleID software (Primer Biosoft), which is illustrated in Table 1. The amplification protocol included 5 min at 94°C for initial denaturation, followed by 40 cycles of 30 s at 94°C for denaturation, 30 s at 60°C for annealing, and final extension at 72°C for 30 s. The data were normalized against the Ct value of the housekeeping gene β2M. The relative quantification (2−ΔΔCt) method was applied to analyze the data.
| Human genes | Primer sequence | Annealing temperature (°C) | Product size (bp) |
|---|---|---|---|
| Pdx1 | Forward: 5′-ATGGATGAAGTCTACCAAAGC-3′ Reverse: 5′-CGTGAGATGTACTTGTTGAATAG-3′ | 60 | 159 |
| Glut2 | Forward: 5′-TCACTGCTGTCTCTGTATTCC-3′ Reverse: 5′-TGCTCACATAACTCATCCAAG-3′ | 60 | 147 |
| Gcg | Forward: 5′-ACCAGAAGACAGCAGAAATG-3′ Reverse: 5′-GAATGTGCCCTGTGAATG-3′ | 59 | 191 |
| Insulin | Forward: 5′-GCTTCTTCTACACACCCAAG-3′ Reverse: 5′-GGTAGAGGGAGCAGATGC-3′ | 60 | 171 |
| FoxA2 | Forward: 5′-GGAGCGGTGAAGATGGAAGG-3′ Reverse: 5′-CGGCGTTCATGTTGCTCAC-3′ | 59 | 93 |
| Sox-17 | Forward: 5′-CAAGATGCTGGGCAAGTC-3′ Reverse: 5′-TGGTCCTGCATGTGCTG-3′ | 59 | 100 |
| β2m | Forward: 5′-ATGCCTGCCGTGTGAAC-3′ Reverse: 5′-ATCTTCAAACCTCCATGATG-3′ | 60 | 91 |
2.12 Immunofluorescence assay
The differentiated hADSCs in both 2D and 3D groups were fixed in 4% (w/v) paraformaldehyde (Sigma) in PBS for 20 min at 4°C, and then rinsed several times in PBS. In the next step, they were permeabilized with 0.4% Triton X–100 (Sigma–Aldrich) at room temperature (RT) for 5 min. After washing with PBS for several times, the cells were incubated with blocked (5% goat serum in PBS) at RT for 60 min. After discarding the blocking reagent, it was then incubated overnight with primary antibodies at 4°C. The primary antibodies were rabbit anti-human PDX1 (1:2000, #ab47267) and rabbit anti−human insulin (1:200, #ab181547, Abcam, Cambridge, MA). After incubation, the cells were rinsed with PBS for several times. Subsequently, the cells were incubated with Fluorescein isothiocyanate-labeled secondary antibodies for 90 min at RT away from darkness. Afterward, they were washed with PBS. The nuclei were stained with 0.1 µg/ml DAPI (Sigma–Aldrich) (Enderami et al., 2016). Images were captured under a phase contrast fluorescent microscope (Nicon, Tokyo, Japan).
2.13 Insulin and C-peptide release and insulin content assay
The differentiated hADSCs in both 2D and 3D groups were washed two times with PBS and were pre-incubated in freshly prepared Krebs–Ringer Bicarbonate (KRB) buffer containing 25 mM NaHCO3, 5 mM KCl, 2.5 mM CaCl2, 1.1 mM MgCl2, 120 mM NaCl, and 0.1% BSA (all from Sigma) in glucose-free for 2 hr. Then, the differentiated cells were incubated for 2 h in KRB buffer with glucose in a variety of concentrations. The supernatant were collected at −70°C until analysis. In order to assay the secreted insulin and C-peptide, we utilized the Ultrasensitive ELISA Kit (#10-1132-01, #10-1141-01, Mecodia, Uppsala, Sweden) according to the manufacturer's protocols (Enderami et al., 2016). In addition to the measurement of intracellular insulin, the cells were washed three times with PBS, extracted in 0.2 ml acid alcohol (10% glacial acetic acid in absolute ethanol) at 4°C overnight, and then the cells were subsequently sonicated three times for 15 s each at 40–60 W, and centrifuged (13,000 RPM, 15 min, 4°C). Then, the supernatant was collected and stocked at −70°C to be assayed. Total protein concentration was determined by the BCA protein assay system and 50 µg of protein was used for detection of intracellular insulin in each well of ELISA kit.
2.14 Statistical analysis
All studies were performed at least triplicate independently, and the data obtained were reported as a mean ± standard deviation (SD). The results were analyzed by one-way ANOVA, and Bonferroni's post-hoc test was used for comparison by GraphPad Prism 6 software (GraphPad Software Inc., La Jolla, CA). In the compared groups, p-values less than 0.05 was considered statistically significant.
3 RESULTS
3.1 Characterization of undifferentiated hADSCs
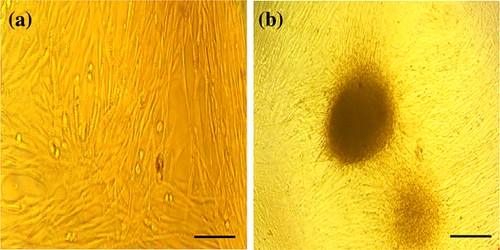
Under an inverted microscope, the hADSCs were observed to be attatched in spindle shaped at passage 3. As it is seen in Figure 1a, flow cytometric analysis of cell surface markers at passage 3 revealed a high expression of CD73 (99.9%), CD90 (85.2%), and CD105 (99.9%), whereas very low expression of CD45 (0.45%) and CD34 (0.73%) was shown. In this study hADSCs induced to differentiate into osteoblasts and adipocytes. We observed mineral deposition and osteogenic differentiation in hADSCs of culture in osteogenic media after 21 days as confirmed by morphological observation and alizarin-red staining (Figure 1c). After 21 days, adipogenic differentiation was confirmed by vacuoles observation and oil-red staining (Figure 1d). These results confirmed the multi-potential capability of hADSCs to form mesodermal lineages.

3.2 Morphological changes of hADSCs differentiation into IPCs
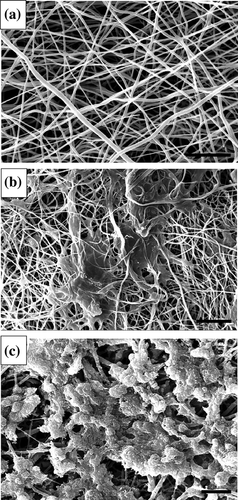
To promote the differentiation of hADSCs into IPCs, the strategies were applied according to previously published differentiation protocols (Seyedi, Farsinejad, Moshrefi, & Nematollahi-Mahani, 2015; Zhang & Dou, 2014) with some modifications, such as obtaining IPCs in a short time of differentiation and adding the PRP. Under the inverted microscope and scanning electron microscope, the induction of hADSCs differentiation into IPCs and cell morphologies were recorded at the end of differentiation stages (Figures 2 and 3). SEM studies indicated that the nanofiber scaffold contained numerous interconnected pores (Figure 2a). The seeding undifferentiated hADSCs on culture plates and nanofiber scaffolds was followed by the sequential addition of differentiation medium I, II, and III for 18 days. hADSCs formed sheets of cells and filled the pores of the PVA scaffolds in both experimental and control 3D culture. In the control group, the morphologies of hADSCs were elongated and flat (Figures 2b and 3a), while the cells in differentiation media in the experimental group showed round-shaped cluster structure and morphology similar to pancreatic islet (Figures 2c and 3b). Finally, the clusters formed at the final stage of the differentiation process were evaluated for other experiments.
3.3 Cell viability and cell proliferation assessment
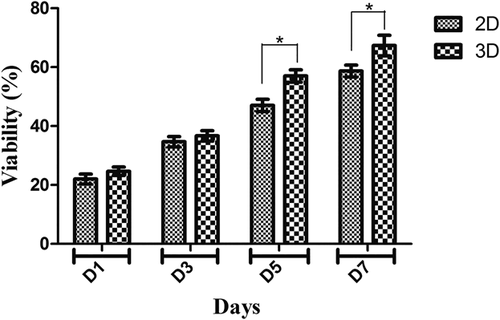
MTT test indicated the cell proliferation and biocompatibility rate on the 3D scaffold. In this study, 2D cell culture as the control group and 3D scaffold as the experimental group were studied by MTT assay to understand the biocompatibility of PVA scaffold. Cell viability on the PVA scaffold was measured by MTT assay 1, 3, 5, and 7 days after seeding. As shown in Figure 4, the viability and adhesion of the hADSCs on scaffold were significantly higher than the 2D culture group. Cell viability on PVA nanofibrous scaffolds indicates a significant increase compared with the sample of 2D culture on the 5th and 7th days, however, on the 1st and 3th days of the culture, the proliferation of scaffold did not show a statistically significant increase compared with 2D culture group.
3.4 Expression of definitive endoderm markers in derived IPCs
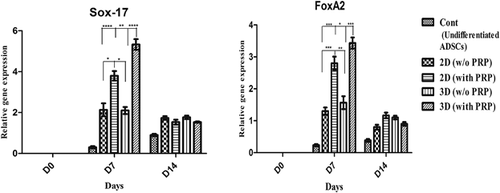
We extracted total RNA from undifferentiated and differentiated hADSCs on days 0, 7, and 14 after pancreatic induction under differentiation conditions, and the markers of definitive endoderm were examined. These markers appeared in the early mesendoderm stage, and its expression level increased in the definitive endoderm and late mesendoderm stages. Then, its expression level reduced with further differentiation into the pancreatic endoderm stage. The expressions of endodermal-related genes like FoxA2 and Sox-17 were detected by real-time PCR. The maximum levels of FoxA2 and Sox-17 transcripts were detected on the 7th day. We observed significant expression of the mentioned genes in the 3D group with PRP compared to 3D without PRP, 2D with PRP and 2D without PRP groups (Figure 5). Recent results have demonstrated that 3D condition with PRP can enhance ADSCs to differentiate into definitive endoderm cells.
3.5 Gene expression pattern of pancreatic endocrine genes in derived IPCs
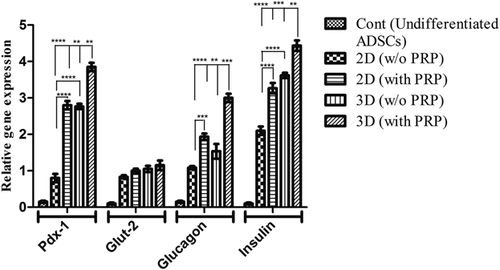
To indicate whether the hADSCs had been derived into IPCs, the endocrine gene expression profiles for pancreatic cell differentiation markers were evaluated using real-time PCR. As the gene expression pattern analysis showed no statistically significant differences between the two controls, all results were combined into one control group. As shown in Figure 6, low expression of Pdx-1, Insulin, Glucagon, and Glut-2 genes were detected in undifferentiated hADSCs (control). The expression pattern of these genes was significantly increased in the 2D culture of hADSCs-derived IPCs in comparison with the control group. We observed high expression of the mentioned genes in PVA scaffold-seeded differentiated cells. Compared with the 2D culture group with PRP, the expression of Pdx-1, Glucagon, and Insulin genes in hADSCs-derived IPCs at the 3D group with PRP increased nearly a 1.4-, 1.3-, and 1.4-fold, respectively, (p < 0.01, p < 0.001). Overall, these data further demonstrated that the presence of scaffold in the induction of culture media contributes to islet-like cluster effective differentiation of hADSCs and identifies PVA nanofiber as one of the synthetic polymer that improve IPCs' development.
3.6 Immunofluorescence staining
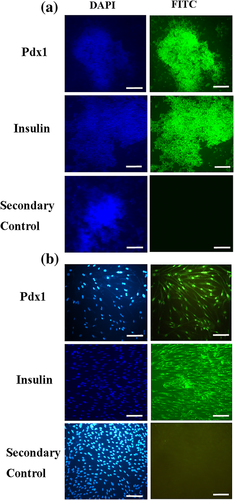
To confirm pancreatic differentiation of hADSCs into IPCs, the expression of insulin protein and Pdx1 transcription factor were shown as green color in the immunostaining assay (Figure 7). No expression of Pdx1 and insulin were observed in both 3D and 2D control groups (data not shown). In the 2D experimental culture group, weak, or moderate immune-reactivity was observed in the nuclei localization of Pdx1 and cytoplasmic localization of Insulin in differentiated IPCs on day 18. In 3D experimental culture group, strong immune-reactivity was observed in the nuclei localization of Pdx1 and cytoplasmic localization of insulin in differentiated IPCs on day 18. Qunter-staining of the nucleus (blue) was performed by DAPI. The IPCs were also analyzed for the secondary controls wherein the IPCs were just incubated with secondary antibody without primary staining. These results are shown in Figure 7.
3.7 Insulin and C-peptide release in response to glucose stimulation
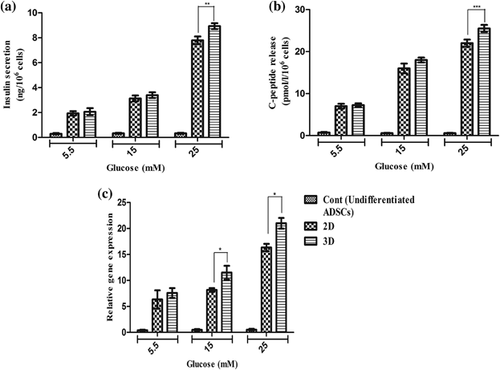
The researchers employed glucose challenge testes for the future study of the functional status of final differentiated cells to release C-peptide and insulin in response to various concentrations of glucose by utilizing an ELISA kit. The cultured hADSCs in control groups indicated very low levels of insulin and C-peptide in the presence of low or high glucose concentrations. Neither of the derived IPCs mediated through the protocol with 2D and 3D had a significant response to concentrations of glucose stimulation from 5.5 to 15 mM compared to each other (p > 0.05). However, in differentiated IPCs with 3D and 2D, insulin secretions increased by a concentration of 25 mM glucose, secreted 8.76 ± 0.25 and 7.76 ± 0.31 ng/106 cells (n = 6) per 60 min and the amounts of C-peptide were 26 ± 1.15 pmol/L and 22 ± 0.91 pmol/L (n = 6), respectively. Thus, our results in Figures 8a and 8b showed that differentiated IPCs with 3D in concentration of 25 mM glucose stimulation could significantly produce more C-peptide and insulin compared to the differentiated IPCs with 2D (p < 0.001, p < 0.01). Moreover, the insulin content in the differentiated IPCs was measured. The values for insulin in 5.5, 15, and 25 mM glucose concentrations in 2D group were 5.5 ± 2.5 ng/mg, 7.16 ± 5.32 ng/mg, 16.38 ± 1.6 ng/mg, and the values in 5.5, 15, and 25 mM glucose for 3D group were 6.7 ± 1.2 ng/mg, 10.6 ± 1.4 ng/mg, 20.6 ± 1.2 ng/mg, respectively. Thus, the obtained results indicated that maturated IPCs in 3D group could produce significant amount of insulin as compared to 2D group (p < 0.5). These results are shown in Figure 8c.
4 DISCUSSION
Recent studies have revealed that the human mesenchymal stem cells (hMSCs) have a potential to differentiate into various tissues such as, neural, musclular, bone, liver, etc (Caplan, 2015; Shahrezaie et al., 2017). When the hMSCs were isolated from different types of tissues with current protocols, researchers all over the world were looking for methods and differentiation protocols for T1DM treatment. The hADSCs are suitable for cell therapy and regenerative medicine in a diabetic's autologous transplantation because hADSCs can be isolated from the patient's own adipose tissues. Thus, many studies reported that the hADSCs were applied for differentiating into IPCs with various differentiation methods in vitro (Dave, 2014; Seyedi et al., 2015). Currently, many scientists are encouraged to do hMSCs differentiation to IPCs in stepwise stages into the differential growth medium in 3D compared to 2D culture systems. In recent studies, 2D culture strategies were not successful because of non-functional and immature IPCs production. Cell aggregation in 3D culture demonstrates an important role in enhancing the differentiated function and viability of stem cells-derived IPCs in vitro mimicking the ultrastructure and morphology of the native pancreas islet. In comparison to 2D culture systems, 3D culture improves islet like morphology, differentiation, and function. These results correspond with the previous findings of the studies on the types of stem cells (Aloysious & Nair, 2014; Hoveizi et al., 2014; Khorsandi, Khodadadi, Nejad-Dehbashi, & Saremy, 2015). The role of hADSCs in tissue engineering and regenerative medicine is promising; however, bioartificial pancreas based on tissue engineering still appears to be fantastic. Up to recent years, the IPCs differentiation of hADSCs has mostly been performed in 2D culture and fully functional and mature islet-like clusters maintaining their features in the condition of 3D culture have not been developed. In addition, natural and polymeric synthetic scaffolds provide a suitable substrate and surface area for cell seeding and attachment as well as developing and improving stem cell proliferation and differentiation capability (Amer, Mahoney, & Bryant, 2014; Mahmoodinia et al., 2017). The combination of hADSCs with nanofibrous scaffolds has many useful effects including the slow diffusion of growth factors and metabolites, as well as the fabrication of pancreas architecture for pancreas tissue engineering purposes in the future (Javad et al., ). In the present study, our results indicated that hADSCs differentiate into IPCs in presence of PVA nanofibers as well as PRP, which is probably effective as a differentiation protocol. When the PRP is stimulated, it releases a lot of adhesive molecules and growth factors involved in the differentiation of stem cells to IPCs such as EGF, bFGF, HGF, VEGF-A, and fibronectin (Burnouf, Strunk, Koh, & Schallmoser, 2016). The bFGF was previously found improving the generation of islet-like clusters from pluripotent stem cells (Lumelsky et al., 2001; Soria, 2001). The EGF has facilitated the proliferation of Pdx1-positive pancreatic progenitors and enhanced the final stages of insulin expression (Jiang et al., 2008; Zhang et al., 2009). The HGF can facilitate the beta cell proliferation, beta cell markers of differentiation, glucose sensing, and total insulin production (Garcia-Ocana et al., 2001). The VEGF-A is highly produced in islet cells compared to other pancreatic cells, and it also plays an important factor in the formation of islet structure (WataDa, 2010). The fibronectin (as a component of extracellular matrix) is applied for enhancing adult and pluripotent stem cell differentiation (Lumelsky et al., 2001; Wei et al., 2013). Thus, the PRP was used to induce hADSCs into pancreatic differentiation and the IPCs generations. The multiple growth factors released from PRP may play a synergistic and important role in the differentiation of hADSCs to IPCs. The methods of preparation of PRP vary from lab to lab and exact composition of PRP is unknown and varies from lot to lot. For these reasons, it is likely that the differentiation protocol presented in this study does not show similar results in another lab and by other researchers, which could be a defect for our protocol. In this regard, and in order to achieve more conclusive results on the effectiveness of our differentiation protocol, it is suggested that this protocol be evaluated in other studies by other researchers. Although other researchers have applied the PRP for stem cells differentiation to different types of tissue cells, no research laboratory to use PRP in facilitating the differentiation of hADSCs to IPCs was reported. For this purpose, we used PRP with an optimized three-stage protocol with 3D culture system for pancreatic differentiation.
The results of this study demonstrated that 3D culture system by using PVA can effectively enhance differentiation of hADSCs into IPCs compared to 2D culture. In the current study, the existence of IPCs was confirmed by gene and protein expression analysis of islet specific genes, morphology analysis, as well as insulin and C-peptide secretion. The induced IPCs were morphologically and structurally similar to pancreatic islet like cells in the 3D compared to 2D culture. One of the remarkable points of our study was 3D morphology of differentiated IPCs in 3D group at day 14 that was completely similar to spherical shaped islets of Langerhans. This morphology was not seen in the same studies at day 14 (Aloysious & Nair, 2014; Khorsandi et al., 2015). The results of immunofluorescence staining in the 3D culture showed strong immune-reactivity in the nuclei localization of Pdx1 and cytoplasmic localization of insulin in differentiated IPCs compared to 2D culture. In this study, the difference between both experimental groups was statistically significant in pancreatic specific cell markers in mRNA level. The insulin, Pdx1, Glut-2, and Glucagon pancreatic gene expression increased in the mRNA level. The increase of the genes expression of differentiated IPCs in the 3D culture was more significant than the 2D culture, but in the Glut-2 gene, the difference between the two experimental groups was not statistically significant (p > 0.05). The Glut-2 protein is a glucose carrier in beta cells. In fact, it is a beta cells maturity marker (Khorsandi et al., 2015). However, the results of this study indicated that the Glut-2 gene was expressed in both experimental groups, but, the difference between the two experimental groups was not statistically significant. This result aligned with Enderami et al. (2016). For this reason, the result (Figures 6a and 6b) showed that the difference of C-peptide and insulin releasing in response to the low level of glucose (5.5 mM) concentration between the two experimental groups was not significant. This result probably confirms the insignificance of Glut-2 gene expression between two experimental groups. However, the difference of C-peptide and insulin releasing in response to the high level of glucose (15 mM and 25 mM) concentration between the two experimental groups was significant. Aloysious and Nair fabricated a 3D nanofiber scaffold comprised of natural polymers for differentiation of hADSCs to islet-like clusters (Aloysious & Nair, 2014). Insulin secretion in response to glucose stimulation of IPCs on the nanofiber scaffold was significantly higher than the 2D culture system. In the present study, insulin secretion in response the high level of glucose stimulation of IPCs on the PVA scaffold was significantly higher than the 2D culture system. In this study, the observed results of pancreatic specific markers expression in mRNA and protein level assessment were aligned with the other studies in the field of stem cell differentiation to IPCs (Enderami et al., 2016; Khorsandi et al., 2015; Zhang et al., 2009). One of the advantages of 3D culture system in the long process of differentiation is increasing cell proliferation and viability compared with the 2D culture. The results of our study indicate that the differentiated cell group in the differentiation protocol with 3D culture is significantly more viable than the 2D culture. Using 3D culture system and protocol optimized with PRP, the number of days for hADSCs to IPCs differentiation decreased to 14 days. This compares to a normal period of induction of 20 days or even longer for the differentiation of the most adult stem cells in IPCs studies. The exact mechanism of the PVA scaffold on IPCs differentiation from hADSCs was not acquired from this study. It has been presumed that the nascent endocrine progenitor cells differentiate into the surrounding mesenchyme to form the islet-like clusters during pancreatic development. Accordingly, it is a desirable property to grow the cells within 3D nanofiber scaffolds to construct a proper extracellular matrix for cell proliferation, attachment, migration, and interaction with each other. It has been demonstrated in 3D culture system that not only temporal synthesis but also spatial distribution of growth factors within the extracellular matrix enhances the lineage-specific differentiation of stem cells (Watt & Hogan, 2000; Wong, 2011). Extracellular matrix networks and interactions have been shown to optimize beta-cell proliferation, survival, and function (Amer et al., 2014; Parnaud et al., 2009). In addition, 3D culture systems are likely to enhance interactions between beta cells in the islet and could enhance insulin secretory dynamics.
5 CONCLUSION
In conclusion, we designed a new approach for the differentiation of hADSCs to insulin-producing cells by improving a differentiation protocol with PRP optimization combined with use of PVA scaffold for the first time. The results of our study reveal that these differentiated IPCs have a beta cell pancreatic feature in the mRNA and protein levels; they also respond to glucose challenge test. The differentiation protocol improved in our study is not only an effective method but also a patient's own ADSCs and PRP to be utilized to treat diabetes in the near future. This approach may actualize the dream of personalized medicine procedure to treat diabetic patients. However, more research is required to investigate the function of these IPCs in vivo.
ACKNOWLEDGMENT
We wish to thank Stem Cell Technology Research Center for the use of their laboratory facilities.
CONFLICTS OF INTEREST
There is no conflict of interest in this study.




