Elevated potassium outward currents in hyperoxia treated atrial cardiomyocytes
Abstract
Supplementation of 100% oxygen is a very common intervention in intensive care units (ICU) and critical care centers for patients with dysfunctional lung and lung disorders. Although there is advantage in delivering sufficient levels of oxygen, hyperoxia is reported to be directly associated with increasing in-hospital deaths. Our previous studies reported ventricular and electrical remodeling in hyperoxia treated mouse hearts, and in this article, for the first time, we are investigating the effects of hyperoxia on atrial electrophysiology using whole-cell patch-clamp electrophysiology experiments along with assessment of Kv1.5, Kv4.2, and KChIP2 transcripts and protein profiles using real-time quantitative RT-PCR and Western blotting. Our data showed that induction of hyperoxia for 3 days in mice showed larger outward potassium currents with shorter action potential durations (APD). This increase in current densities is due to significant increase in ultrarapid delayed rectifier outward K+ currents (IKur) and rapidly activating, rapidly inactivating transient outward K+ current (Ito) densities. We also observed a significant increase in both transcripts and protein levels of Kv1.5 and KChIP2 in hyperoxia treated atrial cardiomyocytes, whereas no significant change was observed in Kv4.2 transcripts or protein. The data presented here further support our previous findings that hyperoxia induces not only ventricular remodeling, but also atrial electrical remodeling.
1 INTRODUCTION
Cardiovascular diseases (CVD) are a major cause of deaths in United States and Worldwide. According to the American Heart Association (AHA), more than 2,200 deaths attributed to CVD are reported daily, with an average of one death every 39 s (Members et al., 2012). Cardiomyopathy is reported in many diseased conditions including acute lung injury, diabetes, obesity, hypertension, and cancer (Blanco et al., 2012; Heather & Clarke, 2011; Howden et al., 2012; Lim, Bergin, & Ragosta, 2008). Administration of 100% oxygen is the primary approach for the treatment of patients in intensive care units (ICU) to deliver higher amounts of oxygen into systemic circulation. However, recent studies show that hyperoxia induces cardiac injury due to dysfunctional lung and compromised pulmonary function (Hovaguimian, Lysakowski, Elia, & Tramer, 2013). Hyperoxia-treated healthy adults are reported to have decreased cerebral blood flow by 11–33% (Floyd et al., 2003; Johnston, Steiner, Gupta, & Menon, 2003). Also, normobaric hyperoxia treatment in normal subjects and in patients with coronary artery disease or chronic heart failure demonstrate decreased coronary blood flow by 8–20% (Farquhar et al., 2009). A multicenter cohort study, using critical care database of ICUs at 120 hospitals within the United States, showed that atrial hyperoxia was independently associated with increased in-hospital mortality in patients admitted to the ICU following resuscitation from cardiac arrest compared to either normoxia or hypoxia (Kilgannon et al., 2010). Another cohort study, in which 2,894 stroke patients were exposed to either hyperoxia, normoxia or hypoxia, reported a significant association between hyperoxia and in-hospital deaths (Rincon, Kang, Maltenfort, et al., 2014). Yet another retrospective cohort study, by the same investigators, showed an independent association between arterial hyperoxia and in-hospital mortality in ventilated traumatic brain injury patients admitted to ICU when compared to normoxia (Rincon, Kang, Vibbert, et al., 2014). Animal models for hyperoxia have been well-established by many investigators in mice, rats, rabbits, guinea pigs, and hamsters, for various time periods (from 3 days up to 1 week) (Frank, Bucher, & Roberts, 1978; Groseclose & Frank, 1982; Johnston, Wright, Reed, & Finkelstein, 1997; Powers, Planck, Berger, Wall, & Rosenbaum, 1994). Much of these animal studies have been focused on investigating the effects of hyperoxia (both normobaric as well as hyperbaric) on acute lung injury, in neonates and in adults (Chen et al., 2015, 2016; Howden et al., 2012; Matute-Bello, Frevert, & Martin, 2008; Wagenaar et al., 2014). However, until recently, there are no published models concerning the impact of hyperoxia on cardiac injury/remodeling. Our laboratory was the first to investigate the effect of hyperoxia in cardiac remodeling and toxicity (Chapalamadugu, Panguluri, Bennett, Kolliputi, & Tipparaju, 2015; Panguluri et al., 2013). Previous studies from our laboratory investigated hyperoxia-induced cardiac remodeling at biochemical, molecular, and physiological levels. Our reports clearly demonstrated that 3 days of hyperoxia exposure causes ion channel remodeling and hypertrophy in ventricles, prolonged monophasic action potential (MAP) duration, bradycardia, and arrhythmia along with prolonged rate corrected QT intervals. We also reported elevated levels of serum cardiac markers, such as cardiac troponin (cTnl) and lactate dehydrogenase (LDH), as well as altered physical parameters such as increased heart weights and decreased body weights. Ultrarapid delayed rectifier outward K+ current or (IKur) play a major role in determining the length of cardiac action potentials, and it was been previously studied regarding its role in arrhythmia (Lee, Hahn, & Choi, 2016). In our current study, we have investigated the effect of hyperoxia in atrial Kv currents using patch-clamp electrophysiology, mRNA and protein profiles.
2 MATERIALS AND METHODS
2.1 Animals
For this study we used adult male wild type mice (C57BL/6 strain), 8–10 weeks of age obtained from Jackson Laboratories (Chicago, IL) and subjected to room air (normoxia) or >90% oxygen (hyperoxia). All animal work was approved by the Institutional Animal Care and Use Committee at the University of South Florida (Tampa, FL).
2.2 Oxygen exposure
Male mice, aged 8–10 weeks, were placed in cages in an airtight chamber (50 × 50 × 30 cm) and exposed to >90% oxygen for 72 hr. The oxygen concentration in the chamber was monitored with an oxygen analyzer (Vascular Technology, Chelmsford, MA) as described previously (Panguluri et al., 2013). Whereas, control group mice were exposed to normal air or normoxia for 72 hr in animal facility.
2.3 Isolation of atrial myocytes
Single left atria myocytes were isolated from beating hearts of wild type mice under normal conditions or under hyperoxia conditions. Mice were heparinized (1 U/kg; IP), followed by 10–15 min deep anesthetization with isoflurane and O2. Beating hearts were then quickly removed and mounted on a Langendorf apparatus and perfused 2 ml/min through the aorta with modified Tyrode's solution in following order (from A–D solutions in mM): (A) 130 NaCl, 5.4 KCl, 1 MgCl2, 0.333 Na2HPO4, 10 HEPES, 11.6 glucose, 20 taurine for 2 min; (B) the same like A plus 0.4 CaCl2; (C) the same like A plus 0.3 EGTA and 20 BDM; (D) the same like A plus 180 U/ml collagenase type 2. Oxygen was applied constantly during perfusion. Finally, hearts were perfused for 5 min in KB solution containing (in mM): 100 K+-glutamate, 10 K+ aspartate, 25 KCl, 10 KH2PO4, 2 MgSO4, 20 taurine, 5 creatine base, 0.5 EGTA, 5 HEPES, 0.1% BSA, 20 glucose (pH adjusted to 7.2 with KOH). The left atrium was then dissected from each heart and then tissue titrated to free single atrial myocytes in KB solution. Dissociated myocytes were collected by low-speed centrifugation at 100g and the pellet was re-suspended and stored in KB solution at room temperature for 4 hr.
2.4 Electrophysiological recordings
Whole cell voltage-clamp and current-clamp recordings were obtained at room temperature (21 ± 2°C) using an Axopatch 200B amplifier. Currents were low pass filtered at 5 kHz and digitized using digidata 1,322A digitizer driven by Clampex 9.2. Recording pipettes were fabricated from borosilicate glass and had resistance 2–4 MΩ when filled with intracellular solution (ICS) containing (in mM): 10 EGTA, 10 HEPRS, 1 MgATP, 5 glucose, 135 KCl, 4.1 K2ATP, pH 7.2. This ICS was used for both experiments with action potentials and K-currents. The extracellular solution for Ca2+-independent, depolarization activated K-currents contained (in mM): 10 HEPES, 10 glucose, 136 NaCl, 0.5 CaCl2, 1 MgCl2, 0.23 CdCl2, 5.4 KCl, 0.02 TTX. For Action Potentials the bath solutions were composed of (in mM): 10 HEPES, 10 glucose, 130 NaCl, 1 CaCl2, 1 MgCl2, 5.4 KCl, 0.33 Na2HPO4.
Series resistance and pipette capacitance were compensated (≤85%) electronically. The capacitance of each myocyte was measured by integrating capacitive transients during ± 10 mV steps from holding potential −75 mV. Membrane capacitance of normoxia left atrial myocytes 55.16 ±5.49 pF (n = 10) was larger than that of hyperoxia which was 45.04 ± 7.8 pF (n = 7). Current amplitudes were normalized to membrane capacitance for each cell. Ca2+ independent voltage-gated K+ currents were recorded in response to 4.5 s voltage steps to potentials between −100 mV and 50 mV in 10 mV increments from holding potential −70 mV.
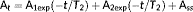
Action potentials were recorded in voltage-clamp mode in response to 125% of threshold AP which consisted of 400–650 pA depolarizing current pulses of 4 ms duration. Stimuli were applied at 1 Hz. Threshold potentials were determined by injection of increasing positive current in 100 pA increments for 4 ms. Action potential duration (APD) was measured from peak to 25%, 50%, 75%, 90% repolarization levels. Refractory period was estimated as time when an overshooting AP that preceded at least two more similar APs was elicited, where 125% of threshold current was injected and next pulses were in 8 ms increment.
2.5 Quantitative real-time-PCR (qRT-PCR)
Total RNA was isolated from control (normoxia) and hyperoxia-treated hearts using the exiqon mercury RNA isolation kit (Exiqon, Woburn, MA) according to manufacturer's instructions. Total RNA concentration and 260/280 ratios were determined using the Synergy H4 hybrid Reader (Biotek, Winooski, VA). One microgram RNA was sequentially treated with DNAseI and cDNA generated using maxima reverse transcriptase (ThermoFisher, Grand Island, NY).
Ion channel genes Kv4.2, Kv1.5, and their interacting protein KChIP-2 along with housekeeping control 18SrRNA were selected to study their relative expressions by qRT-PCR using a MyiQ single color real-time PCR detection system (Bio-Rad Laboratories, Hercules, CA). All qRT-PCR procedures were performed according to our previously published protocol (Panguluri et al., 2013). Briefly, qRT-PCR was performed using 2 μl of 1:80 dilution of cDNA, 0.5 μl of 1:20 dilution of primers, 2.5 μl supermix (Genecopoeia, Rockville, MD). Reaction conditions for qPCR include initial denaturation at 95°C for 10 min followed by 43 cycles of 95°C for 10 s, 58°C for 1 min, 72°C for 1 s. qPCR was completed by incubation at 65°C for 31 s, followed by cycling 61 times with 0.5°C increase per cycle and ramp of 0.5°C/s. A fluorescent SYBR based reading determined the extent of amplification at the end of each cycle. All samples were measured in quadruplets with six animals in each group. Experimental gene expression was defined as the Delta–Delta cycle threshold (ΔΔCt) and calculations were made using 18SrRNA as house-keeping gene.
2.6 Western blotting
For this experiment, dissected atria were homogenized in T-PER tissue protein extraction buffer (Pierce, Rockford, IL) supplemented with DTT (10 mM), protease inhibitors, 1:100 (Sigma–Aldrich, St. Louis, MO) and phosphate inhibitors, 1:100 (Sigma–Aldrich). Then the extract was centrifuged at 14,000 rpm at 4°C and supernatant was collected and stored at −80°C until further use. The protein lysates were quantified by using Pierce BCA protein assay kit (ThermoFisher) at 562 nm according to the manufacturer's protocol by using the Synergy H4 hybrid Reader (Biotek). About 50 µg of protein was loaded with SDS sample buffer (Bio-Rad) and resolved using 12% TGX precast protein gels (BioRad, Hercules, CA). Subsequently, the proteins were transferred on to the PVDF membrane for immune detection of candidate proteins. Antibodies for Kv4.2 (1:1000), and KChIP2 (1:1000) were obtained from Millipore (Billerica, MA), Kv1.5 (1:200) and β-actin (1:1000) from Santa Cruz Biotechnology (Dallas, TX). Secondary antibodies were either rabbit anti-mouse (Millipore) or goat anti-rabbit or rabbit anti-goat (ThermoFisher) at a dilution of 1:5000. The blots were developed by using Pierce ECL Western blotting substrate kit (ThermoFisher) and visualized using ChemiDoc imaging system (BioRad). Bands were analyzed using ImageJ software for quantification, and normalization was completed using β-actin band intensities.
2.7 Statistical analysis
For comparison of electrophysiological parameters from normoxia and hyperoxia, student t-test was used and presented as means ± SE (n = 6 mice). In each case, p < 0.05 was considered significant. For all qRT-PCR experiments, results were expressed as mean ± SE (n = 6 mice). Student t-test was used to compare quantitative data and value of p < 0.05 was considered statistically significant unless otherwise specified. For Western blotting experiments, all bands (n = 3 mice per group) were quantified by using ImageJ software. Student t-test was used to compare normalized band intensities and value of p < 0.05 was considered statistically significant.
3 RESULTS
As in our previous studies, all mice exposed to hyperoxia treatment for 72 hr showed a significant reduction in body weight and an increase in heart weight, as normalized by tibia lengths, when compared to normoxia controls (Chapalamadugu et al., 2015; Panguluri et al., 2013). In our current study, we investigated whole-cell patch-clamp electrophysiological recordings in atrial cardiomyocytes, since we have already reported physical, hemodynamic parameters, serum cardiac bio-markers, ECG, Echocardiogram, and ventricular monophasic action potentials (MAPs) using our ex vivo experiments.
3.1 Effect of hyperoxia on action potential durations
We investigated the effect of hyperoxia on action potential (AP) characteristics including AP amplitude, APD at 25%, 50%, 75%, and 90% of repolarization in single cells isolated from left atrium of Wt mice. The resting membrane potential recorded from normoxia and hyperoxia were not significantly different and consisted of −70.6 ± 0.3 mV (n = 6 mice) for normoxia and −72.8 ± 2.1 mV (n = 6 mice) for hyperoxia, respectively. Single AP waveforms are depicted in Figure 1a, which demonstrate that AP duration after treatment with oxygen was shorter. Summarized data of AP amplitude, APD at 25%, 50%, 75%, and 90% of repolarization and refractory period of normoxia and hyperoxia mice were presented in Figure 1b. Action potential durations (APDs) at 25, 50, and 75 ms showed that the oxygen treated group had significantly shorter APDs compared with normal air controls (Figure 1b). Analysis of AP current-clamp experiments showed that the refractory period in myocytes isolated from normoxia was significantly shorter (124 ± 4.6 ms; n = 6 mice) than hyperoxia (150 ± 3.5 ms; n = 6 mice).
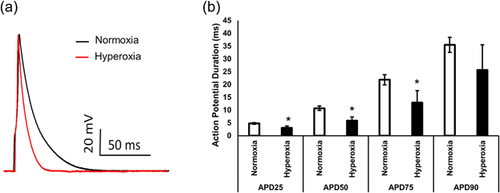
3.2 Effect of hyperoxia on outward currents
Since we observed significant shortening of APDs in atrial cardiomyocytes, we investigated further in order to understand if differences in APDs are due to differences in potassium currents (K-currents). To investigate voltage dependent K-currents, we used whole cell patch-clamp method in voltage-clamp mode during voltage steps to potentials ranging from −120 to +50 mV (as described in heading number 2).
Representative outward current density waveforms recorded from normoxia or hyperoxia myocytes isolated from left are shown in Figure 2. This indicates a presence of inward rectifying current, a rapidly activating and inactivating (Ito); a rapidly activating, slowly inactivating current (IKur); and a slowly activating, non-inactivating current (Iss). As shown in Figure 2 inward rectified currents which are due to activation IK1 channels in response to hyperpolarization to −70 mV also increased after treatment with hyperoxia but not significant. Current densities in hyperoxia were significantly higher compare to normoxia (Figure 2c). Mean peak current densities obtained at +50 mV from normoxia and hyperoxia treated groups (Figure 2c) were 18.6 + 1.2 pA/pF (n = 6 mice) and 35.8 + 8.2 pA/pF (n = 6 mice) (p < 0.05), respectively.
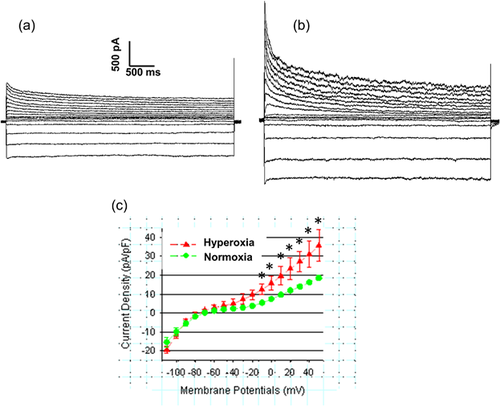
3.3 Effect of hyperoxia on IKur
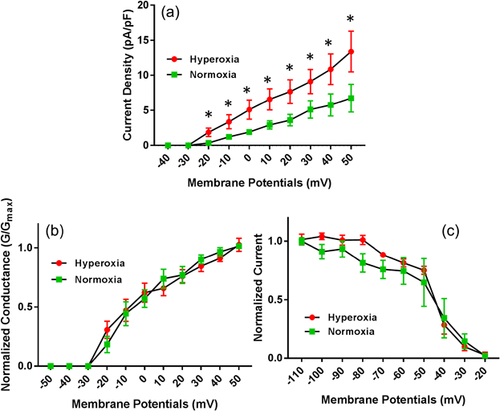
Hyperoxia treatment significantly elevated IKur currents in atrial cardiomyocytes (Figure 3). The current-voltage relations for IKur is illustrated in Figure 3a. This data showed that the mean current densities for IKur from atrial myocytes exposed to hyperoxia were significantly higher compared to normoxia treated myocytes when recorded at membrane potentials between −40 mV to +50 mV. As most of these changes in the voltage-dependent and kinetic properties can account for the differences in IKur current densities observed in hyperoxia treated mice, we further assessed voltage-dependent activation of IKur which did not show any significant differences between hyperoxia and normoxia treated atrial myocytes (Figure 3b). Similarly, the voltage-dependent steady-state inactivation of IKur by double exponential fit to decay of total outward current did not show any significant differences between hyperoxia and normoxia treated atrial myocytes (Figure 3c). The decay phases for Ikur in mice atrial myocytes are well described by two exponential functions and mean inactivation time constant (T decay) which is 1369 ± 137.4 ms in normoxia group and increased to 1,406 ± 158.7 ms in hyperoxia group, but not significant.
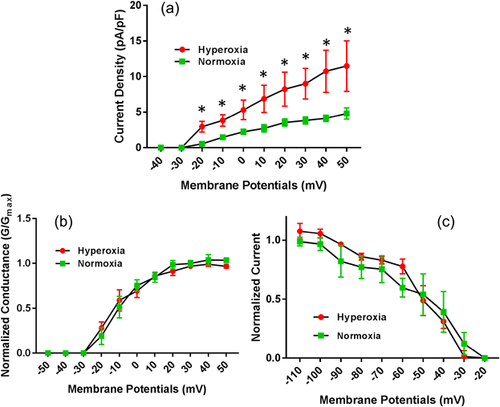
3.4 Effect of hyperoxia on Ito
Similar to IKur currents, Ito currents in atrial cardiomyocytes were also increased significantly in hyperoxia-treated mice atrial cardiomyocytes (Figure 4a). As shown in the data, the mean current densities for Ito from atrial myocytes exposed to hyperoxia were significantly higher compared to normoxia treated myocytes when recorded at membrane potentials between −40 mV and +50 mV. Further assessment of voltage-dependent activation of Ito did not show any significant differences between hyperoxia and normoxia treated atrial myocytes (Figure 4b). Similarly, the voltage-dependent steady-state inactivation of Ito by double exponential fit to decay of total outward current did not show any significant differences between hyperoxia and normoxia treated atrial myocytes (Figure 4c). The decay phases for Ito by two exponential functions and mean inactivation time constant (T decay) is 79.7 ±15.9 ms in normoxia group and increased to 96.3 ± 5.4 ms in hyperoxia group, but did not reach significance.
3.5 Effect of hyperoxia on gene expression
To determine if elevation of Kv1.5 and Kv4.2 transcripts may increase the mean current densities for IKur and Ito, we investigated mRNA expression of Kv1.5, Kv4.2, and its interacting protein KChIP2 in atria of both normoxia- and hyperoxia-treated hearts using quantitative real-time PCR. Our qRT-PCR data showed that there is no significant change in mRNA expression for Kv4.2 in hyperoxia hearts compared to normoxia, whereas significant increase in Kv1.5 and KChIP2 transcripts in hyperoxia treated atrial samples compared to normoxia controls (Figure 5a).
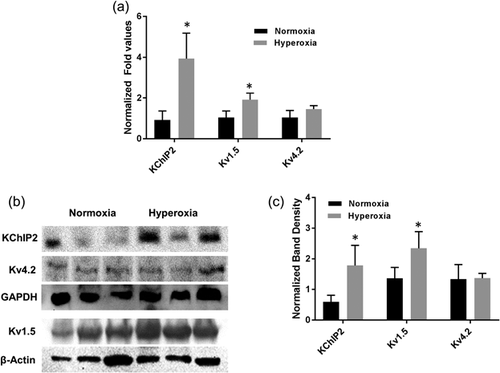
To further validate the differential expression of these transcripts, we examined the protein profiles of Kv1.5, Kv4.2, and KChIP2 along with internal control β-actin gene. As expected, our Western blotting data showed a significant increase in both Kv1.5 and KChIP2 proteins in hyperoxia treated atria compared to normoxia controls, but no significant difference in Kv4.2 expressions (Figure 5b). Using ImageJ software, band intensities for each gene were calculated and normalized with internal control β-actin band intensities and mean (±SE) were presented as bar diagram (Figure 5c).
4 DISCUSSIONS
Mechanical ventilation is one of the most common interventions implemented in intensive care units (ICU) or critical care units. The length of stay in ICU varies between 24 hr to 132 days (Arabi, Venkatesh, Haddad, Al Shimemeri, & Al Malik, 2002), with an average length of stay of about 3.3 days (Hunter, Johnson, & Coustasse, 2014). The average daily cost of stay in ICU represents one third of total health care cost, and is steadily increasing (Shorr, 2002). Several studies, in both animal models and in humans, have outlined the adverse effects of prolonged exposure to hyperoxia to include pulmonary toxicity and an increased risk of poor outcome (Baleeiro, Wilcoxen, Morris, Standiford, & Paine, 2003; Barazzone, Horowitz, Donati, Rodriguez, & Piguet, 1998; Davis et al., 2009; de Jonge et al., 2008; Farquhar et al., 2009; Janz, Hollenbeck, Pollock, McPherson, & Rice, 2012; Kilgannon et al., 2010; Li, Liao, Ko, Lee, & Quinn, 2007; Nagato et al., 2009). Given the relevance of hyperoxia in ICU, we utilized hyperoxia treatment in mice to induce mimic cardiac remodeling in the clinical setting (Chapalamadugu et al., 2015; Panguluri et al., 2013).
Our previously published study showed that exposure of mice to hyperoxia induced arrhythmias (Chapalamadugu et al., 2015) and we know that electrical remodeling plays an important role in arrhythmias (Krogh-Madsen, Abbott, & Christini, 2012). Also we know that left atrial (LA) dysfunction and remodeling are commonly observed in patients with heart failure, and impaired LA function can impose greater hemodynamic stress promoting remodeling (Melenovsky et al., 2015). Additionally, in one simulation study it was shown that the electrical remodeling in LA alone may be the driver of atrial fibrillation (Krogh-Madsen et al., 2012). It has also been shown that the ultra-rapid delayed rectifier current, (IKur), plays an important role in governing early repolarization of action potentials in atrial and ventricular myocytes (Nakamura et al., 2010). The pore-forming sub-unit of this current is encoded by Kv1.5 in humans. Also, a recent report showed that atrial Kv1.5 is the main ion channel responsible for the increase of in-current under shear stress (Boycott et al., 2013). Other studies suggest that either increased or decreased expressions of Kv1.5/IKur enhance the arrhythmogenic effect (Christophersen et al., 2013).
As we previously observed electrical remodeling in ventricles of hyperoxia-treated mice, we chose in this study to determine if hyperoxia can also induce electrical remodeling in atrial myocytes. The patch-clamp electrophysiology data from atrial myocytes in this study clearly shows that the action potential durations (APDs) at 25, 50, and 75 ms are significantly shorter in hyperoxia exposed myocytes compared to its normoxia controls (Figure 1). Similarly, the refractory period in AP current-clamp experiments are significantly shorter in hyperoxia myocytes, when compared to normoxia controls, whereas, no significant difference in resting membrane potential and action potential amplitude in hyperoxia treated myocytes when compared to normoxia. We also observed significant increase in current densities in hyperoxia treated atrial cardiomyocytes when compared to its normoxia controls (Figure 2). This data are in agreement with a previous reports demonstrating that angiotensin II significantly reduces atrial APDs without changing RMPs (Sonoyama et al., 2005), and that angiotensin II has a direct effect on cardiac function by activating atrial renin-angiotensin system, thereby contributing to atrial arrhythmia (Goette et al., 2000). In another study of atrial fibrillation in chronic heart failure, it was shown that the atrial APDs and refractory periods were significantly shorter in a heart failure (HF) group compared to their controls, due to increase in KChIP2 expression, increased Ito currents, and reduced IKur currents (Sridhar et al., 2009).
Earlier reports on hyperthyroidism associated atrial arrhythmias showed that two delayed rectifier currents IKur and Iss were significantly increased with significant reduction of APDs and elevated expressions of both mRNA and proteins of Kv1.5 and 2.1 in hyperthyroid mice group compared to their controls (Hu, Jones, & Dillmann, 2005). Similarly, in another study of ZFHX3 knockdown cells, increased IKur currents and reduced APDs and increased arrhythmogenesis was observed (Kao et al., 2016). Given that we observed increased K+ outward currents in hyperoxia treated atrial cardiomyocytes, we also investigated ultra-rapid delayed rectifier current (IKur), which is a major contributor of atrial action potentials. Similar to previous studies, our data also showed significantly increased IKur currents in hyperoxia treated atrial myocytes compared to their normoxia controls, but no significant difference in voltage-dependent activation or voltage-dependent steady state inactivation (Figure 3a–c).
Both expression and electrophysiological properties of Ito in atrial myocytes were previously reported by many studies (Amos et al., 1996; Bertaso, Sharpe, Hendry, & James, 2002; Escande, Loisance, Planche, & Coraboeuf, 1985; Fermini, Wang, Duan, & Nattel, 1992; Wang, Fermini, & Nattel, 1995). We previously reported Kv channel remodeling in ventricle of hyperoxia treated hearts due to the differential expression of Kv4.2 gene along with its interacting protein KChIP2 in both ventricles (Panguluri et al., 2013), in this study we investigated if hyperoxia can also induce differential expression and functional properties of Kv4.2 in atrial myocytes. Similar to IKur currents, we also observed increased current densities of Ito in hyperoxia-induced atrial cardiomyocytes (Figure 4a–c). From these observations it is evident that the increased Kv1.5 and Kv4.2 currents and shortening of APDs may contribute the arrhythmias in hyperoxia induced mice hearts reported earlier in our study (Chapalamadugu et al., 2015).
Given the significant increase in mean current densities for IKur, and Ito in hyperoxia treated atrial myocytes, we chose to determine if this increase is due to the increase in its transcript levels. Our quantitative real-time PCR demonstrated no significant difference in Kv4.2 transcript levels, but we observed a significant increase in transcripts of Kv1.5 and KChIP2 (Figure 5a). This data were further validated by Western blotting experiments, which showed significant increase in protein profiles of Kv1.5 and KChIP2 in hyperoxia treated atrial myocytes, but no significant change in Kv4.2 protein (Figures 5b and 5c). No significant change in Kv4.2 protein levels in atrial myocytes compared to Kv1.5 and KChIP2 protein levels may be due to the fact that the relative abundance of Kv4.2 and Kv1.5 in atria are different. It is noted that Kv4.2 expressions are relatively two-fold higher in ventricular muscle compared to atria, whereas abundance of Kv1 subfamily (Kv1.5) is observed in atria compared to ventricle (Dixon & McKinnon, 1994). As we know that regulation of KChIP2 mRNA transcripts alone by NFĸB is sufficient enough to significantly change Ito currents without affecting Kv4.2 or Kv4.3 expression (Panama et al., 2011), the increase in Ito currents in hyperoxia treated atrial myocytes in our study, without any change in Kv4.2 protein levels may be due to increase in KChIP2 protein levels in these myocytes.
In this study, for the first time, we have shown that hyperoxia not only induces ion channel remodeling in ventricles, but also in atria, by differential expression of Kv1.5 and KChIP2, thereby affecting potassium outward currents.
5 CONCLUSIONS
The data presented here has improved our understanding of the pathophysiology of hyperoxia-treated hearts, by examining the electrophysiological functionality of atrial cardiomyocytes under hyperoxic conditions. Our previously published study demonstrated reduced body weights, increased heart weights, cardiac hypertrophy, ventricular ion channel remodeling, QTc prolongation, prolonged monophasic action potential durations in hyperoxia-treated mice (Chapalamadugu et al., 2015; Panguluri et al., 2013). In our current study, we observed an induction of atrial ion channel remodeling by increasing Kv1.5 and Kv4.2 currents, shortening APDs, all of which demonstrate an increased risk for atrial arrhythmias. Importantly, we also showed that the observed increase in Kv currents are due to elevated expressions of Kv1.5 and KChIP2 genes under hyperoxic conditions, similar to our previous findings.
AUTHORS’ CONTRIBUTION
The experiments were designed, executed, data analysis and interpretation, and writing manuscript by PSK. Whole-cell patch-clamp experiments was done by VZ. Patch-clamp data analysis was done by CB. Isolation of mRNA, cDNA synthesis, and real-time qRT-PCR was done by SE. Extraction of proteins and Western blotting was done by TX and RJL. Treatment of hyperoxia and necessary equipment for hyperoxia was provided by KN and MS. Troubleshooting for electrophysiological experiments, data interpretation, and equipment of patch-clamp electrophysiology was provided by BES.




