The MIP-1α autocrine loop contributes to decreased sensitivity to anticancer drugs
Abstract
Several autocrine soluble factors, including macrophage inflammatory protein-1α (MIP-1α), tumor necrosis factor-α, and hepatocyte growth factor, promote cell survival and growth in multiple myeloma (MM) cells. We hypothesized that inhibition of the MIP-1α autocrine loop may enhance the cytotoxic effect of anticancer drugs in MM cell lines. In the present study, an MIP-1α neutralizing antibody suppressed cell proliferation and enhanced the cytotoxic effect of melphalan or bortezomib on MM cells. In addition, melphalan resistance cells (RPMI8226/L-PAM and HS-sultan/L-PAM cells) secreted MIP-1α and neutralizing antibody of MIP-1α partially overcame melphalan resistance. Moreover, combination treatment with MIP-1α neutralizing antibody and melphalan or bortezomib inhibited extracellular signal regulated kinase 1/2 (ERK1/2), Akt, and mammalian target of rapamycin (mTOR) activation, Bcl-2, Bcl-xL, and Survivin expression, and upregulated the expression of Bim and cleaved Poly (ADP-ribose) polymerase (PARP). Treatment of IM9 cells with MIP-1α siRNA suppressed the activation of ERK1/2, Akt, and mTOR, and enhanced the cytotoxic effect of melphalan and bortezomib. These results indicate that MIP-1α neutralizing antibodies or MIP-1α siRNA enhance the cytotoxic effect of melphalan and bortezomib by suppressing the chemokine receptor/ERK and chemokine receptor/Akt/mTOR pathways. The inhibition of MIP-1α may thus provide a new therapeutic approach to control tumor progression and bone destruction in patients with MM.
1 INTRODUCTION
Multiple myeloma (MM) is a clonal B-cell malignancy characterized by the accumulation of malignant plasma cells in the bone marrow in close contact with stromal cells, and represents about 13% of all hematopoietic tumors. Melphalan is one of the most active chemotherapeutic agents used to treat MM, and it is used in high doses for therapy in younger patients before autologous stem cell transplantation, as well as in non-transplant candidates or elderly patients as a part of first-line combination regimes (Gullà et al., 2016). Bortezomib, a first-in-class proteasome inhibitor, targets chymotrypsin and caspase-like active sites of proteasomes, thereby suppressing the protein degradation and inducing the unfolded protein response (Dick & Fleming, 2010). Bortezomib suppresses the proteosomal degradation of the inhibitor of κB that leads to the inhibition of nuclear factor κB (NF-κB), and exhibits overall response rate as a single agent against MM (Du et al., 2013). Combination regimes of melphalan with bortezomib and/or lenalidomide have improved progression-free survival and overall survival in MM patients. However, acquisition of drug resistance to these drugs is conducive to MM relapse (Palumbo et al., 2014); therefore, MM remains incurable.
Several autocrine or paracrine soluble factors, such as interleukin-6 (IL-6), tumor necrosis factor-α, macrophage inflammatory protein-1α (MIP-1α), vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), and insulin-like growth factor can promote myeloma cell survival and growth (Chiron et al., 2013; Ferrucci et al., 2014; Hideshima, Chauhan, Schlossman, Richardson, & Anderson, 2001; Klein et al., 2003). Several studies have shown that these cytokines and growth factors play a crucial role in the pathogenesis of MM and are elevated in the plasma of patients with MM, compared with healthy donors (Chiron et al., 2013; Ferrucci et al., 2014; Kuku et al., 2005; Lauta, 2003).
MIP-1α is a member of the chemotactic cytokine (CC) chemokine family, which promotes cell migration against immune cells such as monocytes and lymphocytes (Lee et al., 2000). MIP-1α is secreted by MM cells isolated from patients and MM cell lines (Abe et al., 2002). It has been shown that MIP-1α levels are elevated in the blood plasma of patients with MM, and such patients with high MIP-1α serum levels have a poorer prognosis than patients with low MIP-1α serum levels (Terpos, Politou, Viniou, & Rahemtulla, 2005). It has also been reported that MIP-1α promotes receptor activator of NF-κB ligand in osteoblast and bone marrow stromal cells, which directly induces osteoclast formation, thus leading to increased bone resorption (Tsubaki et al., 2007, 2010). Moreover, MIP-1α promotes cell proliferation in MM cells lines (Lentzsch et al., 2003; Tsubaki et al., 2008). These functions lead to the activation of the mitogen activated protein kinase kinase (MEK)/extracellular signal regulated kinase (ERK) pathway and phosphoinositide 3-kinase (PI3K)/Akt pathway by binding to the CC receptor 1 (CCR1) and/or CCR5 (Tsubaki et al., 2007; Walker, Lawson, Buckle, Snowden, & Chantry, 2014). Activation of MEK/ERK and PI3K/Akt pathways is involved in MM development and progression, as well as drug resistance in MM (Fuchs, 2013; Ocio et al., 2015; Ramakrishnan et al., 2012; Tsubaki et al., 2016). In addition, activated ERK1/2 and Akt increase the transcript levels of the anti-apoptotic Bcl-2 and IAP family of proteins, resulting in prolonged cell survival and acquisition of drug resistance (Fuchs, 2013; Kfir-Erenfeld, Sionov, Spokoini, Cohen, & Yefenof, 2010; Tsubaki et al., 2015). Thus, MIP-1α-induced ERK1/2 and Akt activation could provide a promising molecular target for the treatment of MM.
In the present study, we investigated the effect of MIP-1α neutralizing antibody and siRNA on MM cell growth and its possible enhancement of the cytotoxic effects of melphalan and bortezomib in MM cells.
2 MATERIALS AND METHODS
2.1 Materials
Melphalan was purchased from Sigma (St. Louis, MO). Bortezomib was purchased from Selleck Chemicals (Houston, TX). These reagents were dissolved in dimethyl sulfoxide and diluted in phosphate buffer saline (PBS; 0.05 M, pH 7.4), filtrated through syringe filters (0.45 µm, IWAKI GLASS, Tokyo, Japan), and used for various assays.
Human soluble MIP-1α and anti-MIP-1α neutralizing antibody were purchased from R&D Systems (Minneapolis, MN). These reagents were dissolved in PBS (0.05 M, pH 7.4) and used for various assays as described below.
2.2 Cell culture
The IM9 and RPMI 8226 cell lines were obtained from Health Science Research Resources Bank (Osaka, Japan). HS-sultan cells were obtained from Riken Cell Bank (Ibaraki, Japan). ARH-77 cells were obtained from DS Pharma Biomedical (Osaka, Japan). The PRMI8226/L-PAM variant and HS-sultan/L-PAM variant are resistant to melphalan. All cells were cultured in RPMI 1640 medium (Sigma) supplemented with 10% fetal calf serum (Gibco, Carlsbad, CA), 100 µg/ml penicillin (Gibco), 100 U/ml streptomycin (Gibco), and 25 mM HEPES (pH 7.4; Wako, Tokyo, Japan) and were maintained in an atmosphere containing 5% CO2.
2.3 Trypan blue dye exclusion assay
The effect of various reagents on cell survival/proliferation was determined using the trypan blue dye exclusion assay. Prior to each experiment, cells (2 × 103 cells/well) were plated onto 96-well plates. After culturing for 24 hr, the cells were exposed to anti-cancer drugs for various times. Equal volumes of cell suspension and 0.4% trypan blue solution were mixed gently, loaded into a hemocytometer, and the viable cells (unstained) and dead cells (stained blue) were counted. Each experiment was performed in triplicate. Results are reported from an average of at least five independent experiments.
2.4 Western blotting
The cellular proteins were extracted with the ProteoExtract Subcellular Proteome Extraction kit (Calbiochem, San Diego, CA). The protein content in the lysates was quantified with a BCA protein-assay kit. The extracts (40 µg of protein) were fractionated on SDS–polyacrylamide gels and transferred onto PVDF membranes (GE Healthcare, Buckinghamshire, UK). The membranes were blocked with a solution containing 3% skimmed milk and incubated overnight at 4°C with each of the following antibodies: anti-phospho-NF-κB p65 (#3031), anti-phospho-ERK1/2 (#9101), anti-phospho-Akt (#9271), anti-phospho-mTOR (#2971), anti-NF-κB p65 (#3034), anti-ERK1/2 (#9102), anti-Akt (#9272), anti-mTOR (#2972) anti-Survivin (#2803), anti-XIAP (#2042) (from Cell Signaling Technology, Beverly, MA), anti-Bcl-2 (C21), anti-Bcl-xL (H62), anti-Bim (H191), anti-Bax (N20), anti-cleaved cleaved Poly (ADP-ribose) polymerase (PARP) (194C1439), and anti-ubiquitin (FL-76) (Santa Cruz Biotechnology, CA). Subsequently, the membranes were incubated with horseradish peroxidase-coupled sheep anti-rabbit IgG (GE Healthcare) for 1 hr at room temperature. The reactive proteins were visualized using Luminata Forte (Millipore, Bedford, MA) according to the manufacturer's instructions. As an internal standard, an anti-β-actin mouse monoclonal antibody (Sigma) was used as the primary antibody to detect β-actin protein.
2.5 Quantitative real-time polymerase chain reaction (PCR)
Total RNA was isolated using RNAiso (Takara Biomedical, Shiga, Japan). One microgram of purified total RNA was used for real-time PCR analysis with the PrimeScript First-Strand Synthesis System (Takara Biomedical). cDNA was subjected to quantitative real-time PCR using SYBR Premix Ex Taq (Takara Biomedical) and the Thermal Cycler Dice Real Time System (TAKARA Bio, Ohtsu, Japan) in a 96-well plate according to the manufacturer's instructions. The PCR conditions for GAPDH and MIP-1α were 94°C for 2 min, followed by 40 cycles of 94°C for 0.5 min, 50°C for 0.5 min, and 72°C for 0.5 min. The following primer sequences were used: MIP-1α, 5′-CAG CGA GTA CCA GTC CCT TTT-3′ (5′-primer) and 5′-CCT CGC TGC CTC CAA GA-3′ (3′-primer); and GAPDH, 5′-AAG GTC GGA GTC AAC GGA TT-3′ (5′-primer) and 5′-CTC CTG GAA GAT GGT GAT GG-3′ (3′-primer). GAPDH was used as an internal control for normalization of the results of each sample. Cycle threshold (Ct) values were established, and the relative difference in expression from control cells was determined by the 2−ΔΔCt method of analysis.
2.6 Enzyme-linked immunosorbent assay (ELISA)
Cells were cultured at 1 × 104 cells/ml and conditioned media were harvested. The MIP-1α levels were measured using Quantikine MIP-1α enzyme immunoassay kits (R&D Systems), according to the manufacturer's instructions.
2.7 RNA interference
The double-stranded small interfering RNAs (siRNAs) targeting MIP-1α (MU-007841-03-0002) was synthesized and purified by GE Healthcare/Darmacon. Stealth™ RNAi negative control duplex (low GC content) (Invitrogen, Calrsbad, CA) was used as a negative control. Transfection of siRNAs was performed according to the manufacturer's protocol using the Lipofectamine™ 2000 reagent (Invitrogen). Briefly, 4 µl of 20-µM siRNA was mixed with 200 µl of Opti-minimum essential medium (MEM)®. Lipofectamine™ 2000 (4 μl) was diluted in 200 µl of Opti-MEM® and incubated at room temperature for 5 min. After incubation, the diluted Lipofectamine™ 2000 was mixed with diluted siRNA and further incubated for 20 min at room temperature. In total, 400 µl of the siRNA-Lipofectamine™ 2000 complex was applied to each well containing cultured IM9 cells at approximately 50–70% confluence in six-well microplates.
2.8 Statistical analysis
All results are expressed as mean ± S.D. of several independent experiments. Multiple comparisons of the data were done by ANOVA with Dunnett's test. P-values less than 5% were considered significant. Drug interactions were analyzed by the combination index (CI) based on the method described by Chou and Talalay (1984). CI values less than 1.0 indicate synergy, while CI values greater than 1 indicate antagonism.
3 RESULTS
3.1 Expression of MIP-1α in IM9 and ARH-77 cells, and the inhibitory effect of its neutralizing antibody on IM9 and ARH-77 cell growth
To assess whether different MM cell lines express MIP-1α mRNA, the MIP-1α levels were measured by real-time PCR. The expression of MIP-1α in IM9 and ARH-77 cells was higher than RPMI 8226 and HS-Sultan cells (Figure 1a). To confirm these findings, the MIP-1α secretion in these cells was analyzed by ELISA, which showed very low levels of MIP-1α secretion in RPMI 8226 and HS-Sultan cells (Figure 1b).
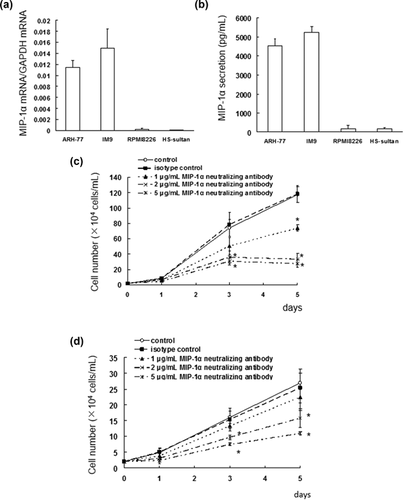
Investigation of the effect of a MIP-1α neutralizing antibody on MIP-1α-dependent IM9 and ARH-77 cell growth showed that the antibody suppressed the cell growth of IM9 and ARH-77 cells in a concentration-dependent manner (Figures 1c and 1d).
3.2 MIP-1α-neutralizing antibody enhances the cytotoxic effects of melphalan
Next, we investigated the cytotoxic effects of combination treatment with melphalan and MIP-1α neutralizing antibody in IM9 cells. IM9 cells were treated with MIP-1α neutralizing antibody (1–5 µg/ml) in combination with melphalan (0.5–5 µM), and the IM9 cell viability was assayed. The MIP-1α neutralizing antibody greatly enhanced the cytotoxic effect of the anticancer drugs (Figure 2a–c). The interaction between MIP-1α neutralizing antibody and melphalan was analyzed using the Chou–Talalay method. At the combination drug doses indicated earlier, the CI ranged from 0.357 to 0.027, showing the synergistic effect of these combinations (Figure 2a–c).
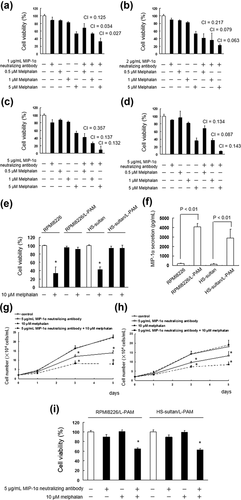
To validate these observations, we also investigated the cytotoxic effects of combination treatment with melphalan and MIP-1α neutralizing antibody in ARH-77 cells. We observed similar combined effects on ARH-77 following co-treatment with 5 µg/ml MIP-1α neutralizing antibody and melphalan (Figure 2d).
Next, we investigated the overcoming melphalan resistance of MIP-1α neutralizing antibody in melphalan resistant RPMI8226 (RPMI8226/L-PAM) and HS-sultan (HS-sultan/L-PAM) cells. We found that secretion of MIP-1α increased melphalan resistant cells than parent cells (Figures 2e and 2f). MIP-1α neutralizing antibody suppressed the cell growth and enhanced cytotoxic effect of melphalan in RPMI8226/L-PAM and HS-sultan/L-PAM cells (Figure 2g–i).
3.3 Combination treatment with MIP-1α neutralizing antibody and melphalan enhanced the inhibition of ERK1/2, Akt, and mTOR activation, and regulated the expression of Bcl-2, Bcl-xL, Survivin, and Bim
To examine the activity of the MIP-1α signaling pathway in IM9 cells, ERK1/2, Akt, mTOR, and NF-κB levels were assessed by immunoblotting in cell lysates from MIP-1α-stimulated IM9 cells. ERK1/2, Akt, and mTOR phosphorylation was detected after 15, 30, and 60 min of MIP-1α stimulation. There was no substantial change in the level of phosphorylated NF-κB in the MIP-1α-stimulated cells compared with that in the control cells (Figures 3a and 3c).
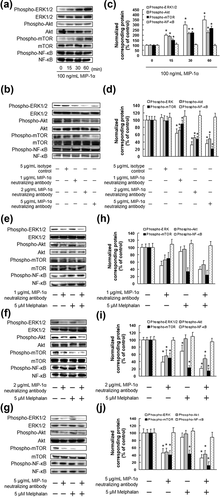
We next investigated the effect of treatment using the MIP-1α neutralizing antibody on signaling molecule activation in IM9 cells. The MIP-1α neutralizing antibody suppressed ERK1/2, Akt, and mTOR activation in a concentration-dependent manner (Figures 3b and 3d). We also examined the effect of combination treatment with MIP-1α neutralizing antibody and melphalan on MIP-1α signaling in IM9 and ARH-77 cells. Treatment with MIP-1α neutralizing antibody alone inhibited ERK1/2, Akt, and mTOR activation. In addition, melphalan alone inhibited ERK1/2 and mTOR activation (Figure 3e–j). Combination treatment with MIP-1α neutralizing antibody and melphalan significantly inhibited ERK1/2, Akt, and mTOR activation (Figure 3e–j).
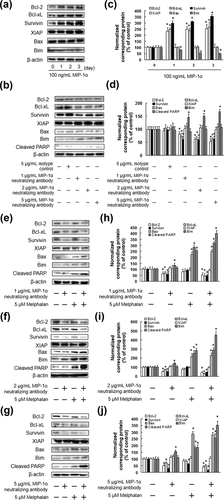
To elucidate the mechanisms of IM9 cell death associated with the targeted inhibition of ERK1/2, Akt, and mTOR activity, we assessed the changes in the expression of the Bcl-2 family proteins, Survivin, and XIAP, a caspase inhibitor. MIP-1α increased the expression of Bcl-2, Bcl-xL, and Survivin, and decreased the expression of Bim (Figures 4a and 4c). In addition, the MIP-1α neutralizing antibody suppressed the expression of Bcl-2, Bcl-xL, and Survivin, and enhanced the expression of Bim (Figures 4b and 4d). The results showed that co-treatment with the MIP-1α neutralizing antibody and melphalan enhanced the expression of Bim and cleaved PARP, and decreased the expression of Bcl-2, Bcl-xL, and Survivin in IM9 and ARH-77 cells. In contrast, there were no significant differences in the levels of the XIAP and Bax proteins following treatment with melphalan, or co-treatment with the MIP-1α neutralizing antibody and melphalan (Figure 4e–j).
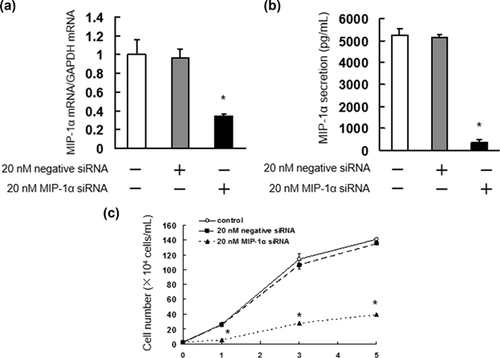
3.4 Enhanced cytotoxic effect of melphalan in combination with MIP-1α siRNA in IM9 cells
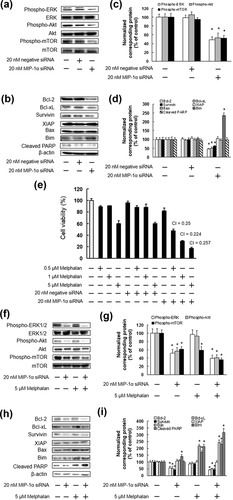
As our results indicated that the enhanced cytotoxic effects of MIP-1α neutralizing antibody and melphalan are mediated by inhibition of ERK1/2, Akt, and mTOR activation, IM9 cells were treated with MIP-1α siRNA to determine whether the suppression of ERK1/2, Akt, and mTOR enhanced the cytotoxic effect of melphalan. The treatment with MIP-1α siRNA significantly suppressed IM9 cell proliferation, which showed a similar effect as MIP-1α neutralizing antibody (Figure 5). In addition, we found that MIP-1α siRNA suppressed ERK1/2, Akt, and mTOR activation, and regulated Bcl-2, Bcl-xL, Survivin, and Bim expression (Figure 6a–d). The combination of MIP-1α siRNA and melphalan considerably induced cell death, similar to that observed after the co-administration of MIP-1α neutralizing antibody and melphalan (Figure 6e). Furthermore, co-treatment of MIP-1α siRNA and melphalan significantly inhibited ERK1/2, Akt, and mTOR activation, expression of Bcl-2, Bcl-xL, and Survivin, as well as increasing Bim and cleaved PARP expression (Figure 6f–i).
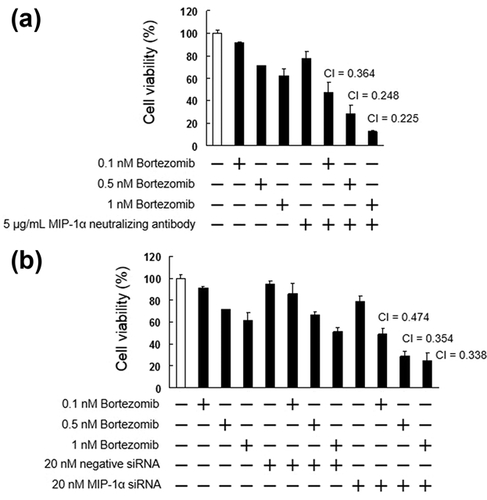
3.5 Inhibition of MIP-1α enhances the cytotoxic effects of bortezomib
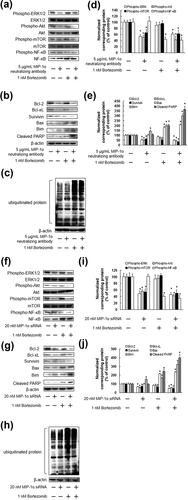
Bortezomib is used clinically for the treatment of MM and mantle cell lymphoma. As bortezomib could be beneficial as a combination drug, we investigated the cytotoxic effects of bortezomib when administered together with MIP-1α neutralizing antibody or MIP-1α siRNA. As shown in Figure 7, MIP-1α neutralizing antibody or MIP-1α siRNA significantly enhanced the cytotoxic effect of bortezomib. In addition, combination of bortezomib and either the MIP-1α neutralizing antibody or the siRNA suppressed the activation of ERK1/2, Akt, mTOR, and NF-κB, suppressed the expression of Bcl-2, Bcl-xL, and Survivin, and enhanced the expression of Bim, cleaved PARP, and ubiquitinated protein (Figures 8). In contrast, there were no significant differences in the levels of Bax proteins following treatment with bortezomib and co-treatment with either the MIP-1α neutralizing antibody or the siRNA and bortezomib (Figure 8).
4 DISCUSSION
In this study, we demonstrated that MM cells secreted MIP-1α, and treatment with MIP-1α neutralizing antibody suppressed MIP-1α-dependent MM cell growth. In addition, MIP-1α neutralizing antibody and MIP-1α siRNA increased the cytotoxic effect of melphalan and bortezomib. Moreover, MIP-1α neutralizing antibody partially overcame melphalan resistance in high secretion of MIP-1α cell lines (RPMI8226/L-PAM and HS-sultan/L-PAM cells). Although it has been reported that IL-6, VEGF, and HGF promote cell growth and survival of MM via autocrine and paracrine mechanisms (Lentzsch et al., 2004), we showed that MIP-1α may be a useful molecular target for the therapy of MM.
MIP-1α promotes cell growth and survival by binding to chemokine receptor 1 (CCR1) and CCR5, and activating ERK1/2 and Akt (Lentzsch et al., 2003; Tsubaki et al., 2007, 2010). In the present study, we investigated the mechanism of enhanced cytotoxicity of melphalan with MIP-1α neutralizing antibody. Our results showed that MIP-1α activated ERK1/2, Akt, and mTOR but did not affect NF-κB activation, suggesting that MIP-1α may promote cell growth and survival through the activation of ERK1/2, Akt, and mTOR in MM cells. We also demonstrated that the combined treatment of cells with melphalan and either the MIP-1α neutralizing antibody or siRNA inhibited ERK1/2, Akt, and mTOR activation. In addition, bortezomib inhibited NF-κB activation, but did not affect the activation of ERK1/2, Akt, or mTOR, and co-treatment with either a MIP-1α neutralizing antibody or siRNA and bortezomib suppressed ERK1/2, Akt, mTOR, and NF-κB activation. Our previous study indicated that multidrug resistance against adriamycin, vincristine, dexamethasone, and melphalan was related to the activation of Src in MM cell lines (Tsubaki et al., 2014). Src promotes the activation of downstream signaling pathways such as ERK1/2, Akt, and mTOR, which in turn induce cell proliferation, survival, and drug resistance (Abrams et al., 2010; Martelli, Evangelisti, Chiarini, & McCubrey, 2010; Penuel & Martin, 1999). In addition, it has been indicated that inhibition of the Akt pathway by siltuximab enhances cytotoxicity of melphalan in MM cells (Hunsucker et al., 2011). It has also been reported that activation of the PI3K/Akt/mTOR pathway is related to melphalan and bortezomib resistance (Du et al., 2015). Inhibition of the MEK/ERK pathway by AS703026, a MEK inhibitor, enhanced sensitivity to melphalan in MM cells (Kim et al., 2010). Moreover, activation of the MEK/ERK pathway via overexpression of CKS1B promotes bortezomib resistance in MM cells (Shi et al., 2010). These results suggest that the enhanced cytotoxic effects of melphalan and bortezomib by the MIP-1α neutralizing antibody or siRNA may be related to the inhibition of ERK1/2, Akt, or mTOR activation.
Activation of ERK1/2, Akt, or mTOR plays an important role in the expression of survival factors. ERK1/2 has been shown to induce the expression of the Bcl-2 family proteins, which play a role in mitochondrial apoptosis; and the IAP family proteins, which are intrinsic caspase inhibitors (Balmanno & Cook, 2009; Tsubaki, Itoh, et al., 2013; Tsubaki et al., 2015). In addition, Akt is regulated by the expression of the Bcl-2 and IAP family proteins (Kim et al., 2016; Srinivasan et al., 2009; Stiles, 2009). Moreover, mTOR promoted the expression of the Bcl-2 and IAP family proteins (Brouxhon et al., 2013; Huang, Lyu, Wang, & Liu, 2014; Tsubaki, Itoh, et al., 2013; Tsubaki, Komai, et al., 2013). Furthermore, overexpression of Survivin or downregulation of Bim, a member of the Bcl-2 family, were involved in multidrug resistance in cancer cells (Tsubaki et al., 2012, 2014). In the present study, the combination treatment with either the MIP-1α neutralizing antibody or siRNA and either melphalan or bortezomib suppressed the expression of Bcl-2, Bcl-xL, and Survivin, enhanced the expression of Bim, and cleaved PARP to a greater extent than single treatment with either the MIP-1α neutralizing antibody, MIP-1α siRNA, melphalan, or bortezomib. It has been reported that MEK inhibitor suppresses Bcl-xL and increases the expression of Bim in acute myeloid leukemia cells (Airiau et al., 2016). It has also been reported that inhibition of ERK1/2 and Akt activation decreases Bcl-2 protein levels in ovarian carcinoma cells (Jang, Kim, Myung, Kim, & Lee, 2011). In addition, suppression of the PI3K/Akt/mTOR pathway inhibited Survivin expression (Dey et al., 2016). Moreover, ERK1/2, Akt, and mTOR have been shown to regulate the expression of Bim (Tsubaki, Itoh, et al., 2013; Tsubaki, Komai, et al., 2013). In bortezomib-treated patients with relapsed/refractory MM overexpressed Bcl-2, and obatoclax, a BH3 mimetic compound, led to the disruption of the Bcl-2/Bim complex and enhanced the patient's sensitivity to bortezomib and melphalan (Ailawadhi et al., 2012; Pérez-Galán et al., 2008; Trudel et al., 2007). Inhibition of Akt suppressed Survivin and Bcl-2 expression, and enhanced the sensitivity of bortezomib (Ding, Yuan, & Chen, 2017). Moreover, LBH589, an HDAC inhibitor, suppressed Bcl-2 and Bcl-xL expression, and synergized with bortezomib, dexamethasone, and melphalan to decrease MM cell number (Maiso et al., 2006). It was reported that suppression of Bcl-xL and Survivin increased the sensitivity of MM cells to melphalan, vincristine, and adriamycin (Takeda et al., 2016). These findings suggest that enhancing the cytotoxic effects of melphalan and bortezomib by using MIP-1α neutralizing antibodies and siRNA may be related to the suppression of Bcl-2, Bcl-xL, and Survivin expression and enhanced expression of Bim and cleaved PARP.
In conclusion, our data show that the cytotoxic effects of melphalan and bortezomib were enhanced by MIP-1α neutralizing antibody or MIP-1α siRNA in MM cells, and this effect is mediated by the inhibition of MIP-1α binding to its receptor, which inhibits the activation of the MEK/ERK and Akt/mTOR pathways. MIP-1α neutralizing antibody and MIP-1α siRNA may therefore be promising therapeutic agents for the suppression of MIP-1α-dependent myeloma cell growth.
ACKNOWLEDGMENTS
This work was supported in part by a Grant-in-Aid for Scientific Research (C) (Grant number 15K08116), Grant-in-Aid for Young Scientists (B) (Grant number 16K18965) from the Japan Society for the Promotion of Science (JSPS) and by Ministry of Education, Culture, Sports, Science, and Technology (MEXT)-Supported Program for the Strategic Research Foundation at Private Universities, 2014-2018 (Grant number S1411037).
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.




