CpG oligodeoxynucleotide preconditioning improves cardiac function after myocardial infarction via modulation of energy metabolism and angiogenesis
Abstract
Unmethylated CpG oligodeoxynucleotide (CpG-ODN), a Toll-like receptor 9 (TLR9) ligand, has been shown to protect against myocardial ischemia/reperfusion injury. However, the potential effects of CpG-ODN on myocardial infarction (MI) induced by persistent ischemia remains unclear. Here, we investigated whether and how CpG-ODN preconditioning protects against MI in mice. C57BL/6 mice were treated with CpG-ODN by i.p. injection 2 hr prior to MI induction, and cardiac function, and histology were analyzed 2 weeks after MI. Both 1826-CpG and KSK-CpG preconditioning significantly improved the left ventricular (LV) ejection fraction (LVEF) and LV fractional shortening (LVFS) when compared with non-CpG controls. Histological analysis further confirmed the cardioprotection of CpG-ODN preconditioning. In vitro studies further demonstrated that CpG-ODN preconditioning increases cardiomyocyte survival under hypoxic/ischemic conditions by enhancing stress tolerance through TLR9-mediated inhibition of the SERCA2/ATP and activation of AMPK pathways. Moreover, CpG-ODN preconditioning significantly increased angiogenesis in the infarcted myocardium compared with non-CpG. However, persistent TLR9 activation mediated by lentiviral infection failed to improve cardiac function after MI. Although CpG-ODN preconditioning increased angiogenesis in vitro, both the persistent stimulation of CpG-ODN and stable overexpression of TLR9 suppressed the tube formation of cardiac microvascular endothelial cells. CpG-ODN preconditioning significantly protects cardiac function against MI by suppressing the energy metabolism of cardiomyocytes and promoting angiogenesis. Our data also indicate that CpG-ODN preconditioning may be useful in MI therapy.
1 INTRODUCTION
Myocardial infarction (MI) remains the leading cause of death and long-term disability worldwide despite great advances in treatment strategies in recent decades. At present, there is no effective treatment for MI or ischemic heart disease. The innate immune and inflammatory responses mediated by Toll-like receptors (TLRs) have been previously demonstrated to play important roles in ischemic injury (Christia & Frangogiannis, 2013; Prabhu & Frangogiannis, 2016). Recent studies have indicated that TLRs may be important targets for the development of new treatment approaches for ischemic heart disease (Wang et al., 2016; Yang et al., 2016).
TLRs were originally identified as receptors for exogenous pathogens, thereby initiating the inflammatory response by immune cells. Among the TLRs, TLR9 is the only member that detects endogenous or exogenous DNA (Hemmi et al., 2000). Although TLR9 was originally found as a sensor for bacterial DNA containing abundant unmethylated CpG dinucleotides (Hemmi et al., 2000), subsequent evidence has demonstrated that synthetic unmethylated CpG oligodeoxynucleotides (CpG-ODNs) can active TLR9 (Krieg, 2006; Kumagai, Takeuchi, & Akira, 2008). With the exception of immune cells, increasing evidence has demonstrated that TLR9 is expressed in nonimmune cells, including cardiomyocytes (Boyd, Mathur, Wang, Bateman, & Walley, 2006), neurons (Crack & Bray, 2007), fibroblasts (Ohm, Alfsnes, et al., 2014), and stem cells (Nurmenniemi et al., 2010). Therefore, the exact roles and potential regulatory functions of TLR9 in nonimmune cells has attracted increasing attention from scientists. Interestingly, both in vitro and in vivo studies have indicated that activation of TLR9 induced by CpG-ODN protects cardiomyocytes against ischemia by modulating energy metabolism (Paladugu et al., 2004; Shintani et al., 2014, 2013). The benefit of TLR9 activation for cardiac function was also reported in a cardiac hypertrophy mouse model. Velten et al. (2012) demonstrated that CpG-ODN 1668 treatment attenuates cardiac hypertrophy following pressure overload by modifying the inflammatory response, thereby reducing cardiac growth and fibrosis as well as delaying the loss of cardiac function. Moreover, it has also been reported that the TLR9 activation induced by CpG-ODN attenuates myocardial ischemia/reperfusion injury (Cao et al., 2013; Markowski et al., 2013). However, TLR9-stimulation upon the onset of ischemia and subsequent reperfusion does not provide any benefit for cardiac ischemia/reperfusion injury (Ohm, Gao, et al., 2014). These different results indicate that CpG-ODN/TLR9 signaling may be inconsistent for the treatment of cardiac ischemia/reperfusion injury and that the treatment time point is more important.
Here, we investigated the effects of the preconditioning of CpG-ODNs, including KSK-CpG and 1826-CpG, on MI and the underlying biological mechanism. We observed that administration of CpG-ODN preconditioning significantly improves cardiac function after MI, at least in part, through suppressing energy metabolism in cardiomyocytes and promoting angiogenesis in infarcted myocardium.
2 MATERIALS AND METHODS
2.1 Vector construction and lentivirus production
To construct the lentiviral vector expressing an shRNA targeting mouse TLR9, an shRNA expression cassette (7SK-shRNA-TLR9) was isolated from the psiRNA-TLR9 plasmid (InvivoGen, San Diego, CA) using ClaI and XbaI double digestion, which was inserted into the pLOXCMV-E/P lentiviral vector (Qi et al., 2015) to replace the CMV promoter by ClaI and SpeI (SpeI and XbaI has the same cohesive terminus) double digestion. The pLOXshRNA-TLR9 lentiviral vector was then constructed. For TLR9 overexpression in the lentiviral vector, the ORF of the mouse TLR9 gene was amplified by PCR primer pairs containing the EcoRI and XhoI restriction sites from the template of the pUNO1-mTLR9 plasmid (InvivoGen). The amplified fragments were then digested and ligated into the pLOXCMV-E/P vector linearized by the EcoRI and XhoI enzymes, thereby constructing the overexpression lentiviral vector pLOXCMV-TLR9 (pLOX-TLR9).
Lentiviruses were prepared in HEK293T cells by co-transfection of pLOX-TLR9 or pLOXshRNA-TLR9 together with the packaging plasmids pCMVR8.74 (#22036, Addgene, Cambridge, MA) and pMD2.G (#12259, Addgene) using the LipoFiter™ Liposomal Transfection Reagent (Hanbio Biotechnology, Shanghai, China). The viral supernatant was concentrated with the Lenti Virus Concentration Reagent (Biomiga, San Diego, CA) and titrated in HEK293T cells. High titer viruses (1 × 109 PFU/ml) were re-suspended in PBS. A large number of small stock aliquots (10 µl) were made and frozen at −80°C for myocardium injection in mice. For animal experiments, a total of 30 µl of lentivirus was injected into three sites in the infarct zone of MI mouse models through an insulin syringe with an incorporated 29 G needle (BD).
2.2 Animal protocols
Male C57BL/6 mice at 8–10 weeks of age were randomized to receive an intraperitoneal (i.p.) injection of CpG-ODNs (1 mg/kg body weight) 2 hr prior to left anterior descending artery (LAD) ligation surgery as previously described (Lu et al., 2014). For certain experiments confirming the efficiency of lentiviral infection in vivo, lentiviruses were injected into the myocardium 3 days prior to MI induction to first upregulate or downregulate TLR9 expression. CpG-ODNs were provided by MWG-Biotech AG (Ebersberg, Germany) and completely phosphorothioate modified as previously described (Qi, Zheng, et al., 2013). The sequences and structures are as follows: KSK-CpG (5′-TCG TCG TTT TCG TCG TCG TTT T-3′), 1826-CpG (5′-TCC ATG ACG TTC CTG ACG TT-3′), and non-CpG (5′-TCC AGG ACT TCT CTC AGG TT-3′). The CG dinucleotides are indicated by italics and underlines. All mouse surgical and experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Jinan University. Sham controls were the same as the MI operation but without the LAD ligation. Animals were anaesthetized with ketamine (100 mg/kg, i.p.).
2.3 Echocardiography
Two weeks after the ligation of LAD, transthoracic echocardiography was performed using the Vevo® 2100 ultrasound system (Visualsonics, Toronto, Canada) equipped with a high-frequency (30 MHz) linear array transducer. Parasternal long-axis, short-axis, and two apical four-chamber views were used to obtain two-dimensional and M-mode images. The left ventricular (LV) ejection fraction (LVEF) and LV fractional shortening (LVFS) were automatically calculated by the Vevo® 2100 ultrasound system.
2.4 Histological analysis
Following serum collection, hearts were rapidly excised and transected into two segments through the infarct zone. One segment was fixed in 4% paraformaldehyde for 48 hr and embedded in paraffin. The other segment was embedded in Tissue-Tek optimal cutting temperature compound (OCT) (Sakura) for frozen sections. Paraffin sections (5 µm) were prepared and stained with hematoxylin and eosin (H&E) or with Masson trichrome for morphological and fibrosis analysis, respectively. The fibrotic area in each section was calculated by Image-Pro Plus version 6.0 software.
2.5 Serum VEGF levels
After echocardiography analysis, serum was collected and measured with a mouse VEGF ELISA Kit (R&D Systems, Minneapolis, MN) to evaluate the level of VEGF in the serum.
2.6 Cell culture
Primary cardiomyocytes were isolated from 2- to 3-day old mice (C57BL/6) using enzymatic tissue digestion as previously described (Ehler, Moore-Morris, & Lange, 2013; Shintani et al., 2013). Briefly, hearts were incubated with 0.05% trypsin/EDTA at 4°C overnight followed by digestion with collagenase type II. Cardiomyocytes were then collected based on a differential plating method, and they were cultured with Dulbecco's-Modified Eagle Medium (DMEM) supplemented with 5% fetal bovine serum (FBS) at 37°C. Adult primary cardiomyocytes were isolated as described previously (Ackers-Johnson et al., 2016).
H9C2 cells were cultured in DMEM supplemented with 10% FBS at 37°C. The culture media was changed to DMEM containing 2% FBS for CpG-ODN administration. For H/Iexperiments, both primary cardiomyocytes and H9C2 cells were cultured in DMEM medium containing 2% FBS and incubated in a hypoxic incubator adjusted at 1% O2 and 5% CO2. For CpG-ODN preconditioning, primary cells and the cell line were pretreated with CpG-ODNs (1 μg/ml) for 1 hr under normoxic conditions. Fresh media was then changed and followed by continuous hypoxic or normoxic incubation. Mouse cardiac microvascular endothelial cells (CMECs) were isolated and cultured as previously described (Qi et al., 2015). NIH-3T3 cells were cultured in DMEM supplemented with 10% FBS at 37°C and used to examine mouse TLR9 knockdown or overexpression mediated by lentiviral infection.
2.7 Cell viability assay
H9C2 cell viability was analyzed using the CCK-8 Cell Counting Kit (Beyotime Biotechnology, China) according to the manufacturer's instructions as previously described (Qi, Li, et al., 2013). For primary cardiomyocytes, a semi-quantitative method of immunofluorescence staining for anti-cTnT antibody was used. Briefly, surviving cardiomyocytes were specifically identified by cTnT antibody staining. Cell viability was evaluated by the relative ratio of cTnT-positive cells to all nuclei.
2.8 Intracellular ATP measurement
Intracellular ATP levels were measured using the Enhanced ATP Assay Kit (Beyotime Biotechnology, China) according to the manufacturer's instructions. Values were normalized by protein content.
2.9 Immunofluorescence staining
Immunocytochemistry was performed as previously described (Qi et al., 2015). Briefly, cells were fixed, permeabilized, washed, blocked, and incubated with anti-TLR9, anti-cTnT and anti-SERCA2 antibodies (Cell Signaling) overnight at 4°C. The cells were then washed and incubated with goat anti-rabbit IgG-FITC or goat anti-rabbit IgG-Cy5 (Santa Cruz, CA) for 1 hr at room temperature, and nuclei were stained for 10 min with DAPI (10 mg/ml; Sigma–Aldrich Co., St. Louis, MO). The stained cells were observed by fluorescence microscopy (Olympus, Tokyo, Japan). Image-Pro Plus version 6.0 software was used to quantify the fluorescent intensity of the cells.
For tissues samples, frozen sections (4 µm) were incubated with the primary antibodies directed against alpha-smooth muscle actin (α-SMA) (1:100 dilution, Abcam, CA), cleaved caspase 3 (1:400 dilution, Cell Signaling), or TLR9 (1:400 dilution, Cell Signaling) at 4°C overnight. After washing, the sections were incubated with the goat anti-rabbit IgG-FITC or goat anti-rabbit IgG-Cy5 (Santa Cruz) at room temperature for 2 hr. Nuclei were counterstained with DAPI. α-SMA signaling was used to evaluate the density of the capillary vessels. In addition, EGFP expression was directly examined using frozen sections to monitor the lentiviral infection in vivo.
2.10 In vitro capillary-like tube formation
Prechilled 48-well microtiter plates were coated with 100 µl growth factor-reduced Matrigel (BD Biosciences, San Jose, CA) and incubated for 2 hr at 37°C. Where indicated, CMECs (5 × 104) were loaded into each well and incubated in DMEM containing 2% FBS for 16 hr. Tube formation was analyzed with an inverted light microscope.
2.11 Western blotting
Whole cell extract preparations were obtained using Cell Lysis Buffer (Beyotime Biotechnology, China) as previously described (Qi et al., 2015). Briefly, after separation by SDS-PAGE and transference, PVDF membranes were then blocked with 5% nonfat milk, washed briefly, incubated with primary antibodies (against TLR9 and β-actin) at 4°C overnight, and then incubated with corresponding HRP-conjugated secondary antibodies for 1 hr at room temperature. Protein bands were visualized by incubating the membranes with chemiluminescence reagents prior to exposure to a gel imaging system.
2.12 RT-PCR analysis
Total RNA was isolated using TRI Reagent (Molecular Research Center Inc., Cincinnati, OH). The quantity and purity of RNA was verified by measuring A260 and A280. cDNA was synthesized from total RNA (2 µg) with oligo (dT)18 primers (0.5 µg) using the ReverTra Ace® qPCR RT Kit (Toyobo, Japan). The primers used in this study are as follows: TLR9: 5′-GAC TTA CTG TTG GAG GTG CAG ACC-3′ (forward) and 5′-GAA CAC CAC GAA GGC ATC ATA GG-3′ (reverse) and β-actin: 5′-GCA CCA CAC CTT CTA CAA TGA G-3′ (forward) and 5′-TTG GCA TAG AGG TCT TTA CGG A-3′ (reverse). β-actin was used as an internal control. All samples were pre-denatured for 5 min at 94 °C according to the PCR Master Mix instructions (Thermo Fisher Scientific, Hudson, NH). The conditions for PCR amplification were as follows: 28 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s. Amplified DNAs were further extended by an additional extension at 72 °C for 7 min. The PCR products were subjected to electrophoresis in 2% agarose gels and visualized by staining with ethidium bromide.
2.13 Statistical analysis
All data are presented as the mean ± SEM. Statistical analyses were performed using the unpaired Student's t-test. A p value less than 0.05 was considered statistically significant.
3 RESULTS
3.1 CpG-ODN preconditioning improves cardiac function after MI
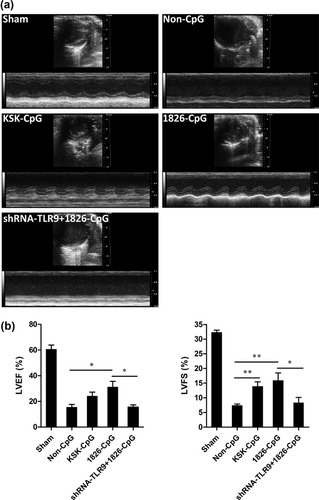
To examine the role of CpG-ODN preconditioning in MI, we administered CpG-ODN and non-CpG-ODN (non-CpG, control) to mice 2 hr prior to MI induction. For some experiments, lentiviruses containing TLR9 shRNA were injected into the heart 3 days prior to MI induction. Cardiac function was evaluated by echocardiography 2 weeks after MI. As shown in Figure 1a, MI-induced decreases in LV systolic function were improved to various degrees by the preconditioning of CpG-ODNs but not by non-CpG. The LVEF was significantly increased by the preconditioning of 1826-CpG compared with non-CpG controls. Although the difference was not statistically significant, KSK-CpG also increased the LVEF values compared with non-CpG. Moreover, LVFS was significantly higher in mice preconditioned by CpG-ODNs compared with the non-CpG control (Figure 1b). However, TLR9 knockdown by lentivirus significantly blocked the cardiac protection of 1826-CpG against MI (Fiure. 1).
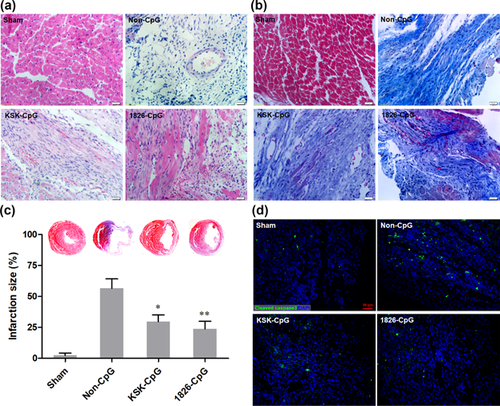
To evaluate the myocardium morphology and fibrosis, H&E and Masson trichrome staining were performed. As shown in Figure 2a, almost no integral cardiomyocytes were observed in the infarct zone in the non-CpG preconditioning group. However, integral cardiomyocytes were observed in CpG-ODN, particularly 1826-CpG, preconditioned mice. Masson trichrome staining showed that CpG-ODN preconditioning significantly attenuated the development of cardiac fibrosis in mice with MI (Figures 2b and 2c). Moreover, CpG-ODN preconditioning could reduce the apoptosis level in the infarcted myocardium, which was confirmed by cleaved caspase three staining (Figure 2d). Taken together, these results demonstrate that CpG-ODN preconditioning improves cardiac function after MI.
3.2 CPG-ODN reconditioning protects cardiomyocytes from hypoxia
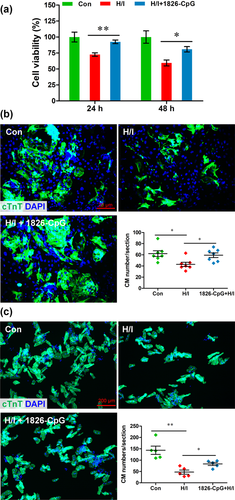
Given that cardiomyocyte cellular injury is mainly induced by hypoxia and ischemia during MI, the potential protective effects of CpG-ODN preconditioning on cardiomyocytes were further examined in vitro. Both the H9C2 cell line and primary cardiomyocytes were pretreated with 1826-CpG (1 μg/ml) for 1 hr prior to H/I induction. As shown in Figure 3a, H/I induced marked injury of H9C2 cells from 24 to 48 hr, but the decrease in cell survival was significantly abolished by 1826-CpG preconditioning. For primary cardiomyocytes, both neonatal and adult cardiomyocyte viability were markedly suppressed by H/I treatment for 48 hr, which is consistent with results in H9C2 cells. However, preconditioning of 1826-CpG significantly increased the cell survival under H/I (Figures 3b and 3c). These findings suggest that CpG-ODN preconditioning protects cardiomyocyte survival against hypoxic and ischemic conditions.
3.3 CpG-ODN preconditioning increases TLR9 expression in cardiomyocytes accompanied by metabolism regulation
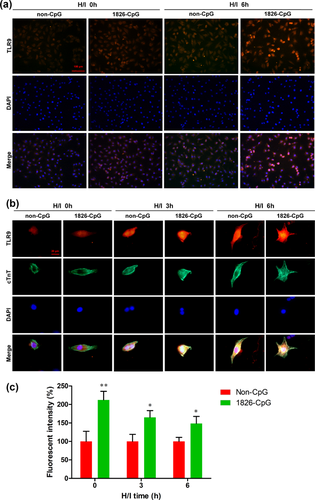
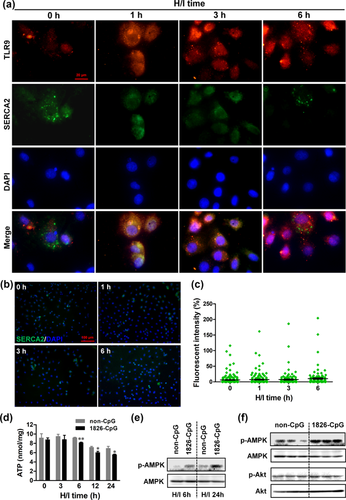
We next examined the expression of TLR9 in cardiomyocytes treated with CpG-ODN preconditioning by immunocytochemistry. As shown in Figure 4a, 1826-CpG preconditioning promoted TLR9 expression in H9C2 cells even under normoxic conditions. Moreover, marked TLR9 expression was observed in H9C2 cells preconditioned by 1826-CpG 6 hr after H/I induction. Consistent with the cardiomyocyte cell line, 1826-CpG preconditioning also increased the expression of TLR9 in neonatal primary mouse cardiomyocytes under both normoxic and H/I conditions (Figures 4b and 4c). These results indicated that TLR9 may be involved in CpG-ODN-mediated cardiomyocyte protection against H/I treatment.
Indeed, it has been reported that TLR9 plays important roles in the cellular protection of cardiomyocytes by regulating SERCA2/AMPK pathway-mediated cellular metabolism (Shintani et al., 2014, 2013). We therefore examined the expression of SERCA2 in cardiomyocytes preconditioned with 1826-CpG. As shown in Figure 5a, SERCA2 expression was decreased in H9C2 cells treated with 1826-CpG under H/I although the expression of TLR9 was increased. However, there was no difference in SERCA2 expression in H9C2 cells directly subjected to H/I (Figures 5b and 5c). Given that SERCA2 is an Ca2+ ATPase that governs ATP production and energy metabolism (Shintani et al., 2014), ATP production was also analyzed in H9C2 cells treated with 1826-CpG. As shown in Figure 5d, 1826-CpG preconditioning significantly decreased ATP production in H9C2 cells under H/I from 6 to 24 hr compared with non-CpG. Previous studies have determined that decreases in ATP is a key trigger for the activation of AMP-activated kinase (AMPK) in cardiomyocytes, which thereby increases cellular tolerance against metabolic stress (Shintani et al., 2014, 2013). AMPK activation was subsequently analyzed by western blotting to further confirm whether the SERCA2/ATP/AMPK pathway was involved in CpG-ODN-mediated cardiomyocyte protection. We found that the preconditioning of 1826-CpG increased AMPK phosphorylation in H9C2 cells treated with H/I (Figure 5e). Importantly, a great increase in AMPK phosphorylation was also observed in infarcted myocardium tissues after preconditioning with CpG-ODN, which is in addition to the slight Akt phosphorylation (Figure 5f). These findings further reveal that the SERCA2/ATP/AMPK pathway may play an important role in the CpG-ODN preconditioning-mediated cellular protection of cardiomyocytes through TLR9 activation.
3.4 CpG-ODN preconditioning promotes angiogenesis after MI
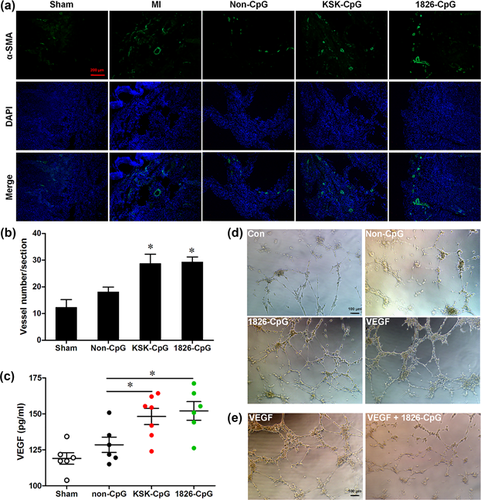
It is known that angiogenesis plays a critical role in the repair of damaged hearts. However, a series of studies have suggested that continuous treatment with CpG-ODNs suppresses angiogenesis under different pathological or physiological statuses (Wu, Cui, Dick, & Liu, 2014; Wu et al., 2016; Yan et al., 2015). To further explore the potential involvement of CpG-ODN preconditioning in angiogenesis after MI, angiogenesis was analyzed by immunofluorescence staining for α-SMA. As shown in Figures 6a and 6b, CpG-ODN preconditioning significantly promoted angiogenesis compared with non-CpG although MI also induced angiogenesis to a certain extent compared with the sham group. Moreover, serum VEGF production was also increased by CpG-ODN preconditioning compared with non-CpG (Figure 6c). The in vitro angiogenesis of endothelial cells preconditioned by CpG-ODN was further examined by tube formation assays. We found that 1826-CpG preconditioning promoted the tube formation of CMECs as VEGF-treated endothelial cells (Figure 6d). However, the VEGF-mediated tube formation of endothelial cells was suppressed by continuous treatment with 1826-CpG (Figure 6e). These results indicate that different CpG-ODN-mediated effects on angiogenesis depend on the administration method, including preconditioning or continuous treatment.
3.5 Persistent expression of TLR9 fails to protect cardiac function from MI
Finally, we examined whether persistent TLR9 activation had the same protective effects on MI using lentiviral-mediated stable expression of the TLR9 gene. Surprisingly, overexpression of the TLR9 gene around the infarcted zone had no effect on improving cardiac function compared with the control group, which was injected only with PBS. Furthermore, TLR9 knockdown by lentiviral-mediated shRNA also had no influence on cardiac function compared with the control group. Both LVEF and LVFS were not significantly regulated by the persistent overexpression or knockdown of TLR9 compared with the control group (Figures 7a and 7b). The efficient overexpression and knockdown of TLR9 was verified both in vitro (NIH-3T3 cells) and in vivo (mice) (Figure 7c–f). Furthermore, the efficient infection of lentiviruses in vivo was further confirmed by expression of the EGFP reporter gene in the myocardium (Figure 7g). These findings further indicate that the persistent activation of TLR9, the target of CpG-ODNs, failed to improve cardiac function. However, TLR9 overexpression suppressed the tube formation of endothelial cells, indicating that persistent TLR9 activation inhibits angiogenesis (Figure 7h).

4 DISCUSSION
This study has shown that the preconditioning of CpG-ODN, a TLR9 ligand, to mice 2 hr prior to LAD ligation significantly improves cardiac function and angiogenesis following MI. Moreover, CpG-ODN preconditioning protects cardiomyocyte survival under hypoxic and ischemic conditions through the regulation of the SERCA2/ATP/AMPK pathway. In addition, tube formation was increased by CpG-ODN preconditioning in vitro. However, persistent stimulation of CpG-ODN or stable overexpression of TLR9 failed to protect cardiomyocyte survival and improve angiogenesis. Thus, our data suggest that preconditioning, but not persistent stimulation, of CpG-ODN improves cardiac function after MI by increasing cardiomyocyte survival and angiogenesis.
Although TLR9 plays important roles in immune responses, its potential benefit for the treatment of ischemic diseases independent of inflammation has recently attracted attention. It has been reported that CpG-ODN preconditioning induces protection against cerebral and myocardial ischemia/reperfusion injury by activating the PI3K/Akt pathway (Cao et al., 2013; Lu et al., 2014). Moreover, CpG-ODN postconditioning also improves cardiac function after myocardium ischemic/reperfusion injury by activating the TLR9/PI3K pathway (Kim et al., 2014). In addition, CpG-ODN preconditioning attenuates cardiac hypertrophy following pressure overload and delays the loss of cardiac function. These studies have revealed that CpG-ODN cardioprotection depends on the method and time of administration. Consistent with these studies, our results have indicated that CpG-ODN preconditioning protects cardiac function following myocardial infarction (Figure 1) although we used a MI instead of ischemic/reperfusion injury model. However, the TLR9 activation induced by CpG-ODN during the onset of myocardial ischemia does not influence cardiac function, including the infarct size in a myocardial ischemia/reperfusion model (Ohm, Gao, et al., 2014). Thus, these studies further demonstrate that CpG-ODN/TLR9 preconditioning (administration prior to injury) may induce a certain cascade of reactions prior to the onset of tissue injury, thereby abolishing injury related pathways and protecting damaged tissue or cells. Indeed, our data show that persistent TLR9 overexpression fails to protect cardiac function after MI (Figure 7).
Ischemia and hypoxia-induced cell damage or cell death is the main reason for myocardium injury following MI. Therefore, promoting cardiomyocyte survival and angiogenesis plays a critical role in therapy for MI. Although severe hypoxia in vivo (13% oxygen) induced cardioprotection (Mohammed Abdul, Jovanovic, Du, Sukhodub, & Jovanovic, 2015), our severe in vitro hypoxia (1% oxygen) and ischemia (2% FBS) induced the cell death of cardiomyocytes (Figure 3). Under our H/I conditions, we found that CpG-ODN preconditioning significantly promotes the survival of cardiomyocytes under hypoxic and ischemic conditions depending on the regulation of cardiomyocyte energy metabolism (Figures 3-5). In fact, it has been demonstrated that TLR9 activation suppresses SERCA2 expression and activation, inhibits ATP production and increases AMPK activation, thereby enhancing the tolerance of cardiomyocytes to stress (Shintani et al., 2014, 2013). These previous studies further confirmed our results demonstrating that the SERCA2/ATP/AMPK pathway may play an important role in CpG-ODN preconditioning-mediated cardioprotection (Figure 5). Indeed, it has been demonstrated that AMPK activation induces cardioprotection by activating and recruiting sarcolemmal KATP channels, or by up-regulating the expression of SUR2A, a cardioprotective protein (Mohammed Abdul, Jovanovic, & Jovanovic, 2017; Sukhodub et al., 2007).
Many studies have reported that the CpG-ODN/TLR9 signal contributes to angiogenesis inhibition in different models. For instance, CpG-ODN treatment efficiently inhibited corneal angiogenesis in a mouse model (Wu et al., 2014, 2016). Moreover, CpG-ODN administration significantly inhibited tumor growth in a rat model by suppressing angiogenesis (Yan et al., 2015). These results are different from our data showing that CpG-ODN preconditioning greatly promotes angiogenesis in vivo and in vitro (Figure 6). The possible reasons for these different results may be explained by the method and time of CpG-ODN administration. In fact, anti-angiogenetic effects in the cornea were observed after persistent CpG-ODN stimulation from the onset of suturing injury (Wu et al., 2014, 2016). For the anti-angiogenetic effects of CpG-ODN in tumors, CpG-ODN was also administered at the same time as tumor cell injection (Yan et al., 2015). Consistently, we also found that persistent CpG-ODN stimulation or stable TLR9 overexpression indeed suppressed angiogenesis in vitro (Figures 6e and 7h). Together these results appear to suggest that CpG-ODN preconditioning promotes angiogenesis, but the persistent stimulation of CpG-ODN results in anti-angiogenetic effects.
In summary, CpG-ODN preconditioning significantly promotes cardiac function after MI through the protection of cardiomyocyte survival and angiogenesis under hypoxic and ischemic conditions. However, persistent CpG-ODN stimulation does not protect against cardiomyocyte injury, and stable overexpression of TLR9 fails to improve cardiac function following MI. CpG-ODN cardioprotection may depend on the enhanced stress tolerance mediated by the SERCA2/ATP/AMPK pathway. Furthermore, increased VEGP production in serum contributed, at least in part, to CpG-ODN preconditioning-mediated angiogenesis in myocardium following MI. Our data suggest that CpG-ODN preconditioning may be a novel approach for managing and treating cardiac function decrease following MI.
ACKNOWLEDGMENTS
This work was supported by grants from the National Key R and D Program of China (2016YFE0204700 and 2017YFA0103302), the National Natural Science Foundation of China (81570222, 81770240, 91649203, 81670422, and 81270183), the Guangdong Natural Science Funds for Distinguished Young Scholar (2014A030306011), the Guangdong Science and Technology Planning Project (2014A050503043 and 2016A020221034), the New Star of Pearl River on Science and Technology of Guangzhou (2014J2200002), the Top Young Talents of Guangdong Province Special Support Program (87315007), the Foundation for Distinguished Young Talents in Higher Education of Guangdong Province (2013LYM0026), the Fundamental Research Funds for the Central Universities (21617436), Young Taishan Scholars Program of Shandong Province (tsqn20161045) and the SRF for ROCS, SEM (2013-693), China.
CONFLICTS OF INTEREST
There are no conflicts of interest.
AUTHORS CONTRIBUTION
XFQ and LZ designed research and wrote the paper. DCZ, YHS, FQJ, and JBX contributed animal experiments and histology analysis. HYW established primary cardiomyocytes and CMECs cultures. ZSC contributed cell viability and ATP assay. WTP contributed plasmids construction. XFQ, DCZ, and JBX analyzed data. GHS, KSP, SKK, and DQC reviewed the manuscript.




